Abstract
Klotho gene is an important gene involved in calcium homeostasis, and polymorphisms of this gene may render the individual prone to renal stone formation. We evaluated G395A single nucleotide polymorphisms (SNPs) of Klotho gene at rs1207568 in renal stone patients of North India. This was a prospective study involving 150 patients of renal stone disease (aged 15–60 years) and 100 age- and sex-matched controls. The DNA was isolated and subjected to polymerase chain reaction (PCR) for identifying the G395A Klotho SNPs at rs1207568. Confronting two pair primers were used, and gel electrophoresis showing two bands at 175,252 bp was considered as GG genotype, three bands at 121,175 and 252 bp as GA and two bands at 121 and 252 bp as AA genotype. The association between genotype and cases was evaluated by using Chi-square test and logistic regression analysis. Cases and controls were well matched for age (40.65 vs 42.06, p = 0.063) and sex (p = 0.420). Significantly high proportion of patients with renal stones had GG genotype as compared to controls (odds ratio (OR) 2.37(1.39,4.03), p = 0.001). None of the participants (cases and controls) had homozygous recessive AA genotype. The risk of stone formation was significantly higher in the population carrying G allele {OR 1.94 (1.225–3.073), p 0.004}. Mean serum calcium was higher in stone formers with GG genotype as compared to those with GA genotype (9.16 mg/dl vs 8.91 mg/dl; p = 0.06). GG genotype of G396A Klotho gene SNPs is associated with renal stone formation. The G allele carrier is twice at risk of renal stone formation. The absence of AA genotype in north-western Indian population remains a curiosity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Urolithiasis, one of the oldest maladies suffered by the human race, is responsible for significant cost burden on both the patient and health care services. Its prevalence varies in different geographical areas from 3.5 to 18.5% [1]. Supersaturation of urine with inorganic ions like calcium and phosphorus is a prerequisite for stone formation with calcium stones being the most common type. The factors responsible for calcium metabolism may directly or indirectly contribute to urolithiasis and are therefore a key area of research. Genes responsible for calcium homeostasis can result in hypercalciuria and hence formation of calcigerous urinary stones by virtue of mutation or polymorphisms. Various genes such as Klotho gene, vitamin D receptor (VDR), claudin 14 (CLDN14) and calcium sensing receptor gene (CaSR) have been implicated in the pathogenesis of calcium urolithiasis [2, 3].
North-western India has been identified as one of the endemic regions for urolithiasis [4], the reason for which is multifactorial like race, geographical location, dietary habits and genetic makeup. The incidence of calcium oxalate stones in this part of the country is greater compared to the rest of the world. One probable reason could be a genetic cause linked to this geographical area which is being explored. Klotho gene is an important gene involved in calcium and phosphorus homeostasis, and it may likely play a role in etiopathogenesis of renal stones. We investigated single nucleotide gene polymorphisms (SNPs) of Klotho gene in renal stone disease patients of north-western India.
Materials and methods
This was a case–control study conducted at a tertiary care centre in northern India which caters lot of renal stone disease patients coming from states located in north-western region of the country. The study protocol was approved by the Institute Ethical committee, and informed written consent was taken from all study participants. In case of minors, informed consent was obtained from their parents or legally authorized representative. All patients of calcigerous renal stone disease (aged 15–60 years) without any known comorbidities undergoing endoscopic stone clearance with percutaneous nephrolithotomy were enrolled as cases. Patients suffering from known diseases altering the calcium and phosphorus metabolism like gout, chronic kidney disease and hyperparathyroidism were excluded. Patients with uric acid and infective stones were excluded. All cases were subjected to serum calcium, blood urea, serum creatinine, iPTH (intact parathyroid hormone), vitamin D3 and 24-h urinary calcium and phosphorus estimation. Imaging such as intravenous pyelography and non-contrast computed tomography scan of KUB region was done as perceived necessary. They underwent endoscopic percutaneous nephrolithotomy for renal stones as per the decision of the urologist, and the stones retrieved were subjected to Fourier transform infrared (FTIR) analysis to know its chemical composition. Age- and sex-matched healthy subjects without renal stones served as controls.
