Abstract
Biofertilizers are currently considered the only alternative of chemical fertilizers. In addition, improving the effect of biofertilizers can make chemical fertilizers less demanded, thereby leading to the reduction of adverse environmental effects. In the present study, three rice rhizosphere bacteria (Bacillus megaterium DD-2, Bacillus aryabhattai DD-3 and Bacillus subtilis DD-4) were isolated and then identified from the soil of a farm in Northeast China. As revealed from the results, all isolates exhibited the ability to dissolve potassium and phosphorus. To be specific, DD-4 was capable of dissolving potassium to the greatest extent (1.37 mg/L), DD-2 could maximally dissolve inorganic phosphorus (288.33 mg/L) and DD-3 exhibited the maximum solubility of organic phosphorus (31.58 mg/L). All isolates were capable of synthesizing Indole-3-acetic acid (IAA) (55.66 ~ 75.89 mg/L), Gibberellic acid (GA) (16.33 ~ 23.58 mg/L) and siderophores (38.77 ~ 52.88%). As suggested from the results of the pot experiment, the isolates were capable of promoting the shoot and underground part of rice seedlings. With the increase in the concentration of isolates and the time of addition, the overall effect of the isolates to boost rice seedling growth first increased and then leveled off. The mentioned isolates were observed to exhibit great potential for being biologically applied. However, the impact of isolates on rice required more specific assessments under biotic and abiotic stress before being recommended as biofertilizers.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rice refers to one of the critical crops worldwide and the staple food crop for half of the world's population (Das and Osborne 2020). The total rice planting area and global rice production, respectively, reach 16.25 million hm2 and 74.09 million t in the world. It is a grain as well as a lifeline (Sun et al. 2020). To achieve the optimal yields of rice, high fertilizer rates are required. Excessive use of synthetic fertilizers, however, can result in soil, air and water pollution (Kang et al. 2017). Moreover, most chemical fertilizers applied to plants are easy to exploit, and they are leached away or fixed to plant-unavailable forms in the soil (Fahad et al. 2014). The rice rhizosphere covers various plant growth boosting bacteria. Furthermore, it is considered to be essential to gradually replace synthetic fertilizers with biological fertilizers and beneficial microorganisms (Lu et al. 2018).
There have been numerous studies on the efficiency of biofertilizers in rice production and agricultural production (Bakhshandeh et al. 2020). Besides, several biofertilizer formulations have been developed by well-known research institutions, especially in developing nations, and these institutions are constantly seeking for effective microorganisms in biofertilizer formulations (Du et al. 2019). Plants absorb a range of mineral elements as nutrients to achieve their ideal growth. To be specific, phosphorus (P) and potassium (K) are considered the major elements required for plant survival (Lee et al. 2019). In the soil, there are considerable P and K elements in the form of silicate and phosphate, whereas they exhibit poorly assimilable forms (Mitra et al. 2018). Biofertilizers refer to inoculants of live microorganisms, capable of dissolving silicate and phosphate to boost plant growth (Naseer et al. 2020). In addition, microorganisms secrete stimulating hormones (e.g., gibberellins, auxins and siderophores) (Fahad et al. 2015). Thus, a growing number of farmers and scientists come to realize that using biofertilizer is effective for sustainable agriculture and capable of maintaining environmental-friendly nutrition management and ecosystem functions (Banik et al. 2019).
If biofertilizer affects rice growth, the addition concentration and time are of critical importance (Sakoda et al. 2019). Overall, chemical fertilizers should be applied 2 to 3 times or more, and applied after 7 or 10 days of rice growth (Patra and Haque 2011). It has been demonstrated extensively that the fertilizer added on the 10th day of the seedlings grew more significantly, compared with the fertilizer added on the 12-, 14- and 16-day, and the above and below-ground characteristics of the rice seedlings varied with the concentration of the added fertilizer (Xiao et al. 2019). Accordingly, to obtain the optimal rice growth, the appropriate time and attention of biofertilizer application should be determined. The present study adopted soil in Northeast China as the material to isolate and identify the rhizosphere bacteria of rice. The potential of the isolates to promote rice growth was verified by quantitatively analyzing the isolates physiological and biochemical properties, silicate and phosphate solubilization capacity and plant hormones (IAA, GA and siderophores). Furthermore, the effect of isolates as inoculants on the growth of rice in pots was studied, and the concentration and time conditions of isolates as rice microbial fertilizers were further determined.
