Abstract
Hypocitraturia is a profound risk for kidney stone formation and recurrence. Sodium-dicarboxylate cotransporter-1 (NaDC-1) is a main transporter responsible for citrate reabsorption in renal proximal tubules. To investigate an association of sodium-dicarboxylate cotransporter-1 (NaDC-1) polymorphism with hypocitraturia in Thai patients with nephrolithiasis (NL). Exonic SNPs in NaDC-1 were screened in peripheral blood DNA of 13 NL patients. The rs11567842 (A/G) variant was found and further genotyped in 145 NL patients and 115 non-stone forming controls. NL patients had significantly lower level of urinary citrate than the controls. Based on logistic regression, hypocitraturia was significantly associated with urinary stone formation (adjusted OR 8.34, 95% CI 4.63–15.04). Significant association of urinary citrate level with rs11567842 genotype was found only in the NL group. NL patients with GG genotype had significantly higher urinary citrate than those with AA and AG genotypes. GG carrying patients had significantly reduced risk for hypocitraturia (adjusted OR 0.15; 95% CI 0.05–0.48, AA as reference). In selected 15 calcium oxalate stone patients, AA carriers had significantly higher intrarenal NaDC-1 mRNA than GG and AG carriers. Homozygous GG of rs11567842 SNP in NaDC-1 gene was a protective genotype for hypocitraturia in kidney stone patients. The findings suggested that patients with AA genotypes were more susceptible to hypocitraturia than those with GG, hence carrying a higher risk for kidney stone recurrence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Low urinary citrate excretion or hypocitraturia is a profound metabolic risk factor for urinary stone formation and recurrence, therefore, elevation of urinary citrate is a key therapeutic target. Urinary stone disease is a multifactorial condition involving both genetic and environmental risk factors. Genetic susceptibility of urolithiasis (Taguchi et al. 2017) and hypocitraturic phenotype (Mossetti et al. 2003; Shah et al. 2005; Zhu et al. 2010) have been documented. Renal handling of urinary citrate excretion is mainly regulated by expression and function of sodium-dicarboxylate cotransporter 1 (NaDC-1) that is encoded by SLA13A2 gene (Unwin et al. 2004). Urine acidification and increased NaDC-1 expression accelerate reabsorption of citrate and subsequently cause hypocitraturia. However, majority of hypocitraturic stone formers have normal urine pH, indicating that other factors are involved in the regulation of citrate reabsorption (Strohmaier et al. 2012). Evidence shows that potassium citrate drug successfully rises urinary citrate through two main mechanisms- the first mechanism is its alkalinizing action (by converting citrate into bicarbonate) and the second one is increased excretion of unmetabolized citrate (Sakhaee et al. 1992). Potassium citrate is capable of down-regulating NaDC-1 expression in the kidneys of ethylene glycol (EG)-induced nephrolithic rats, and hence elevating urinary citrate excretion (He et al. 2004). In contrast, increase in renal expression of NaDC1 mRNA and decreases in urinary pH and citrate were found in the nephrolithic rats induced by chronic acid ingestion (Okamoto et al. 2007a). Our recent immunohistochemical study showed that intrarenal NaDC-1 expression in nephrolithiasis (NL) patients who had low urine pH was significantly higher than those with high urine pH (pH 7.4) (Chuaypen et al. 2013). Furthermore, we found that NaDC-1 protein expression in the acid-treated HK-2 cells (pH 6.8) was significantly higher than that in the untreated control cells. In addition, genetic polymorphism of NaDC-1 (I550V or rs11567842 SNP) is associated with urinary citrate excretion as demonstrated in the Japanese calcium stone formers (Okamoto et al. 2007b). In vitro experiment shows that this rs11567842 SNP has a modest effect on the function of NaDC-1, as it causes decreases in protein abundance (about 20%) and transport activity (Pajor and Sun 2010). Hitherto, association of NaDC-1 polymorphism with its intrarenal expression in NL patients has not been reported.
In this study, we investigated the association of rs11567842 SNP with hypocitraturia in Thai NL patients and non-stone forming individuals. Intrarenal expression of NaDC-1 mRNA in the kidney tissues of selected calcium oxalate (CaOx) stone patients was determined to preliminarily find the association of NaDC-1 mRNA expression with the rs11567842 genotypes.