Two millilitres of blood sample for genotyping was obtained in a EDTA vial from both cases and controls and stored at – 20 ℃ for further analysis. DNA was isolated from the blood sample using the QIAmp blood mini kit according to the instructions given by the manufacturer (Qiagen™, Germany). The amount of DNA and the purification ratio was calculated using the SCANIT software. The DNA so isolated was subjected to polymerase chain reaction (PCR) for identifying the G395A SNPs at restriction site 1207568. The genotyping of G395A of Klotho protein was performed using PCR with confronting two pair primers assay. In this assay, confronting pairs of primers (four primers in all) are used as shown below:
Forward primer 1: GTTTCGTGGACGCTCAGGTTCATTCTC.
Forward primer 2: GAGAAAAGGCGCCGACCAACTTTC.
Reverse primer 1: GATCCCGCCCCCAAGTCGGGA.
Reverse primer 2: GTCCCTCTAGGATTTCGGCCAG.
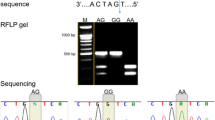
The region containing this polymorphism was amplified by PCR with these primers with the initial denature at 95 °C for 5 min followed by 35 cycles at 95 °C for 1 min, at 65 °C for 1 min, at 72 °C for 1 min and additionally at 72 °C for 5 min. PCR products were then visualized on a 2% agarose gel with ethidium bromide staining. Gel electrophoresis showing two bands at 175,252 bp was considered as GG genotype, three bands at 121,175 and 252 bp as GA and two bands at 121 and 252 bp as AA genotype (Figs. 1 and 2).
We compared the genotype and allele distribution in cases and controls. We also looked for the association of serum calcium, vitamin D3, 24-h urinary calcium and phosphorus levels with the specific genotype of cases. Statistical analysis was done using SPSS software version 20. Continuous variables were expressed as mean ± standard deviation Pearson’s Chi-square test, and odds ratio (OR) was applied to test the significance of genotype distribution among the study population. A p value of ≤ 0.05 was considered as significant.
Results
A total of 250 participants, consisting of 150 cases and 100 controls, were enrolled into the study of which 160 (64%) were males and 90 (36%) were females. Out of 150 cases with calcigerous renal stones, 133(88.7%) had calcium oxalate stones and the remaining had mixed calcium oxalate and phosphate stones. Both cases and controls were comparable in terms of age and sex. Twenty-four-hour urinary calcium and phosphorus levels in cases and controls were 240.27 ± 99.08 mg/TV vs 182.43 ± 69.93 mg/TV and 528.57 ± 97.45 mg/TV vs 497.23 ± 94.25 mg/TV, respectively. The 24-h mean urinary calcium and phosphorus levels were significantly higher in cases as compared to controls (Table 1). Mean serum vitamin D3 level in cases was 15.42 + 7.39 ng/ml.
The genotype distribution and frequency of G and A allele were in accordance with the Hardy Weinberg equilibrium. Significantly high proportion of patients with renal stones had GG genotype as compared to controls (odds ratio (OR) 2.37(1.39,4.03), p = 0.001). None of the participants (cases and controls) had homozygous recessive AA genotype. The distribution of alleles of G395A among cases and controls is also shown in Table 2. The risk of stone formation was twofold in population carrying G allele {odds ratio = 1.94; CI(1.225–3.073)}. The mean serum calcium was higher in stone formers with GG genotype as compared to those with GA genotype (9.16 mg/dl Vs 8.91 mg/dl; p = 0.06). There was no significant difference in serum vitamin D3 levels in GG or GA genotypes (16.80 ± 7.73 ng/ml vs 15.44 ± 6.43 ng/ml, p = 0.312). The 24-h urinary calcium and phosphorus excretion were comparable among GG or GA genotype in stone formers (Table 3).