Materials and Methods
Description of the Study Site and Soil Samples
The rhizosphere soil for isolation of growth-promoting bacteria was collected from a rice field (grown for 180 days) of organic Farm in Jiamusi district (latitude 47°48′ N, longitude 131°75′ E), and the farm is located in northeastern China. The main climate of the farm consists of low temperature and drought in spring, warm and rainy summer, easy waterlogging and early frost in autumn, as well as cold and long winter (Fang et al. 2013). The soil used in the pot experiment was taken from 0 ~ 10 cm soil layer of the farm. The air-dried sample went through a 4-mm sieve. The initial soil physical and chemical properties included pH 5.89, organic matter 20.67 g/kg, total nitrogen 1.18 g/kg, available phosphorus 2.43 mg/kg and available potassium 50.7 mg/kg. The rice seed (Longjing 31) employed in the present study was offered by the Beidahuang Seed Industry in China.
Isolation and Culture of Rhizospheric Bacteria
Ten grams of soil samples in 90 mL of sterile water was suspended and diluted sixfold for bacterial isolation. In addition, the isolates were isolated with Luria–Bertani (LB) agar medium, cultured at 30 °C for 3 days and then isolated from independent colonies. Subsequently, the isolated microorganisms were re-streaked and then cultured in LB medium for 3 days (Saleemi et al. 2017).
Assessment of the Ability to Solubilize Insoluble Silicate and Phosphate
The bacterial isolates were inoculated into Alexander Rove solution and cultured at 30 ℃ and centrifugation 120 rpm for 168 h. Subsequently, the solubilization of silicate was determined by flame spectrophotometry (Zhang and Kong 2014); the bacterial isolates were inoculated in Pikovskaya medium (inorganic phosphorus) and Mengjinna medium (organic phosphorus), and then, they were cultured at 30 °C and centrifugation 120 rpm for 168 h. Next, the solubilization of available phosphorus was determined by phospho-molybdate spectrophotometry (Munira et al. 2018). Among the screened isolates, three isolates exhibiting the largest silicate and phosphate solubilizing ability were selected for further analysis.
Biochemical and Phenotypic Characteristics
The morphology and structure of bacterial cells were observed under an optical microscope. The phenotypic characteristics were characterized by complying with the standard method described by Bergey's Manual of Systematic Bacteriology (Majeed et al. 2015).
Molecular Characterization
DNA was extracted using genomic DNA extraction kit (Meiji, Shanghai). The 16S rDNA was sequenced by Shanghai Meiji Company. The sequencing results were analyzed using NCBI database for homologous sequences of 16S rDNA. Phylogenetic analysis was performed using MEGA 5.0 software and an adjacency algorithm (Thurasi et al. 2018).
Determination of Optimum Growth of Bacterial Isolates
To investigate the influence of temperature and pH on the growth of isolates, the isolate was inoculated into a flask of 150 mL LB medium, with the pH value controlled at a constant value of pH 7, while the temperature was kept at 24, 26, 28, 30 and 32 °C. The samples were cultured at 120 rpm for 24 h, and then, the amount of the isolates in the culture medium was measured with an ultraviolet–visible spectrophotometer (PG-instrument T6, Beijing, CHN) to try to measure the optimal temperature. The fermentation temperature was kept constant at 30 °C, while the culture was performed at various constant pH values of 6.0, 6.5, 7.0, 7.5 and 8.0. The number of isolated bacteria in the fermentation broth was measured using an ultraviolet–visible spectrophotometer at 120 rpm for 24 h, and the optimal initial pH was determined (Amoozegar et al. 2018).
Growth-Promoting Potential of Bacterial Isolates
Production of Indole Acetic Acid
The isolated bacteria were inoculated into LB broth supplemented by 50 g/mL tryptophan and then cultured at 30 °C for 24 h. The bacterial culture solution was centrifugated at 10,000 rpm (centrifuge, Hitachi CT15RE, Tokyo, Japan) for 10 min. The supernatant was collected, and then, 4 mL Salkowski reagent was added (50 mL 35% HClO4, 1 mL 0.5 M FeCl3). The mix was subsequently kept in the dark for 60 min. The optical density was measured at 540 nm, and the concentration of IAA synthesized was determined by using the standard curve method (Kumari et al. 2018).
Production of Gibberellic Acid
The bacterial isolates were inoculated into the LB culture solution and then cultured at 30 °C for 48 h. The bacterial culture solution was centrifugated at 4000 rpm (centrifuge, Hitachi CT15RE, Tokyo, Japan) for 10 min. Subsequently, the supernatant was collected, add concentrated sulfuric acid into the supernatant and adjust the volume to 200 mL. The optical density was measured at 412 nm, and the concentration of GA synthesized was determined by using the standard curve method (Mussa et al. 2018).
Production of Siderophore
The bacterial culture solution was centrifugated at 4000 rpm for 10 min at 4 °C. Cell-free supernatant was assayed for the qualitative detection of siderophore by using Chrome Azurol S (CAS) shuttle assay (Bhatt and Maheshwari 2020a, b).