Materials and methods
Subjects and specimen collection
One hundred and forty-five NL patients and 115 non-stone forming individuals were recruited for the study. Stone patients were admitted to Khon Kaen Hospital, Khon Kaen province, and Sunpasit Prasong Hospital, Ubon Ratchathani province. All patients had positive KUB X-ray imaging or intravenous pyelogram (IVP) or both for diagnosing kidney stones. Control subjects had no history of urinary stone formation. Non-stone forming status of the controls were ensured by direct interview and the questionnaire containing questions related to sign and symptom of kidney stone disease. All control subjects verbally confirmed that they had no history of urinary stone disease. Subjects with chronic recurrent urinary infection, genetic stone disease and malignancies were excluded. The 24-h urine samples (pre-operation) were collected from all patients and controls using thymol as preservative. Urinary citrate levels were measured by standard enzymatic methods. Heparinized blood samples were collected for DNA extraction. Some patients had stone specimens available for stone type analysis (by Fourier transform infrared spectroscopy, FTIR). Fifteen patients with CaOx stones had renal tissues available for analysis of NaDC-1 mRNA expression.
Written informed consents were obtained from all participants prior to specimen collection. The research protocol was approved by the ethics committee of Faculty of Medicine, Chulalongkorn University, of Sunpasit Prasong Hospital and of Thai traditional and alternative medicine, Ministry of Public Health, Thailand.
Genotyping SLC13A2 gene polymorphisms
Genomic DNA was extracted from peripheral blood samples using QIAamp DNA Blood Mini Kits (QIAGEN) according to the manufacturer’s protocol. DNA concentration was determined by absorption at 260 nm (NanoDrop 2000 Spectrophotometer, USA). Initially, 13 DNA samples from NL patients were amplified using specific primers for exonic regions of SLC13A2 gene to screen for candidate SNPs (Supplementary Table 1). The PCR products were electrophoresed in 1.5% agarose gel, purified using the ExoSAP-IT (USP Corporation, USA), and sent to the Macrogen, Inc., Korea for sequencing. The sequences were analyzed by mutation surveyor V4.0.9 software. Based on the sequencing result of these 13 NL patients, three genotypes (AA, AG, GG) of rs11567842 (A/G) SNP in exon 12, and only one genotype (AA) of rs16964363 (A/C) were observed (Supplementary Table 1). We, therefore, opted to investigate only rs11567842 (A/G) SNP in this study.
For the rest of DNA samples from cases and controls, rs11567842 (A/G) SNP were genotyped using PCR-restriction fragment length polymorphism (PCR-RFLP). Exon 12 was amplified by forward primer: 5′-ACGGGAGGACTTCCCAGAGA-3′ and reverse primer: 5′-CAGGCGCACACATATGCGCA-3′ with annealing temperature of 60 °C, 30 PCR cycles. The PCR products (520 bp) were cut by Bcl-I (5′-T˅GATCA-3′, underlined A is a polymorphic base) at 50 °C, overnight. Genotypes were identified according to the band patterns: AA (2 bands; 358 and 162 bp: complete cut), AG (3 bands; 520, 358 and 162 bp: partial cut) and GG (1 band; 520 bp: uncut). The genotypes identified by PCR-RFLP were confirmed by DNA sequencing (Fig. 1).