Discussion
Etiopathogenesis of calcium oxalate stone formation is complex and multifactorial. It is due to a complex interplay of genetic, dietary and environmental factors [5]. Klotho gene is one of the genes involved in altering the calcium and phosphorus homeostasis. It is located at 13q12 chromosome locus, and its product is a transmembrane protein with β glucuronidase activity which modulates calcium reabsorption in the distal renal tubule [6]. β glucuronidase activity of Klotho causes hydrolysis of extracellular N-linked oligosaccharide residue on transient receptor potential vanilloid type 5 (TRPV5) epithelial calcium channel located in the distal collecting tubule of kidney and TRPV6 in the intestine [7, 8]. This will lead to activation of TRPV5 and TRPV6 channels leading to enhanced calcium reabsorption and decreased phosphate absorption. Klotho gene decreases phosphate absorption also by increasing fibroblast growth factor 23(FGF23) signalling. FGF23 also called phosphaturic hormone, inhibits phosphate absorption in the proximal renal tubules [8]. It also promotes calcium reabsorption through activation of TRPV5 channel [9]. The other mechanism postulated for this gene in preventing the formation of stones in the urinary tract is by reducing the oxidative stress due to suppression of insulin/IGF-1 signalling [10]. It is not clear whether alteration of Klotho gene is likely to increase oxidative stress leading to alteration in calculus related proteins such as osteopontin in urine. Klotho gene-deficient mice have shown to have hypercalcaemia, hypercalciuria and hyperphosphatemia [11]. Therefore, single nucleotide polymorphism (SNP) of this gene can alter the calcium and phosphorus homeostasis rendering the individual susceptible to renal stone formation. Studies done to evaluate the association of Klotho gene polymorphisms with calcium oxalate stone formers have shown conflicting results [12, 13]. This may partly be due to different ethnicity and geographical locations of the study population. As compared to western population (66–72%), Indian population have higher percentage of calcium oxalate stones (93%) and north-western region of India is considered as the stone belt region of the country [14]. The reason for the increased incidence of calcium oxalate stones in this geographical area could be due to genetic and environmental causes. It is not known whether Klotho gene polymorphisms are associated with kidney stone formation in this population.
The present prospective case–control study was done to evaluate Klotho gene SNP in calcigerous renal stone formers reporting to a tertiary care centre in northern India. In our study, the male-to-female ratio in 150 enrolled cases of renal stone formers was 1.63:1. This higher preponderance of renal stones in males than females is similar to the previous published literature showing 1.5–2.5 times higher risk of renal stone formation in males as compared to females. In USA the incidence of renal stones in women is increasing slowly every year due to changes in diet and increasing incidence of women with metabolic syndrome [15]. However, in our study cohort as a whole the cases and controls were age- and sex-matched. We observed that GG genotype of G395A Klotho gene was significantly associated with renal stone patients compared to healthy controls (72% vs 52%, p value 0.001). Our results are similar to that observed by Gurel et al. [13] and Telci et al. [16] in Turkey population. We also found that people with G allele are almost twice at risk of stone formation (odds ratio 1.94). The exact mechanism for increased risk of stone formation in patients with GG genotype is not clearly known. G395A SNPs located at the promoter region can lead to altered function of the Klotho protein. Kawano et al. have shown that differential binding patterns were detected between G- and A-bearing alleles [17]. Subjects with G-bearing alleles have higher DNA protein complex formation compared to those with A alleles. This altered affinity pattern of transcription factors with respect to G and A allele might lead to change in expression of the Klotho gene. As a result, alteration in Klotho protein may affect calcium and phosphorus homeostasis leading to increased risk of stone formation. Klotho-deficient mice are hypercalciuric due to decreased expression of TRPV5 channels in the distal collecting tubule of kidney leading to decreased absorption of urinary calcium [18]. Telci et al. have shown that GG genotype of G395A Klotho polymorphism was associated with high serum calcium and urinary phosphorus excretion.
In our study, there was a trend of higher serum calcium level in patients with GG genotype (p = 0.06). We did not find any significant association of GG genotype with 24-h urinary calcium and phosphorus excretion though the 24-h urinary calcium and phosphate excretion was higher in this group. Gurel el al also made a similar observation. The prevalence of AA genotype was reported to be 2–4% in previous studies [19]. Whether the absence of AA genotype in north-western Indian population is a chance finding or a reality needs to be studied.
To the best of our knowledge, the present study is the first to describe the Klotho gene polymorphism in Indian population. Further studies involving other offending genes for renal stone formation would add on to the existing knowledge on genetic implications of urolithiasis.
Conclusion
GG genotype of G396A Klotho gene SNPs is associated with renal stone formation. The G allele carrier is twice at risk of renal stone formation. The absence of AA genotype in north-western Indian population remains a curiosity.