Pot Experiment
The rice seeds were added to 95% ethanol and stirred for 1 min. In addition, the seeds received the 20-min stirring after being added to 1.2% sodium hypochlorite. Then, they were rinsed with sterile water 5 times. The seeds were soaked in sterile water for 2 days, and the 3 growth-promoting bacteria were cultured in LB medium resuspended and then rinsed with sterile water. Afterward, the seeds were diluted to the concentrations of 106 (a), 107 (b), 108 (c) and 109 (d) CFU/mL, respectively. Each nursery pot was filled with nursery soil (250 g), and each pot was sown with 20 germinated seeds. The diluted bacterial solution was soaked with the seeds (10 mL bacterial solution for each seed and 6 h for soaking) for 0, 10, 20, 30 and 40 days (10 mL bacterial solution for each seed directly) into the nursery soil in the standard under 18 h of light, 25 ± 2 °C and 60% relative humidity. Moreover, the seeds were placed in the growth room for 50 days, and then, sterilized water was added to build a control. All treatments were replicated three times, and sterile water was introduced to the nursery pot regularly (Lee et al. 2015). The rice seedlings were measured for 50 days. To be specific, the measurement was conducted for the above-ground indicators (e.g., plant height, stem diameter, above-ground fresh weight and chlorophyll content) and below-ground indicators (e.g., root length, root fresh weight and root activity) (Xiao et al. 2017; Roca et al. 2018; Moretti et al. 2020).
Statistical Analysis
The data were subjected to analysis of variance (ANOVA), and means were separated by the Duncan's multirange test using the SPSS package (version 16.0). Values of P ≤ 0.05 were taken as statistically significant.
Results
Isolation and Culture of Rhizospheric Bacteria
Among all 10 isolates, 3 exhibited the ability to dissolve potassium and phosphorus isolates were selected for further investigation. The surface of the three isolates (DD-2, DD-3 and DD-4) was observed to be smooth in appearance and rod-shaped. In addition, the physiological and biochemical characteristics of the isolates are listed in Table 1.
Phylogenetic Analysis of Isolates
By complying with 16S rDNA gene sequencing of bacterial isolates DD-2, DD-3 and DD-4 (GenBank Accession No. MT672776, MT672779 and MT272777), the closest phylogenetic relationship was demonstrated with Bacillus megaterium strain Z1 (GenBank Accession No. MN830159.1), Bacillus aryabhattai strain HTI21 (GenBank Accession No. MK521057.1) and Bacillus subtilis strain CPM-STR-4 (GenBank Accession No. FJ687601.1), respectively, exhibiting 99.65%, 96.2% and 97.5% sequence similarity. The isolates were, respectively, identified as Bacillus megaterium strain (DD-2), Bacillus aryabhattai strain (DD-3) and Bacillus subtilis strain (DD-4) (Fig. 1).
Neighbor-joining phylogenetic dendrogram based on 16S rDNA sequences displaying relationships between the bacterial isolates and their closest taxa. The numbers at the nodes indicate the levels of bootstrap support based on data for 1000 replicates; values derived greater than 50% are only presented. The scale bar represents the observed number of changes per nucleotide position. Accession numbers are given in parenthesis
Determination of Optimum Growth of Bacterial Isolates
After the bacterial isolates (DD-2, DD-3 and DD-4) were cultured at different temperatures for 24 h, the number of viable bacteria reached over 2.2 × 106 CFU/mL, and the maximum viable bacteria number at 30 °C was 5.8 × 107, 4.6 × 107 and 4.8 × 107 CFU/mL; the isolates were cultured under different initial pH values, and the isolates grew effectively. When the initial pH value was 7, the number of viable bacteria was the maximum, which was 5.2 × 107, 4.4 × 107 and 4.6 × 107 CFU/mL (Fig. 2).
Growth-Promoting Potential of Bacterial Isolates
Figure 3a illustrates the solubilizing capacity of insoluble silicate (K) and phosphate (organic and inorganic P). All the isolates exhibited the ability of potassium and phosphorus solubilization: The isolate DD-4 was capable of maximally solubilizing potassium, i.e., 1.37 mg/L; the isolate DD-2 had the maximum inorganic phosphorus solubilizing capacity, i.e., 288.33 mg/L; and the isolate DD-3 had the maximum organic phosphorus solubilizing capacity, i.e., 31.58 mg/L.
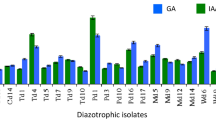
The production of IAA, GA and siderophore is illustrated in Fig. 3b. All the isolates were capable of synthesizing IAA, GA and siderophore: The yield of isolate DD-2 IAA, GA and siderophore, respectively, reached 55.66 mg/L, 16.33 mg/L and 51.22%; the yield of isolate DD-3 IAA, GA and siderophore was up to 68.34 mg/L, 18.52 mg/L and 38.77%, respectively; and the yield of isolate DD-4 IAA, GA and siderophore was 75.89 mg/L, 23.58 mg/L and 52.88%, respectively.