Specific recognition sequence of Bcl-I restriction enzyme highlighted the variation site of rs11567842 (A/G) SNP (upper panel). Representative PCR-RFLP results of AA (358 and 162 bp), AG (520, 358 and 162 bp) and GG (520 bp, uncut) genotypes (middle panel). Representative sequencing data to confirm the validity of RFLP analysis for AA, AG and GG genotypes (lower panel)
NaDC-1 mRNA measurement in stone-containing kidney tissues
Kidney tissues near stones (stones-adjacent renal tissues) were collected during the operation (wedge-resection at surgery) from NL patients who admitted to Khon Kaen Hospital. These patients had large stones and the function of their kidneys were severely impaired. Therefore, opened surgery for stone removal and nephrectomy were performed. Performing opened surgery and kidney tissue collection were dependent upon the urologist’s decision. Fifteen kidney tissue samples obtained from 15 CaOx stone patients were available to investigate intrarenal expression of NaDC-1 mRNA using quantitative RT-PCR. Mean of corrected creatinine clearance (CCr) of these 15 patients were 58.8 ± 23.4 (range 13–113.6) mL/min/1.73 m2. Total RNA was isolated from kidney tissues using RNA isolation kit (Promega, USA), and converted to cDNA using reverse transcriptase. Real time PCR (LigthCycler, Germany) was carried out using forward primer: 5′-GGTGACCAAGCTTGATAATGG-3′ and reverse primer: 5′-CTCATGCACTGGGTGAGGT-3′ (QuantiTect SYBR Green PCR Master Mix, Qiagen, Germany) and yielded 105 bp products. PCR condition was 95 °C, 15 s for denaturation, 57 °C, 20 s for annealing and 72 °C for extension (45 cycles). Beta-2-microglobulin (B2M) was used as reference gene. Calibrator was a sample of non-cancerous renal tissues as described in our previous study (Boonla et al. 2008). The \({{\text{2}}^ - }^{{\Delta \Delta {C_{\text{t}}}}}\) value of each sample indicated a relatively normalized ratio (NR) of NaDC-1 mRNA expression.
Statistical analysis
Data were presented as mean ± SD or median (interquartile range, IQR) as appropriated. Two-samples t-test or Mann–Whitney test was performed for comparing means or median of age, body mass index (BMI) and urinary citrate between NL and control groups. Receiver operating characteristic (ROC) curve analysis was performed to select the appropriate cut-off of urinary citrate level for distinguishing NL patients from control subjects. Association of genotypes with stone development and hypocitraturic phenotype were assessed using Chi square test. Kruskal–Wallis test followed by Dunn’s multiple comparison test was performed to test the difference of urinary citrate among genotypes. Odds ratios (OR) were derived from logistic regression model in order to quantify the strength of association of each genotype with hypocitraturia. GraphPad Prism 5 and Stata 12 were used for graphs and statistical computation. P value < 0.05 was considered as statistically significant.
Results
A total of 260 subjects were recruited for the study, divided into NL patients (n = 145) and non-stone forming controls (n = 115). Sex distribution between patients and controls were not significantly different (Table 1). Mean age of NL patients was higher than controls (50.99 ± 11.66 vs. 44.32 ± 13.81 years, P < 0.001). One hundred and seven controls and 128 patients had body mass index (BMI) data available, and we found that the NL patients had significantly greater BMI than the controls (25.56 ± 4.86 vs. 23.26 ± 3.83 kg/m2, P = 0.001).
Low urinary citrate excretion in NL patients
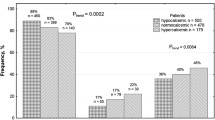
Level of urinary citrate in NL patients was significantly lower than non-stone forming controls (median (IQR): 0.55 (0.66) vs. 1.34 (0.88) mmol/day, P < 0.001) (Fig. 2). ROC curve analysis (Supplementary Fig. 1) revealed an area under curve (AUC) of 0.803 (95% CI 0.749–0.857). This indicates that urinary citrate determination had a moderate accuracy (0.7 < AUC ≤ 0.9) (Greiner et al. 2000) to distinguish NL patients from non-stone forming individuals. Based on our previous hospital-based study and research experiences (Saepoo et al. 2009), we selected cut-off value of 1.04 mmol (200 mg)/day to define hypocitraturia for Thai peoples, and used this cut-off in the subsequent analysis. Additionally, study by Hussein et al. in Malaysian peoples used a similar reference value of urinary citrate (1.05 mmol/day) for defining hypocitraturia (Hussein et al. 2013). In the present study, prevalence of hypocitraturia (< 1.04 mmol/day) were found at 32.17 and 78.62% in control and patient groups, respectively. As expected, hypocitraturia was significantly associated with NL (Chi square test, P < 0.001). Logistic regression adjusted for age and sex revealed that hypocitraturic individuals had approximately 8 times (adjusted OR 8.34, 95% CI 4.63–15.04) higher risk for kidney stone development than those with normocitraturia.
