Abstract
Purpose
Present study was intended to investigate the potential contribution of TRPV5 gene polymorphisms with calcium urolithiasis in the population of West Bengal, India.
Methods
A case–control study was performed with 152 calcium urolithiasis patients and 144 corresponding healthy controls. Epidemiological and clinical parameters were documented as well as peripheral blood sample was collected from each individual, followed by genomic DNA isolation. Then to identify genetic variants of TRPV5, the entire coding region and exon–intron boundaries of the gene were amplified by polymerase chain reaction using specific oligonucleotide primers and then genotypes were determined by bi-directional DNA sequencing and sequence alignment between case and control individuals.
Results
Urinary calcium excretion was found to be significantly high (p value < 0.0001) in urolithiasis patients as compared to controls. A total of 14 SNPs were obtained of which one non-synonymous (rs4236480; p.Arg154His; CGT > CAT), one synonymous (rs4252417; p.Tyr278Tyr; TAC > TAT) and three intronic (rs4252400, rs4252402, rs4236481) SNPs were found to be significantly associated with increased risk of urolithiasis. For non-synonymous SNP rs4236480, ‘A’ was found to be the risk allele (OR 1.77, 95% CI 1.24–2.51; p value 0.001) and genotype frequency analysis revealed that individuals carrying variant genotype AA were more prone to the disease than individuals with wild genotype GG (OR 3.09, 95% CI 1.26–7.59; p value 0.0136), indicating AA as the risk genotype.
Conclusions
The non-synonymous SNP rs4236480 showed significant association with urolithiasis risk in West Bengal population of India. Future translational and larger population-based studies are required to validate our finding.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Urolithiasis or kidney stone disease is one of the most common urological problems affecting about 12% of the world population [1]. Kidney stones result from the growth, nucleation, aggregation and finally retention of crystal forming substances like calcium oxalate, calcium phosphate, uric acid, etc. in any part of urinary tract (kidney, ureter or urinary bladder) leading to intense colicky pain in the back, flank, lower abdomen, groin region along with hematuria and sometimes in bilateral cases, renal failure. Genetic predisposition of an individual plays a major role in stone formation [2] along with environmental, geographic, epidemiological and dietary risk factors. High recurrence rate (10–23% per year, 50% in 5–10 years and 75% in 20 years) [3] of urolithiasis and reports of high frequency rate [32.4% vs 17.3%; p < 0.001] of the disease in monozygotic twins in comparison to dizygotic twins [4] support heritability and genetic basis of kidney stone formation. From the genome-wide association studies and candidate gene studies, several genes like calcium sensing receptor (CaSR), calcitonin receptor (CALCR), vitamin D receptor (VDR), transient receptor potential vanilloid member 5 (TRPV5), matrix gla protein (MGP), klotho, claudin14 (CLDN14) have been found to be associated with increasing risk of urolithiasis in different populations of the world; however, the role of these genes needs to be evaluated in the pathogenesis of the disease in Indian population for better diagnosis and treatment of urolithiasis patients of India.
Approximately, 75% of kidney stones are calcium containing and hypercalciuria is the main risk factor in calcium urolithiasis [5]. In human, dietary calcium is mostly absorbed in duodenum [6] and remaining calcium gets filtered in glomerulus and excreted through urine after renal reabsorption [7]. A minute disturbance in renal calcium reabsorption leads to excess urinary calcium excretion, thereby promoting chance of kidney stone formation. The epithelial channel TRPV5 facilitates apical calcium entry [8] and constitutes the rate-limiting step [9] of distal renal tubular calcium reabsorption. It has been reported that ablation of TRPV5 gene alters renal calcium handling in mice, resulting in diminished active calcium reabsorption and thereby promoting severe hypercalciuria [7], which leads to urolithiasis.
The human gene encoding TRPV5 is located on chromosome 7q35. It consists of 15 exons and translates into a 729 amino acid protein. A full screening of TRPV5 coding region and intron–exon boundaries in 20 renal hypercalciuria patients of Paris revealed three non-synonymous (A8V, R154H, A561T) and five synonymous (L205L, Y222Y, Y278Y, T281T, T344T) polymorphisms [10]. High allele frequency of A8V, R154H, A563T and L712F was reported among African Americans [11]. The nonsynonymous SNP R154H has been reported to be associated with kidney stone multiplicity in 365 patients of Taiwan [12]; whereas, another variant L530R was found to be significantly associated with recurrent kidney stones in 2636 Icelanders [13].