References
Romero V, Akpinar H, Assimos DG (2010) Kidney stones: a global picture of prevalence, incidence, and associated risk factors. Rev Urol 12(2–3):e86
Monico CG, Milliner DS (2012) Genetic determinants of urolithiasis. Nat Rev Nephrol 8(3):151
Amar A, Afzal A, Hussain SA et al (2019) Association of vitamin D receptor gene polymorphisms and risk of urolithiasis: results of a genetic epidemiology study and comprehensive meta-analysis. Urolithiasis. https://doi.org/10.1007/s00240-019-01157-7 (published online ahead of print, Sep 12)
Ganesamoni R, Singh SK (2012) Epidemiology of stone disease in Northern India. In: Talati J, Tiselius HG, Albala D, Ye Z (eds) Urolithiasis. Springer, London, pp 39–46
Pedro RN, Aslam AU, Bello JO et al (2020) Nutrients, vitamins, probiotics and herbal products: an update of their role in urolithogenesis. Urolithiasis 48(4):285–301
Chang Q, Hoefs S, van der Kemp AW, Topala CN, Bindels RJ, Hoenderop JG (2005) The beta-glucuronidase KLOTHO hydrolyzes and activates the TRPV5 channel. Science 310(5747):490–493
Lu P, Boros S, Chang Q, Bindels RJ, Hoenderop JG (2008) The β-glucuronidase klotho exclusively activates the epithelial Ca2+ channels TRPV5 and TRPV6. Nephrol Dial Transpl 23(11):3397–3402
John GB, Cheng CY, Kuro-o M (2011) Role of Klotho in aging, phosphate metabolism, and CKD. Am J Kidney Dis 58(1):127–134
Andrukhova O, Smorodchenko A, Egerbacher M et al (2014) FGF 23 promotes renal calcium reabsorption through the TRPV 5 channel. EMBO J 33(3):229–246
Kuro-o M (2009) KLOTHO and aging. Biochim Biophys Acta 1790(10):1049–1058
Kurosu H, Ogawa Y, Miyoshi M et al (2006) Regulation of fibroblast growth factor-23 signalling by KLOTHO. J Biol Chem 281(10):6120–6123
Ali A, Tursun H, Talat A et al (2017) Association study of KLOTHO gene polymorphism with Calcium oxalate stones in the Uyghur population of Xinjiang, China. Urol J 14(1):2939–2943
Gürel A, Üre İ, Temel HE et al (2016) The impact of KLOTHO gene polymorphisms on urinary tract stone disease. World J Urol 34(7):1045–1050
Ansari MS, Gupta NP, Hemal AK, Dogra PN, Seth A, Aron M et al (2005) Spectrum of stone composition: structural analysis of 1050 upper urinary tract calculi from northern India. Int J Urol 12:12–16
Sorokin I, Mamoulakis C, Miyazawa K, Rodgers A, Talati J, Lotan Y (2017) Epidemiology of stone disease across the world. World J Urol 35(9):1301–1320
Telci D, Dogan AU, Ozbek E et al (2011) KLOTHO gene polymorphism of G395A is associated with kidney stones. Am J Nephrol 33(4):337–343
Kawano K, Ogata N, Chiano M et al (2002) KLOTHO gene polymorphisms associated with bone density of aged postmenopausal women. J Bone Miner Res 17:1744–1751
Kuro-o M, Matsumura Y, Aizawa H et al (1997) Mutation of the mouse KLOTHO gene leads to a syndrome resembling ageing. Nature 390(6655):45–51
Xu C, Song RJ, Yang J et al (2013) KLOTHO gene polymorphism of rs3752472 is associated with the risk of urinary calculi in the population of Han nationality in Eastern China. Gene 526(2):494–497
Funding
None.
Author information
Authors and Affiliations
Contributions
LP contributed to data collection, data analysis and manuscript writing and editing. DSK and SSK was involved in protocol/project development and manuscript writing and editing. SD performed data collection and analysis. KJ performed protocol/project development and data collection.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Statement of human rights
This research involved human participants and the study protocol was approved by the institute’s ethics committee. We certify that the study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Statement on the welfare of animals
This article does not contain any studies with animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lanka, P., Devana, S.K., Singh, S.K. et al. Klotho gene polymorphism in renal stone formers from Northwestern India. Urolithiasis 49, 195–199 (2021). https://doi.org/10.1007/s00240-020-01226-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00240-020-01226-2