Plant Growth-Promoting Ability Assays
Promotion of Growth Parameters of Rice Above-Ground Indicators by Isolates
Compared with the control (Fig. 4), the isolates exhibiting different concentrations and time exerted different promoting effects on the above-ground indicators of rice (ANOVA, P < 0.05). With the increase in isolates concentration and the times of isolates added, the effect to boost rice seedling growth first increased and then leveled off. Compared with the uninoculated control above-ground fresh weight (0.071 g) (Fig. 4a), in the treatment of seed soaking, the optimal growth-promoting capacity was 109 CFU/mL DD-3 (0.24 g); in the treatment of the added isolates on the 0th day, the optimal growth-promoting capacity was 107 CFU/mL DD-4 (0.21 g); in the treatment of the added isolates on the 0 and 10th days, the optimal growth-promoting capacity was 106 CFU/mL DD-4 (0.52 g); in the treatment of the added isolates on the 0, 10 and 20th days, the optimal growth-promoting capacity was 106 CFU/mL DD-3 (0.63 g); in the treatment of the added isolates on the 0, 10, 20 and 30th days, the optimal growth-promoting capacity was 107 CFU/mL DD-3 (1.04 g); and in the treatment of the added isolates on the 0, 10, 20, 30 and 40th days, the optimal growth-promoting capacity was 109 CFU/mL DD-3 (0.52 g).
Effects of the bacterial isolates on different growth parameters of rice plants above-ground indicators in pots. a Effects of the bacterial isolates on rice fresh weight. b Effects of the bacterial isolates on rice stem diameter. c Effects of the bacterial isolate on rice total chlorophyll. d Effects of the bacterial isolate on rice plant height. Data are expressed as means ± standard deviation. In the figure, ***, ** and * indicate P < 0.001, P < 0.01 and P < 0.05, respectively
Compared with the uninoculated control stem diameter (1.4 mm) (Fig. 4b), in the treatment of seed soaking, the optimal growth-promoting capacity was 109 CFU/mL DD-3 (2.09 mm); in the treatment of the added isolates on the 0th day, the optimal growth-promoting capacity was 106 CFU/mL DD-2 (1.87 mm); in the treatment of the added isolates on the 0 and 10th days, the optimal growth-promoting capacity was 106 CFU/mL DD-4 (3.06 mm); in the treatment of the added isolates on the 0, 10 and 20th days, the optimal growth-promoting capacity was 106 CFU/mL DD-4 (2.84 mm); in the treatment of the added isolates on the 0, 10, 20 and 30th days, the optimal growth-promoting capacity was 107 CFU/mL DD-3 (3.53 mm); and in the treatment of the added isolates on the 0, 10, 20, 30 and 40th days, the optimal growth-promoting capacity was 109 CFU/mL DD-4 (2.79 mm).
Compared with the uninoculated control chlorophyll content (1.13 mg/L) (Fig. 4c), in the treatment of seed soaking, the optimal growth-promoting capacity was 106 CFU/mL DD-4 (2.3 mg/L); in the treatment of the added isolates on the 0th day, the optimal growth-promoting capacity was 106 CFU/mL DD-4 (2.04 mg/L); in the treatment of the added isolates on the 0 and 10th days, the optimal growth-promoting capacity was 107 CFU/mL DD-4 (2.72 mg/L); in the treatment of the added isolates on the 0, 10 and 20th days, the optimal growth-promoting capacity was 107 CFU/mL DD-2 (2.52 mg/L); in the treatment of the added isolates on the 0, 10, 20 and 30th days, the optimal growth-promoting capacity was 109 CFU/mL DD-4 (2.57 mg/L); and in the treatment of the added isolates on the 0, 10, 20, 30 and 40th days, the optimal growth-promoting capacity was 108 CFU/mL DD-2 (1.94 mg/L).
Compared with the uninoculated control plant height (23.5 cm) (Fig. 4d), in the treatment of seed soaking, the optimal growth-promoting capacity was 109 CFU/mL DD-3 (36 cm); in the treatment of the added isolates on the 0th day, the optimal growth-promoting capacity was 109 CFU/mL DD-2 (34.13 cm); in the treatment of the added isolates on the 0 and 10th days, the optimal growth-promoting capacity was 106 CFU/mL DD-4 (46.25 cm); in the treatment of the added isolates on the 0, 10 and 20th days, the optimal growth-promoting capacity was 106 CFU/mL DD-4 (47.5 cm); in the treatment of the added isolates on the 0, 10, 20 and 30th days, the optimal growth-promoting capacity was 108 CFU/mL DD-4 (49.75 cm); and in the treatment of the added isolates on the 0, 10, 20, 30 and 40th days, the optimal growth-promoting capacity was 109 CFU/mL DD-4 (49.75 cm).