Comparison of 24-h urine citrate levels between NL patients (n = 145) and non-stone forming controls (n = 115). Patients with kidney stones had significantly lower excretory level of urine citrate than the controls (P < 0.001). Medians (IQR) of urinary citrate levels in NL and control groups were 0.55 (0.66) and 1.34 (0.88) mmol/day, respectively
Association of rs11567842 (A/G) SNP with hypocitraturia in NL patients
Major allele that found in both NL and control groups was A allele. Frequencies of A and G alleles in NL patients were 0.69 and 0.31, while in controls were 0.71 and 0.29, respectively (Table 1). In NL groups, AA, AG and GG genotypes were accounted for 74 (51.03%), 51 (35.17%) and 20 (13.79%) (Hardy–Weinberg equilibrium, P = 0.027). In controls, AA, AG and GG genotypes were accounted for 58 (50.43%), 48 (41.74%) and 9 (7.83%) (Hardy–Weinberg equilibrium, P = 0.831). Association of the genotype with NL was not observed (Chi square test, P = 0.249). Likewise, logistic regression controlled for age and sex showed no association of the genotypes with NL development in G codominance mode (Adjusted OR for AG = 0.86; 95% CI 0.50–1.49, P = 0.599), for GG = 1.76; 95% CI 0.72–4.30, P = 0.214, AA as reference (Table 2). Also, significant association of genotypes with NL development was not observed neither in G dominance nor G recessive modes.
Level of urinary citrate among these three genotypes were significantly different only in the NL group, but not in controls. GG-carried NL patients had significantly higher urinary citrate level than those carried AA or AG (Fig. 3). In control group, urinary citrate levels among AA, AG and GG carriers were comparable.
Urinary citrate levels compared among different rs11567842 (A/G) genotypes in NL and non-stone forming control groups. NL patients with GG genotype had significantly higher level of urinary citrate compared to those with AA and AG genotypes (a). In control group, urinary citrate levels among AA, AG and GG carriers were not significantly different (b). Significant P values (< 0.05) were obtained from Dunn’s multiple comparison test after Kruskal–Wallis test. Hypocitraturia or low urinary citrate excretion is defined as having urinary citrate level less than 1.04 mmol or 200 mg per day
We further categorized subjects into two groups, hypocitraturia and normocitraturia. rs11567842 genotypes was significantly associated with hypocitraturia in NL patients (Chi square test, P < 0.001), but not in controls (Chi square test, P = 0.714). Logistic regression adjusted for age and sex showed that NL patients with GG genotype had reduced risk for hypocitraturia compared with those with AA genotype (Adjusted OR for GG = 0.15; 95% CI 0.05–0.48, P = 0.001, AA as reference in G codominant mode) (Table 3). In other word, NL patients with AA had 6.67 (1/0.15) times higher risk for hypocitraturia than those with GG. In G dominance mode, no significant association of genotype with hypocitraturia was observed. However, in G recessive mode patients with GG had significantly reduced risk for hypocitraturia compared with those carried AA and AG (Adjusted OR for GG = 0.13; 95% CI 0.04–0.38, P < 0.001, AA and AG as reference), meaning that the AA and AG carriers had a greater risk for hypocitraturia (7.68 (1/0.13) times) than the GG carriers (Table 3). Crude OR of each genotype obtained from univariate analysis revealed a similar result (Supplementary Table 2). Our current finding suggests that GG is a protective genotype for low urinary citrate excretion in NL patients.
In non-stone forming controls, protective effect of GG genotype on hypocitraturia was not observed neither in G codominant, G dominant nor G recessive modes (Table 3). Therefore, we conclude that association of rs11567842 (A/G) SNP with urinary citrate excretion is pronounced only in the stone forming individuals.