In India, urolithiasis affects approximately 12% of the population and 50% of the affected people ends up with kidney loss or renal damage [14]. It is absolutely necessary to determine the candidate causes for better diagnosis and prevention. To the best of our knowledge, from India, association of CaSR, CLDN14 [15], CALCR [16, 17], PTH [18] and VDR [16, 19] genes has been studied with urolithiasis. As there is no prior report elucidating the potential role of TRPV5 polymorphisms with kidney stone risk, the present study was aimed to investigate the possible association of TRPV5 gene polymorphisms with increased risk of urolithiasis in the population of West Bengal, India.
Materials and methods
Study participants
Calcium urolithiasis patients (both male and female) admitted in Department of Urology, Institute of Post Graduate Medical Education and Research (IPGME & R), Kolkata were recruited for our study. A total of 152 calcium containing kidney stone patients (both male and female) between the ages of 18 and 75 years with kidney stone(s) of more than 5-mm diameter were enrolled on the basis of stone diagnosis by renal ultrasound (US) or multi-detector computed tomography (MDCT) scan or X-ray of the kidney or the individuals who had reported a spontaneous urinary stone passage or a history of surgical stone removal. The stone composition was checked by chemical test and KSD patients with calcium-containing (calcium oxalate or calcium phosphate or a combination of the two) stones were only recruited for the study. The past medical history of the patient was obtained from available medical records and conversation with the patient. The patients having anatomic abnormality, symptoms of recurrent urinary tract infection, taking drugs like steroids, diuretics which may affect electrolyte or citrate handling in the body were excluded from the study along with those with history of renal failure and known metabolic, gastrointestinal, endocrinological disorders. The controls (n = 144) were selected from the same geographical region and socioeconomic strata who had no history of personal and familial kidney stone and microscopic urinalysis was performed for ruling out urolithiasis and urinary tract infection.
Study method
Detailed information such as age, gender, ethnicity, occupation, BMI, place of living, lifestyle, drinking and dietary habits, daily urine output and family history of kidney stone or other chronic diseases were obtained from each of the study participants through personal interview. Kidney stone samples were collected from the patients after spontaneous stone passage or surgical intervention [12] for analysis of stone composition and only patients with calcium containing stones were enrolled for the present study. Clinical parameters like serum calcium, 24-h urinary calcium, oxalate, citrate, potassium, phosphate, urate and fasting urine pH were acquired from medical records. The analytic procedures used in measuring the clinical parameters are described briefly in our previous work [15]. Peripheral blood samples of nearly 3 ml were collected in EDTA (ethylene diamine tetraacetic acid) vial from all the study participants and genomic DNA was extracted from the blood leucocytes using extraction kit (QIAamp Blood Kit, QIAGEN, Hilden, Germany) according to the manufacturer’s instruction. The purity and concentration of extracted DNA was checked by nanophotometer (Implen, Implen GmbH, Munich, Germany).
Genotyping for TRPV5 polymorphisms
Specific oligonucleotide primers (Supplementary File 1) were designed using integrated DNA technologies and primer3 software. The entire coding region as well as the intron–exon boundaries of TRPV5 gene was amplified by polymerase chain reaction (PCR). PCR amplification was carried out in 30-μl reaction mixture containing 100 ng of genomic DNA, 0.5 μM of forward and reverse primer, 0.2 mM of deoxyribonucleotide triphosphate mix (Invitrogen Carlsbad, CA, USA), 1.5-mM magnesium chloride (50 mmol/l), 1X PCR buffer and 0.5 Unit Taq DNA polymerase (Invitrogen, USA). The PCR was performed in a thermocycler (Applied Biosystems, Veriti 96-well thermal cycler, Model No. 9902) in the following condition: denaturation at 94 °C for 5 min followed by 44 cycles of denaturation for 30 s, annealing at particular temperature (mentioned in Supplementary File 1) for 30 s, extension at 72 °C for 1 min and final extension at 72 °C for 5 min. Forward and reverse strand DNA sequencing of the PCR products was carried out using a Taq Dye Deoxy Terminator sequencing kit (Applied Biosystems, Foster City, CA, USA) on an automated DNA capillary sequencer (Model 3700; Applied Biosystems). Alignment between sequences of case and control individuals was performed to find the best matching piecewise (local) or global alignments of two query sequences with the help of ClustalW program (EMBL-EBI, Hinxton, England).