Promotion of Growth Parameters of Rice Below-Ground Indicators by Isolates
Compared with the control (Fig. 5), the isolates exhibiting different concentrations and time of addition exerted different effects to promote the below-ground indicators of rice (ANOVA, P < 0.05). With the increase in isolates concentration and the time of isolates added, the effect to boost rice seedling growth first increased and then leveled off. Compared with the uninoculated control root fresh weight (0.025 g) (Fig. 5a), in the treatment of seed soaking, the optimal growth-promoting capacity was 106 CFU/mL DD-2 (0.047 g); in the treatment of the added isolates on the 0th day, the optimal growth-promoting capacity was 106 CFU/mL DD-3 (0.04 g); in the treatment of the added isolates on the 0 and 10th days, the optimal growth-promoting capacity was 107 CFU/mL DD-3 (0.098 g); in the treatment of the added isolates on the 0, 10 and 20th days, the optimal growth-promoting capacity was 106 CFU/mL DD-3 (0.17 g); in the treatment of the added isolates on the 0, 10, 20 and 30th days, the optimal growth-promoting capacity was 107 CFU/mL DD-3 (0.38 g); and in the treatment of the added isolates on the 0, 10, 20, 30 and 40th days, the optimal growth-promoting capacity was 109 CFU/mL DD-2 (0.17 g).
Effects of the bacterial isolates on different growth parameters of rice plants below-ground indicators in pots. a Effects of the bacterial isolates on rice root fresh weight. b Effects of the bacterial isolates on rice root length. c Effects of the bacterial isolate on rice root activity. Data are expressed as means ± standard deviation. In the figure, ***, ** and * indicate P < 0.001, P < 0.01 and P < 0.05, respectively
Compared with the uninoculated control root length (4.2 cm) (Fig. 5b), in the treatment of seed soaking, the optimal growth-promoting capacity was 109 CFU/mL DD-2 (8.6 cm); in the treatment of the added isolates on the 0th day, the optimal growth-promoting capacity was 109 CFU/mL DD-2 (6.9 cm); in the treatment of the added isolates on the 0 and 10th days, the optimal growth-promoting capacity was 106 CFU/mL DD-4 (9.3 cm); in the treatment of the added isolates on the 0, 10 and 20th days, the optimal growth-promoting capacity was 109 CFU/mL DD-4 (9.4 cm); in the treatment of the added isolates on the 0, 10, 20 and 30th days, the optimal growth-promoting capacity was 107 CFU/mL DD-3 (10.2 cm); and in the treatment of the added isolates on the 0, 10, 20, 30 and 40th days, the optimal growth-promoting capacity was 106 CFU/mL DD-4 (8.9 cm).
Compared with the uninoculated control root activity (0.025 mg/g·h) (Fig. 5c), in the treatment of seed soaking, the optimal growth-promoting capacity was 108 CFU/mL DD-4 (0.37 mg/g·h); in the treatment of the added isolates on the 0th day, the optimal growth-promoting capacity was 107 CFU/mL DD-3 (1.27 mg/g·h); in the treatment of the added isolates on the 0 and 10th days, the optimal growth-promoting capacity was 109 CFU/mL DD-4 (0.52 mg/g·h); in the treatment of the added isolates on the 0, 10 and 20th days, the optimal growth-promoting capacity was 108 CFU/mL DD-3 (0.23 mg/g·h); in the treatment of the added isolates on the 0, 10, 20 and 30th days, the optimal growth-promoting capacity was 106 CFU/mL DD-3 (0.34 mg/g·h); and in the treatment of the added isolates on the 0, 10, 20, 30 and 40th days, the optimal growth-promoting capacity was 106 CFU/mL DD-4 (0.26 mg/g·h).
Discussion
Rice refers to one of the most cultivated cereals in the world (Lee et al. 2018). Plants absorb a variety of mineral elements as nutrients for their ideal growth. P and K elements are abundant in soil, in the form of silicate and phosphate, whereas they are difficult to absorb (Khaliq et al. 2018). To achieve the optimal yield of rice, a high amount of fertilizer is required. However, excessive use of synthetic fertilizers will result in environmental pollution (Farooq et al. 2018). There are various growth-promoting bacteria in rice rhizosphere. It is considered that synthetic fertilizer should be replaced with biological fertilizer and beneficial microorganism (Ngo et al. 2019). Furthermore, it has been extensively demonstrated that inoculating beneficial microorganisms on rice can significantly increase different parameters (e.g., plant height, stem diameter and root growth) and improve soil properties (Erdogan et al. 2018).