Low intrarenal expression of NaDC-1 mRNA in CaOx NL patients with GG genotype
In 15 CaOx NL patients, expression of NaDC-1 mRNA in stone-adjacent renal tissues was measured. Level of intrarenal NaDC-1 mRNA in patients with AA genotype (n = 9) was significantly higher than those with AG (n = 3) (P = 0.0091) and GG (n = 3) (P = 0.0318) genotypes (Fig. 4). Although sample size was relatively small, the current data suggested that low urinary citrate excretion in patients with AA genotype might be explained by high expression of NaDC-1 mRNA in their kidneys. Also, intrarenal NaDC-1 transcript in patients with AA genotype was significantly greater than those with AG and GG (n = 6) (P = 0.0028). Power analysis for independent sample t-test of these two groups (means: 1.94 vs. 0.18, n: 9 vs. 6) revealed the power of 97.00%.
Discussion
Hypocitraturia is the most common feature found in kidney stone patients. We sorted out whether this stone risk factor had a genetic susceptibility. We screened for exonic variations in NaDC-1 gene in 13 NL patients and found a candidate SNP in exon 12, rs11567842 (A/G). It causes amino acid change from isoleucine (I) to valine (V) at the position of 550, so-called p.I550V. The AA, AG and GG genotypes in NL patients was accounted for 51, 35 and 14%, respectively, indicating that GG was a minor genotype in Thai population (only 8% found in control group). Association of this SNP with NL was not observed in this study similar to the study by Okamoto et al. (2007b). Therefore, it is reasonable to conclude that rs11567842 SNP is likely not a direct genetic risk for kidney stone formation. However, this rs11567842 SNP was strongly associated with hypocitraturic phenotype in NL patients. Our data clearly demonstrated that NL patients with AA had significantly lower urinary citrate level than those with GG. We concluded that AA genotype was susceptible to the hypocitraturic trait, whereas GG was a protective genotype for hypocitraturia. Once hypocitraturia occurred, it increases risk (approximately 8 times) to develop NL compared with normocitraturia. Interestingly, we preliminarily found that AA-carrying patients had significantly higher renal expression of NaDC-1 mRNA than GG-carrying patients. This might explain an increased risk for hypocitraturia in AA carriers. Increased NaDC-1 expression causes increased reabsorption of citrate, hence resulting in low urinary citrate excretion. However, this speculation requires further experimental proof.
Hypocitraturia as a main etiological cause of NL is well recognized. We confirmed again herein that hypocitraturia increased risk for NL development. However, the urinary citrate levels in our study (median (IQR): 105.00 (126.98) mg/day in NL group and 258.36 (169.61) mg/day in control group) were relatively low compared with the western studies that used a cut-off of 320 mg/day to define hypocitraturia (Caudarella and Vescini 2009; Pak 1994). We did ROC analysis and selected an appropriate cut-off for Thai population at 200 mg/day. According to this cut-off, hypocitraturia in our study was found at 32 and 79% in controls and NL patients, respectively. Diversity of stone disease across countries worldwide is likely due to nutritional-environmental and socio-political-economic factors (Pak et al. 1997). A retrospective study by Maloney et al. in the US found that hypocitraturia (< 320 mg/day) was found in all Asian stone formers (100%), whereas in white stone formers hypocitraturia was only accounted for 68% (Maloney et al. 2005). Furthermore, they found that urinary citrate level in Asian patients (333 ± 199 mg/day) was significantly lower than the white patients (520 ± 262 mg/day). Although it is still lack of direct evidence, we speculate that a lower level of urinary citrate in NL patients in Thailand relative to those in western countries might be delineated by differences in ethnicity, lifestyle, dietary habit as well as socioeconomic status.
We previously showed that increase in intrarenal expression of NaDC-1 was associated with decrease in citrate excretion in NL patients and also demonstrated that proton (acid condition) was capable of upregulating NaDC-1 expression in HK-2 cells (Chuaypen et al. 2013). Association of the p.I550V polymorphism in NaDC-1 gene with urinary citrate excretion was well documented in Japanese recurrent renal calcium stone formers (Okamoto et al. 2007b), and AA genotype was associated with low urinary citrate excretion. We speculate that AA-carrying patients predispose to develop acidic urine that leads to upregulation of NaDC-1 and subsequently causes hypocitrauria. However, further experiments are required to warrant this speculation.