Statistical methods
Hardy–Weinberg equilibrium of each SNP in the patients and control individuals were examined using χ2 (Chi-square) test. Student’s t test was used to compare continuous independent variables (age, BMI) within the controls and patients. All data were expressed as mean ± SD (standard deviation) and p value < 0.05 was considered to be statistically significant. The nonparametric variables were analyzed by Mann–Whitney U test. To test the allelic and genotypic associations of each SNP, χ2 or Fisher exact tests were used wherever applicable. The risk for the variant genotype towards kidney stone development was analyzed as odds ratio (OR) with respective 95% confidence intervals (95% CI) and p value using GraphPad InStat software (GraphPad InStat software, San Diego, CA) and SNPassoc version 1.81 software (Catalan Institute of Oncology, Barcelona, Spain). Linkage disequilibrium (LD) pattern of SNPs and haplotype analysis was performed using Haploview 4.2 software.
Results
Characteristics of study participants
We recruited 152 calcium urolithiasis patients (73.68% males and 26.32% females, sex ratio 3:1 approximately) for this study after completing the exclusions (Fig. 1). The stone was found to be recurrent in nature in 32 (21.05%) patients. Our study group, including patients (n = 152) and control individuals (n = 144), was balanced in terms of age, gender, ethnicity, socio-economic status, habitat (urban/rural), food habit (vegetarian/non-vegetarian) and lifestyle (sedentary/nonsedentary). The basic and clinical characteristics of both the patients and controls are presented in Table 1. Among the clinical parameters, urinary calcium excretion level was found to be significantly higher in patients and that was statistically very much significant (p value < 0.0001); however, no significant difference was noted in serum calcium level between control and patient group (p value 0.9378). No other parameter of the 24-h urine showed significant alteration in patients as compared to controls.
TRPV5 genetic polymorphisms
A total of 14 SNPs (representative diagram in Fig. 2) in the TRPV5 gene were obtained in our study population of which eight polymorphisms were detected in the coding and six in the intron regions of TRPV5. The association between all these SNPs of TRPV5 gene and risk of kidney stone was evaluated in this study. The genotype distributions of the SNPs were in HWE (Hardy–Weinberg Equilibrium) except rs755955591 located in exon 4 of the TRPV5 gene. Hence, this SNP was excluded from further study. The allelic and genotypic frequency of the 13 SNPs is summarized in Table 2.
Schematic representation of the position of the SNPs (numbered 1, 2, … 14) of TRPV5 gene. The SNPs and their locations are—1 (rs4252400) in intron 1, 2 (rs4252402) and 3 (rs4236481) in intron 3, 4 (rs755955591) and 5 (rs4236480) in exon 4, 6 (rs4252411) and 7 (rs4252412) in exon 6, 8 (rs4252417) and 9 (rs4252418) in exon 7, 10 (rs4252419) and 11 (rs4252433) in intron 7, 12 (rs4252435) in exon 8, 13 (rs4252499) in exon 13 and 14 (rs4252507) in intron 14
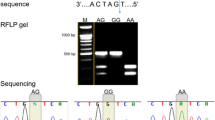
One non-synonymous (rs4236480; p.Arg154His; CGT > CAT), one synonymous (rs4252417; p.Tyr278Tyr; TAC > TAT) and three intronic (rs4252400, rs4252402, rs4236481) SNPs were found to be significantly associated with increased risk of kidney stone disease in our study group. Significant high ‘A’ allele distribution in patient population (0.38) was observed in comparison to healthy controls (0.25) against the non-synonymous polymorphism rs4236480 and our result suggests ‘A’ as the risk allele (p value = 0.001; odds ratio = 1.77; 95% CI 1.24–2.51) for urolithiasis. We had checked the result by applying Bonferroni correction and significant association of this SNP with high risk of urolithiasis in West Bengal population could be verified. The wild GG genotype frequency for control and patients