In the present study, the rhizosphere bacteria of rice were isolated and identified from the soil of a farm in Northeast China. 16S rDNA phylogenetic analysis revealed that the three isolates of bacteria isolated from rice rhizosphere soil consisted of Bacillus megaterium DD-2, Bacillus aryabhattai DD-3 and Bacillus subtilis DD-4. Bacillus enhances plant growth by more than 20 possible mechanisms, of which the most prominent are the release of valuable metabolites, such as phytohormones, antibiotics and siderophores (Adnan et al. 2020). Moreover, bacillus has good stress resistance, and it is widely employed in the biological fertilizer production (Tahir et al. 2018). Under the initial pH value of 7 at 30 °C, the number of viable bacteria was the maximum. Both pH and temperature could affect the growth of the strain and the function of promoting plant growth. At the temperature of 25 ~ 32 °C, the pH value was 6 ~ 8, and the microbial activity was more active. Bacillus velezensis was cultured at 10 ~ 80 °C and pH 5.5 ~ 9.0 (Li et al. 2020). Chelating compounds, siderophores and mineral acids produced by bacteria are also accountable for P and K solubilization in soils (Adnan et al. 2019). Similarly, bacteria produce low-molecular-weight organic acids that acidify the soil and solubilize insoluble inorganic phosphate compounds, such as tricalcium phosphate, dicalcium phosphate, hydroxyapatite and rock phosphate (Adnan et al. 2018). Bacillus decomposes minerals and insoluble silicic acid by releasing silicon and potassium ions to form organic acids and polysaccharides. It is believed that organic acids establish organic ligands and promote silicate dissolution by forming unstable framework surface complexes and metal complexes (Depeng et al. 2018). To be specific, DD-4 exhibited the maximum potassium dissolving capacity, i.e., 1.37 mg/L, DD-2 was capable of dissolving inorganic phosphorus maximally, with the treatment capacity of 288.33 mg/L, and DD-3 had the maximum solubility of organic phosphorus, i.e., 31.58 mg/L. According to the research, a strain of Bacillus methylotrophicus (DD-1) was isolated. Its potassium soluble activity was 1.18 mg/L (Liu et al. 2020). As demonstrated by the qualitative analysis of phosphorus enrichment, Bacillus megaterium YLYP1 produced 716 mg/L of available phosphate within 6 days under the optimal culture (Yu et al. 2019). All isolates are capable of synthesizing IAA, GA and siderophores. At the germination stage, seeds obtain most of their nutrients from internal reserves, but growth hormones like auxins or gibberellins produced by bacteria stimulate the process of germination (Adnan et al. 2020). IAA is an important plant growth-promoting hormone, which is synthesized by rhizobia and leads to root elongation, root hair and lateral root proliferation (Wang et al. 2016). Bacillus cereus, Bacillus pumilus, Bacillus halotolerans and Bacillus vallismortis produce IAA (Efe 2020). Likewise, the beneficial effects of Gibberellin on enhancing plant metabolism and generally synthesized by plants, fungi and bacteria have been extensively reported (Salazar-Cerezo et al. 2018). Bacillus megaterium CDK25 that synthesize siderophores have been shown to promote the growth of Capsicum annuum L (Bhatt and Maheshwari 2020a, b). The amount of IAA produced by microorganisms depends on the internal operating pathway; for example, the amount of IAA produced by the tryptophan-dependent pathway will be higher, while the amount of IAA produced by the tryptophan-independent pathway will be lower (Fahad et al. 2017). Plant growth-promoting rhizobacteria release substances like phosphatases, IAA and gibberellins in addition to different organic and mineral acids, which ultimately improve crop growth (Adnan et al. 2020).
The effects of bacterial isolates on rice plant growth were studied by pot experiment. Under different growth conditions, plant growth can be effectively promoted either by the application of bacteria alone or in combination with chemical fertilizers (Stanojkovic-sebic et al. 2018). The stem length, root length, shoot fresh weight, root fresh weight and chlorophyll content were higher compared with those of the rice plants treated with IP alone. With the increase in the concentration of isolates and the time of addition, the overall effect of the isolates to boost the growth of rice seedlings first increased and then leveled off. Different isolates exerted different effects to promote rice growth, and the more the isolates added, the promotion effect would not be improved. After the strain is applied to rice, the availability of silicon increases, which may increase the amount of deposits in the leaves, thereby strengthening the leaves and increasing their surface area. This can ultimately be attributed to the use of increased photosynthesis to maximize the collection of sunlight (Fahad et al. 2017). In order to make full use of the soluble phosphorus and other nutrients in the soil, plants need to have a well-developed root system to increase the absorption of nutrients and water in the soil. Therefore, phosphate-dissolving rhizosphere bacteria are very important for agriculture, but the bacteria IAA increases the root surface area and length, thereby providing more nutrients and water for plants (Adnan et al. 2018). Many researchers have shown that compared with the fertilizer added on the 12, 14 and 16th days, the fertilizer growth on the 10th day of seedling growth is better, and the above-ground and underground characteristics of rice seedlings change with the fertilizer concentration (Xiao et al. 2019). In the pot experiment, as impacted by the limitation of soil, the effect of the times of adding isolates on the growth of rice will be increasingly less, so in-depth research is required in field conditions.