In control group, the observed allele frequencies of A (0.71) and G (0.29) were different from that found in Japanese non-stone forming controls (0.56 and 0.44, respectively) (Okamoto et al. 2007b). This suggests that allele frequency of rs11567842 SNP varies among ethnic groups. However, our finding was consistent with the result of Okamoto’s study that showed that the AA-carrying NL patients had significantly lower urinary citrate levels than the GG carriers. We think that increased risk for hypocitraturia in patients with AA genotype may lead to increased risk for stone recurrence. Therefore, hypocitraturic NL patients with AA genotype are recommended to increase consumption of citrate-rich diets or adequately receive citrate supplement to correct the hypocitraturic condition and prevent the recurrence of urinary calculi.
Pajor and Sun used COS-7 heterologous expression system and demonstrated that the G variant of rs11567842 impaired the protein localization and caused about 20% decrease in number of NaDC-1 transporter at the plasma membrane resulting in reduction of sodium/citrate transport activity (Pajor and Sun 2010). This might explain an increased urinary citrate level in the GG carriers relative to the AA carriers. We demonstrated that patients with AA had higher expression of NaDC-1 mRNA in their affected kidneys than those with GG. This might imply that AA carriers have higher expression of NaDC-1 protein in renal tubular cells leading to higher reabsorption of citrate and higher risk of hypocitraturia, compared with the GG carriers. Although there is no statistically significant evidence to show association of AA genotype with kidney stone formation, AA genotype is indeed associated with the hypocitraturia. Persistent and long standing hypocitraturia may gradually enhance the development of urinary stones in individuals with AA genotype.
Limitations of the present study should be mentioned. Sample size used to investigate renal expression of NaDC-1 mRNA was relatively small (n = 15), and data of their stone sizes were not available. Protein expression of NaDC-1 in renal tissue samples was not explored. Functional study of rs11567842 variant was not investigated. The data of stone episode was not available. Therefore, correlation between recurrent episode and urinary citrate and genetic variation were not evaluated. We did not measure urine pH of the studied subjects, therefore, association of urine pH with urine citrate could not be assessed. Although the mean age of the studied NL patients was significantly higher than the controls, we controlled this confounding factor by statistic approach using logistic regression to quantify the strength of association of rs11567842 SNP with risks for NL and hypocitraturia. Non-stone forming condition in control group was not ensured by imaging means (e.g., ultrasound). It was only based on direct interview.
In conclusion, we demonstrated that the AA genotype of rs11567842 SNP in NaDC-1 gene was associated with hypocitraturia in Thai NL patients. This was consistent with the study in Japanese patients (Okamoto et al. 2007b). Our preliminary data showed that intrarenal expression of NaDC-1 mRNA in AA-carrying patients was significantly higher than the GG-carrying patients. Although it is a very preliminary finding and requires further verification, it might imply that increased NaDC-1 mRNA expression is responsible for hypocitraturic phenotype in AA carriers. Therefore, NL patients with AA genotype are recommended to increase consumption of high citrate-containing food or prescribed with citrate supplement in order to elevate urinary citrate level and prevent the development of recurrent calculi.