was 55.55% and 38.16%, respectively, and heterozygous GA frequency was 38.19% and 48.68%, respectively. Representative chromatograms for this SNP are presented in Fig. 3. The variant genotype AA was observed in 20 (13.16%) patients, as compared to only 9(6.25%) controls, and the result indicates that individuals with AA genotype were at about three times increased risk (OR 3.09 with 95% CI 1.26–7.59; p value 0.0136) towards urolithiasis. The association between this particular SNP and quantitative variables like age, BMI, serum and urinary calcium level were evaluated (Table 3) and the result shows that urinary calcium level was significantly higher (p value < 0.05) in both patients and controls carrying GA and AA genotypes, as compared to individuals with GG genotype. Although no significant change was observed in the urinary oxalate level for kidney stone patients as compared to healthy controls (Table 1), we have noticed urinary oxalate level was higher for both the patients and controls who were either heterozygous GA or carrying the risk genotype AA for the nonsynonymous SNP rs4236480. This finding is of special interest because higher level of urine oxalate promotes the risk of stone formation in kidney [20]. Our result also revealed that serum calcium level was significantly higher (p value 0.0004) in healthy controls with heterozygous (GA) and variant homozygous (AA) genotypes; however, no significant difference was observed in the patients. Other clinical parameters like urinary citrate, potassium, phosphate, urate, fasting urinary pH and basic parameters like age, BMI were also compared in between individuals with wild genotype GG and individuals with GA and AA genotype, although, no significant alteration was observed both for controls and urolithiasis patients.
For the synonymous polymorphism rs4252417, ‘T’ was found to be the risk allele (C vs T: OR 2.46, 95% CI 1.07–5.69; p value 0.029) towards the development of kidney stone. The heterozygous CT frequency was almost double in patients (10.53%) as compared to controls (5.55%). When we combined the variant CT genotype with the TT genotype (i.e., CT + TT), assuming a dominant genetic model, we observed almost threefold increased risk of developing the disease (OR 2.78, 95% CI 1.12–6.92; p value 0.0231). Moreover, three intronic SNPs (rs4252400, rs4252402 and rs4236481) showed significant association with urolithiasis in our study group. For rs4252400, the risk allele ‘A’ frequency was observed 38% in patients. Individuals carrying heterozygous CA and homozygous variant AA genotype were at 2.20 times (CC vs CA + AA: OR 2.20, 95% CI 1.34–3.63; p value 0.0017) increased risk. For SNP rs4252402 located in intron 3, ‘T’ allele was observed to be associated with the risk of urolithiasis (C vs T: OR 1.78, 95% CI 1.26–2.54; p value 0.001) and individuals carrying TT genotype showed high risk of disease development (CC vs TT: OR 3.19, 95% CI 1.47–6.93; p value 0.0049). For the other SNP rs4236481, ‘G’ was found to be the risk allele and GG the risk genotype in our study group. Assuming a dominant genetic model when combined genotype (TG + GG) was compared with TT genotype, our result predicted about twofold increased risk of urolithiasis (OR 1.94, 95% CI 1.18–3.20; p value 0.0085) in our study group.
Eight more SNPs (four synonymous-Leu205Leu, Tyr222Tyr, Thr281Thr, Thr344Thr; one nonsynonymous-Ala563Thr and three intronic variants-rs4252419: t > g, rs4252433: t > g, rs4252507: g > a) were also observed in our study group; however, no significant differences between controls and kidney stone patients were observed in the distribution of either allelic or genotype frequencies. The linkage disequilibrium (LD) pattern of all the 13 SNPs is presented in Fig. 4. Strong LD association was observed for rs4252433–rs4252435. The association was found to be moderate for rs4236480–rs4236481 and rs4252418–rs4252419.