In brief, three isolates of growth-promoting bacteria were isolated and identified from rice rhizosphere soil in Northeast China, including Bacillus megaterium DD-2, Bacillus aryabhattai DD-3 and Bacillus subtilis DD-4. As revealed from the results, all isolates were capable of dissolving potassium and phosphorus. All isolates exhibited the ability to synthesize IAA, GA and siderophores, which showed the properties to significantly promote the growth of rice. The above-ground indexes (e.g., plant height, stem diameter, above-ground fresh weight and chlorophyll content) and above-ground indexes (e.g., root length, root fresh weight and root activity) of rice seedlings were elevated by the isolates. The bacterial isolates observed in the present study show promising biological applications.
Abbreviations
- IAA:
-
Indole-3-acetic acid
- GA:
-
Gibberellic acid
- LB:
-
Luria–Bertani
- CAS:
-
Chrome Azurol S
- DD-2:
-
Bacillus megaterium DD-2
- DD-3:
-
Bacillus aryabhattai DD-3
- DD-4:
-
Bacillus subtilis DD-4
References
Adnan M, Fahad S, Khan IA, Saeed M, Ihsan MZ, Saud S, Riaz M, Wang D, Wu C (2019) Integration of poultry manure and phosphate solubilizing bacteria improved availability of Ca bound P in calcareous soils. 3 Biotech 9:368
Adnan M, Fahad S, Muhammad Z, Shahen S, Ishaq AM, Subhan D, Zafar-ul-Hye M, Martin LB, Raja MMN, Beena S, Saud S, Imran A, Zhen Y, Martin B, Jiri H, Rahul D (2020) Coupling phosphate-solubilizing bacteria with phosphorus supplements improve maize phosphorus acquisition and growth under lime induced salinity stress. Plants 9:900
Adnan M, Zahir S, Fahad S, Arif M, Mukhtar A, Imtiaz AK, Ishaq AM, Abdul B, Hidayat U, Muhammad A, Inayat-Ur R, Saud S, Muhammad ZI, Yousaf J, Amanullah HMH, Wajid N (2018) Phosphate-solubilizing bacteria nullify the antagonistic effect of soil calcification on bioavailability of phosphorus in alkaline soils. Sci Rep 8:4339
Amoozegar MA, Shahinpei A, Makzum S, Rafieyan S, Nikou MM, Spröer C, Ventosa A (2018) Salipaludibacillus halalkaliphilus sp. nov., a moderately haloalkaliphilic bacterium from a coastal-marine wetland. Int J Syst Evol Microbiol 68:2214–2219
Bakhshandeh E, Pirdashti H, Shahsavarpour LK, Gilani Z, Yaghoubi KM, Crecchio C (2020) Effects of plant growth promoting microorganisms inoculums on mineral nutrition, growth and productivity of rice (Oryza sativa L.). J Plant Nutr 43:1–18
Banik A, Dash GK, Swain P, Kumar U, Mukhopadhyay SK, Dangar TK (2019) Application of rice (Oryza sativa L.) root endophytic diazotrophic Azotobacter sp. strain Avi2 (MCC 3432) can increase rice yield under green house and field condition. Microbiol Res 18:56–65
Bhatt K, Maheshwari DK (2020a) Bacillus megaterium strain CDK25, a novel plant growth promoting bacterium enhances proximate chemical and nutritional composition of Capsicum annuum L. Front Plant Sci 11:1147
Bhatt K, Maheshwari DK (2020b) Zinc solubilizing bacteria (Bacillus megaterium) with multifarious plant growth promoting activities alleviates growth in Capsicum annuum L. 3 Biotech 10:36–46
Dash DM, Osborne JW (2020) Biodegradation of monocrotophos by a plant growth promoting Bacillus aryabhattai (VITNNDJ5) strain in artificially contaminated soil. Int J Environ Sci Technol 17:1475–1490
Depeng W, Fahad S, Saud S, Muhammad K, Aziz K, Mohammad NK, Hafiz MH, Wajid N (2018) Morphological acclimation to agronomic manipulation in leaf dispersion and orientation to promote “Ideotype” breeding: evidence from 3D visual modeling of “super” rice (Oryza sativa L.). Plant Physiol Biochem 135:499–510