References
Boonla C, Hunapathed C, Bovornpadungkitti S, Poonpirome K, Tungsanga K, Sampatanukul P, Tosukhowong P (2008) Messenger RNA expression of monocyte chemoattractant protein-1 and interleukin-6 in stone-containing kidneys. BJU Int 101:1170–1177
Caudarella R, Vescini F (2009) Urinary citrate and renal stone disease: the preventive role of alkali citrate treatment. Arch Ital Urol Androl 81:182–187
Chuaypen N, Boonla C, Dissayabutra T, Predanon C, Ruangvejvorachai P, Waiwijit U, Tosukhowong P (2013) Increased intrarenal expression of sodium-dicarboxylate cotransporter-1 in nephrolithiasis patients with acidic urine pH. Asian Biomed 7:571–577
Greiner M, Pfeiffer D, Smith RD (2000) Principles and practical application of the receiver-operating characteristic analysis for diagnostic tests. Prev Vet Med 45:23–41
He Y, Chen X, Yu Z, Wu D, Lv Y, Shi S, Zhu H (2004) Sodium dicarboxylate cotransporter-1 expression in renal tissues and its role in rat experimental nephrolithiasis. J Nephrol 17:34–42
Hussein NS, Sadiq SM, Kamaliah MD, Norakmal AW, Gohar MN (2013) Twenty-four-hour urine constituents in stone formers: a study from the northeast part of Peninsular Malaysia. Saudi J Kidney Dis Transplant 24:630–637
Maloney ME, Springhart WP, Ekeruo WO, Young MD, Enemchukwu CU, Preminger GM (2005) Ethnic background has minimal impact on the etiology of nephrolithiasis. J Urol 173:2001–2004
Mossetti G, Vuotto P, Rendina D, Numis FG, Viceconti R, Giordano F, Cioffi M, Scopacasa F, Nunziata V (2003) Association between vitamin D receptor gene polymorphisms and tubular citrate handling in calcium nephrolithiasis. J Intern Med 253:194–200
Okamoto N, Aruga S, Tomita K, Takeuchi T, Kitamura T (2007a) Chronic acid ingestion promotes renal stone formation in rats treated with vitamin D3. Int J Urol 14:60–66
Okamoto N, Aruga S, Matsuzaki S, Takahashi S, Matsushita K, Kitamura T (2007b) Associations between renal sodium-citrate cotransporter (hNaDC-1) gene polymorphism and urinary citrate excretion in recurrent renal calcium stone formers and normal controls. Int J Urol 14:344–349
Pajor AM, Sun NN (2010) Single nucleotide polymorphisms in the human Na+-dicarboxylate cotransporter affect transport activity and protein expression. Am J Physiol Renal Physiol 299:F704-711
Pak CY (1994) Citrate and renal calculi: an update. Miner Electrolyte Metab 20:371–377
Pak CY, Resnick MI, Preminger GM (1997) Ethnic and geographic diversity of stone disease. Urology 50:504–507
Saepoo S, Adstamongkonkul D, Tosukhowong P, Predanon C, Shotelersuk V, Boonla C (2009) Comparison of urinary citrate between patientswith nephrolithiasis and healthy controls. Chula Med J 53:51–65
Sakhaee K, Alpern R, Poindexter J, Pak CY (1992) Citraturic response to oral citric acid load. J Urol 147:975–976
Shah O, Assimos DG, Holmes RP (2005) Genetic and dietary factors in urinary citrate excretion. J Endourol 19:177–182
Strohmaier WL, Seilnacht J, Schubert G (2012) Urinary stone formers with hypocitraturia and ‘normal’ urinary pH are at high risk for recurrence. Urol Int 88:294–297
Taguchi K, Yasui T, Milliner DS, Hoppe B, Chi T (2017) Genetic risk factors for idiopathic urolithiasis: a systematic review of the literature and causal network analysis. Eur Urol Focus 3:72–81
Unwin RJ, Capasso G, Shirley DG (2004) An overview of divalent cation and citrate handling by the kidney. Nephron Physiol 98:p15–p20
Zhu C, Ye Z, Chen Z, Xia D, Hu J (2010) Association between vitamin D receptor gene polymorphisms and idiopathic hypocitraturia in the Chinese population. Urol Int 85:100–105
Acknowledgements
The study was partially supported by the National Research Council of Thailand (NRCT), the 90th Anniversary of Chulalongkorn University Fund (Ratchadaphiseksomphot Endowment Fund), the Chulalongkorn Academic Advancement into Its 2nd Century Project and Thailand Research Fund (DPG6180001). The 72nd Anniversary of his Majesty King Bhumibol Adulyadej scholarship from the graduate school, Chulalongkorn University. Thanks to all members in the Biochemistry and Molecular Biology of Metabolic Diseases Research Unit, and the medical team at Urology Clinic, Sunpasit Prasong Hospital.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
Pattarin Udomsilp, Sarawut Saepoo, Rungnapa Ittiwut, Vorasuk Shotelersuk, Thasinas Dissayabutra, Chanchai Boonla and Piyaratana Tosukhowong declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Udomsilp, P., Saepoo, S., Ittiwut, R. et al. rs11567842 SNP in SLC13A2 gene associates with hypocitraturia in Thai patients with nephrolithiasis. Genes Genom 40, 965–972 (2018). https://doi.org/10.1007/s13258-018-0702-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13258-018-0702-4