Linkage disequilibrium (LD) pattern of the 13 SNPs of TRPV5 in the study group. The LD between the SNPs was measured as r2 and shown in the diamond at the intersection of the diagonals from each SNP. r2 = 0 is shown as white and r2 = 100 is denoted by dark red color. The intermediate r2 values are shown by different shades of pink to red
The haplotype frequencies of all the 13 polymorphisms of TRPV5 gene were examined in controls and patients. The haplotypes with a frequency of ≤ 1% were excluded from further analysis. Haploview program predicted totally 13 different haplotypes of which haplotype CCTGCCCATTCGG was found to be the most frequent one. The haplotype distribution of the study participants is presented in Table 4. The haplotype analysis of the SNPs depicted that ATGACCCATTCGG haplotype was significantly associated with kidney stone risk (odds ratio 2.67; 95% CI 1.37–5.22; p value 0.005) in our study group.
Discussion
Calcium homeostasis in the body is maintained by bone resorption, renal tubular reabsorption, intestinal absorption and excretion, which all are controlled by calciotropic hormones. Approximately, 10% of filtered calcium gets reabsorbed in distal convoluted tubule and 5% in the collecting duct of nephron [21]. TRPV5 is an epithelial calcium channel transient receptor that is expressed in the distal convoluted tubule and collecting duct, and mainly involved in renal active calcium reabsorption. TRPV5 protein expression was found to be attenuated in the stone affected region of biopsy kidney tissue (n = 6) as compared to adjacent control tissues (Supplementary Files 2 and 3). So, genetic alteration of TRPV5 might influence urinary calcium excretion, resulting in hypercalciuric kidney stone disease. Significantly high urinary calcium excretion (p value < 0.0001) of urolithiasis patients in our study group prompted us to do a thorough scanning of the TRPV5 gene.
Ours is the first case–control study attempting to find out whether TRPV5 gene can be used as a genetic marker in calcium urolithiasis patients in the population of West Bengal, a major state of eastern India. Screening of all the 15 exons and exon–intron boundaries of the gene revealed synonymous (Leu205Leu, Tyr222Tyr, Tyr278Tyr, Thr281Thr, Thr344Thr) and non-synonymous (Arg154His, Ala563Thr) polymorphisms that were previously reported [10, 11]. SNPs like Ala8Val [10], Leu530Arg [13], Ala561Thr [10] and Leu712Phe [11] were also identified in urolithiasis patients, but these four SNPs showed monomorphic nature in our study population. The discrepancy might be due to the difference in population origin. We had observed another non-synonymous polymorphism (rs755955591; Ala141Ser) in both controls and patients of our study group, but the genotype distribution was not in HWE and hence, this polymorphism was excluded from the further association studies.
There are previous reports of significant association of the missense variant rs4236480 (Arg154His) with kidney stone multiplicity in Taiwanese urolithiasis patients [12], where risk for stone multiplicity was observed to be higher in patients carrying at least one minor allele than the wild-type group. In our study too, the occurrence of the kidney stone disease was found to be higher (OR 1.95 with 95% CI 1.19–3.19; p value 0.0080) in individuals with at least one minor allele, i.e., individuals carrying GA and AA genotypes, than the wild-type group (GG genotype). ‘A’ was the minor allele, the frequency of which was observed to be 0.25 in control individuals, which matches with the allele frequency registered in Ensembl database from South Asian Bengali population (‘A’ allele frequency 26%). We had observed significantly high level (p value < 0.05) of urinary calcium in both patients and controls carrying GA and AA genotypes, as compared to individuals with wild GG genotype. The allele frequency result also indicated ‘A’ as the risk allele. Therefore, the allelic change from ‘G’ to ‘A’ for rs4236480 of TRPV5 can be predicted as a risk factor for kidney stone development. The functional characterization of this non-synonymous variant rs4236480 has already been performed [10, 11]. When the wild-type TRPV5 and TRPV5 mutant (Arg154His) construct were transiently expressed in HEK293 cells and functionally characterized by patch-clamp analysis, electrophysiological characterization did not reveal significant functional changes (in terms of sodium and calcium peak currents and calcium-dependent inactivation curves) in mutant TRPV5 protein as compared to wild-type TRPV5. Similarly when the functional significance of the mutant was analyzed using Xenopus laevis oocyte expression system, then also no significant change was observed. Hence, it seems that the non-synonymous polymorphism rs4236480 does not harbor a pathogenic effect itself but may still be associated with disease development. This particular SNP, rs4236480 was found to be in LD with an intronic variant, rs4236481, which also showed significant association with the disease in terms of genotype frequency in our study group. Among the other intronic variants that were detected in our studied population, one SNP of intron 1 (rs4252400) and another of intron 3 (rs4252402) showed significant association with increased risk of kidney stone. For the variants rs4252400 and rs4252402, ‘A’ and ‘T’ were the risk alleles, respectively. However, none of the three intronic variants (rs4252400, rs4252402, rs4236481) were in a position of splicing acceptor or donor site and hence, the importance of these intronic variants in kidney stone chance seems to be doubtful.
Risk of kidney stone was observed to be almost 2.5-fold high in case of allele ‘T’ than allele ‘C’ for the synonymous polymorphism rs4252417 (Tyr278Tyr). According to 1000 genomes project, the allele frequency of ‘C’ is 92% worldwide and 99% in South Asians. In the present study, we have observed the frequency of allele ‘C’ was 97% in control group and 93% in patient group. ‘T’ was found to be the risk allele (C vs T: OR 2.46, 95% CI 1.07–5.69, p value 0.029). The homozygous TT variant was absent in control; whereas, two patients (1.31%) showed TT genotype. Frequency of the heterozygous CT was almost double in patients (10.53%) as compared to controls (5.55%) and the dominant genetic model also revealed almost threefold increased risk of developing KSD (OR 2.78, 95% CI 1.12–6.92; p value 0.0231). Another polymorphism Ala563Thr (rs4252499) was reported to significantly increase calcium influx by affecting the calcium permeation pathway [11] in Afro-Americans, but in our population no such association was observed between rs4252499 and KSD risk.
From our present study, the non-synonymous polymorphism rs4236480, synonymous polymorphism rs4252417 and three intronic (rs4252400, rs4252402, rs4236481) SNPs were found to be significantly associated with increased risk of kidney stone formation, among which the nonsynonymous polymorphism rs4236480 (Arg154His) seems to be a candidate marker for the susceptibility of calcium urolithiasis in the population of West Bengal, India. For validation of our results, extensive studies with larger sample size supported with functional analysis would be required to provide conclusive evidence.
References
Alelign T, Petros B (2018) Kidney stone disease: an update on current concepts. Adv Urol 2018:3068365
Khan SR, Canales BK (2009) Genetic basis of renal cellular dysfunction and the formation of kidney stones. Urol Res 37:169–180
Moe OW (2006) Kidney stones: pathophysiology and medical management. Lancet 367:333–344
Goldfarb DS, Fischer ME, Keich Y, Goldberg J (2005) A twin study of genetic and dietary influences on nephrolithiasis: a report from the Vietnam Era Twin (VET) Registry. Kidney Int 7:1053–1061
Sayer JA (2017) Progress in understanding the genetics of calcium-containing nephrolithiasis. J Am Soc Nephrol 28:748–759
Khanal RC, Nemere I (2008) Regulation of intestinal calcium transport. Annu Rev Nutr 28:179–196
Hoenderop JG, van Leeuwen JP, van der Eerden BC, Kersten FF, van der Kemp AW, Merillat AM, Waarsing JH, Rossier BC, Vallon V, Hummler E, Bindels RJ (2003) Renal Ca2+ wasting, hyperabsorption, and reduced bone thickness in mice lacking TRPV5. J Clin Investig 112:1906–1914
Hwang I, Jung EM, Yang H, Choi KC, Jeung EB (2011) Tissue-specific expression of the calcium transporter genes TRPV5, TRPV6, NCX1, and PMCA1b in the duodenum, kidney and heart of Equus caballus. J Vet Med Sci 73:1437–1444
De Groot T, Bindels RJ, Hoenderop JG (2008) TRPV5: an ingeniously controlled calcium channel. Kidney Int 74:1241–1246
Renkema KY, Lee K, Topala CN, Goossens M, Houillier P, Bindels RJ, Hoenderop JG (2009) TRPV5 gene polymorphisms in renal hypercalciuria. Nephrol Dial Transplant 24:1919–1924
Na T, Zhang W, Jiang Y, Liang Y, Ma HP, Warnock DG, Peng JB (2009) The A563T variation of the renal epithelial calcium channel TRPV5 among African Americans enhances calcium influx. Am J Physiol Ren Physiol 296:F1042–F1051
Khaleel A, Wu MS, Wong HS, Hsu YW, Chou YH, Chen HY (2015) A single nucleotide polymorphism (rs4236480) in TRPV5 calcium channel gene is associated with stone multiplicity in calcium nephrolithiasis patients. Mediators Inflamm 2015:375427
Oddsson A, Sulem P, Helgason H, Edvardsson VO, Thorleifsson G, Sveinbjornsson G, Haraldsdottir E, Eviolfsson GI, Sigurdardottir O, Olafsson I, Masson G, Holm H, Gudbjartsson DF, Thorsteinsdottir U, Indridason OS, Palsson R, Stefansson K (2015) Common and rare variants associated with kidney stones and biochemical traits. Nat Commun 6:7975
Joseph KC, Parekh BB, Joshi MJ (2005) Inhibition of growth of urinary type calcium hydrogen phosphate dihydrate crystals by tartaric acid and tamarind. Curr Sci 88:1232–1238
Guha M, Bankura B, Ghosh S, Pattanayak AK, Ghosh S, Pal DK, Puri A, Das M (2015) Polymorphisms in CaSR and CLDN14 genes associated with increased risk of kidney stone disease in patients from the eastern part of India. PLoS One 10:e0130790
Bid HK, Chaudhary H, Mittal RD (2005) Association of vitamin-D and calcitonin receptor gene polymorphism in paediatric nephrolithiasis. Pediatr Nephrol 20:773–776
Mitra P, Guha M, Ghosh S, Mukherjee S, Bankura B, Pal DK, Maity B, Das M (2017) Association of calcitonin receptor gene (CALCR) polymorphism with kidney stone disease in the population of West Bengal, India. Gene 622:23–28
Mitra P, Maity B, Pal DK, Das M (2018) Polymorphisms of PTH (parathyroid hormone) gene and risk of kidney stone disease: a case–control study from West Bengal, India. Urology 121:79–85
Relan V, Khullar M, Singh SK, Sharma SK (2004) Association of vitamin D receptor genotypes with calcium excretion in nephrolithiatic subjects in northern India. Urol Res 32:236–240
Taylor EN, Curhan GC (2007) Oxalate intake and the risk for nephrolithiasis. J Am Soc Nephrol 18:2198–2204
Blaine J, Chonchol M, Levi M (2015) Renal control of calcium, phosphate, and magnesium homeostasis. Clin J Am Soc Nephrol 10:1257–1272
Acknowledgements
This work was financially supported by the Department of Science and Technology (DST), Government of India (Ref. No. DST/INSPIRE Fellowship/2016/IF160107, awarded to Pubali Mitra) and Indian Council of Medical Research (Ref No. 5/4/7-6/2015-NCD-II) sanctioned to Prof Madhusudan Das. The authors would like to acknowledge the study participants who gave their consent and collaborated in this study. Dr. Siddharth Saraf and Dr. Alankar Jaiswal, Postdoctoral trainees of Department of Urology, IPGME & R, Kolkata deserves special thanks for their cooperation in sample collection.
Author information
Authors and Affiliations
Contributions
PM and MD conceptualized and designed the research study; PM performed the experiments; PM analyzed the data; MD contributed essential reagents/materials/analysis tools; DKP provided blood samples along with clinical data; PM drafted the manuscript with important intellectual inputs from DKP; MD critically revised the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest and do not have anything to disclose.
Ethical approval
This study was approved by the Institutional Ethics Committee [Memo No. INST/IEC/2016/374 dated 06.06.2016] of Institute of Post Graduate Medical Education & Research (IPGME & R), Kolkata, West Bengal, India and was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
Signed informed consent was obtained from each study participant included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mitra, P., Pal, D.K. & Das, M. Association of TRPV5 gene polymorphism with calcium urolithiasis: a case–control study from West Bengal, India. World J Urol 38, 1311–1322 (2020). https://doi.org/10.1007/s00345-019-02911-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-019-02911-7