Du B, Luo H, He L, Zhang L, Liu Y, Mo Z, Pan S, Tian H, Duan M, Tang X (2019) Rice seed priming with sodium selenate: effects on germination, seedling growth, and biochemical attributes. Sci Rep 9:1–9
Efe D (2020) Potential plant growth-promoting bacteria with heavy metal resistance. Curr Microbiol 77:3861–3868
Erdogan U, Turan M, Ates F, Kotan R, Çakmakçi R, Erdogan Y, Kitir N, Tüfenkçi S (2018) Effects of root plant growth promoting rhizobacteria inoculations on the growth and nutrient content of grapevine. Commun Soil Sci Plan 49:1731–1738
Fahad S, Bajwa AA, Nazir U, Anjum SA, Farooq A, Zohaib A, Sadia S, Nasim W, Adkins S, Saud S, Ihsan MZ, Alharby H, Wu C, Wang D, Huang J (2017) Crop production under drought and heat stress: plant responses and management options. Front Plant Sci 8:1147
Fahad S, Hussain S, Bano A, Saud S, Hassan S, Shan D, Khan FA, Khan F, Chen Y, Wu C, Tabassum MA, Chun MX, Afzal M, Jan A, Jan MT, Huang J (2014) Potential role of phytohormones and plant growth-promoting rhizobacteria in abiotic stresses: consequences for changing environment. Environ Sci Pollut Res 22:4907–4921
Fahad S, Nie L, Chen Y, Wu C, Xiong D, Saud S, Hongyan L, Cui K, Huang J (2015) Crop plant hormones and environmental stress. Sustain Agric Rev 15:371–400
Fang J, Wang X, Liu Y, Tang Z, White PS, Sanders NJ (2013) Multi-scale patterns of forest structure and species composition in relation to climate in northeast china. Ecography 35:1072–1082
Farooq N, Iqbal M, Farooq M, Zahir ZA (2018) Interactive effects of synthetic fertilizer and organic residue inputs on soil fertility and wheat crop under various moisture regimes. Int J Agric Biol 21:244–250
Kang SM, Waqas M, Shahzad R, You YH, Lee IJ (2017) Isolation and characterization of a novel silicate-solubilizing bacterial strain Burkholderia eburnea CS4-2 that promotes growth of japonica rice (Oryza sativa L. cv. Dongjin). Soil Sci Plant Nutr 63:1–9
Khaliq MA, James B, Chen YH, Ahmed Saqib HS, Li HH, Jayasuriya P, Guo W (2018) Uptake, translocation, and accumulation of Cd and its interaction with mineral nutrients (Fe, Zn, Ni, Ca, Mg) in upland rice. Chemosphere 215:916–924
Kumari P, Meena M, Upadhyay RS (2018) Characterization of plant growth promoting rhizobacteria (PGPR) isolated from the rhizosphere of Vigna radiata (mung bean). Biocatal Agric Biotechnol 16:155–162
Lee KE, Radhakrishnan R, Kang SM, You YH, Kim JH (2015) Enterococcus faecium lke12 cell-free extract accelerates host plant growth via gibberellin and indole-3-acetic acid secretion. J Microbiol Biotechnol 25:1467–1475
Lee K, Adhikari A, Kang S, You Y, Joo G, Kim J, Lee I (2019) Isolation and characterization of the high silicate and phosphate solubilizing novel strain Enterobacter ludwigii GAK2 that promotes growth in rice plants. Agronomy 9:144–155
Lee TK, Kang HR, Kim KH (2018) A new feruloyl glyceride from the roots of Asian rice (Oryza sativa). Rev Bras Farmacogn 28:421–424
Li T, Huang L, Li Y, Xu Z, Ge X, Zhang Y, Wang N, Wang S, Yang W, Lu F, Liu Y (2020) The heterologous expression, characterization, and application of a novel laccase from Bacillus velezensis. Sci Total Environ 713:136713
Liu Z, Wang H, Xu W, Wang Z (2020) Isolation and evaluation of the plant growth promoting rhizobacterium Bacillus methylotrophicus (DD-1) for growth enhancement of rice seedling. Arch Microbiol 10:1–11
Lu T, Ke M, Lavoie M, Jin Y, Fan X, Zhang Z, Fu Z, Sun L, Gillings M, Peñuelas J, Qian H, Zhu Y (2018) Rhizosphere microorganisms can influence the timing of plant flowering. Microbiome 6:1–12
Majeed A, Abbasi MK, Hameed S, Imran A, Rahim N (2015) Isolation and characterization of plant growth-promoting rhizobacteria from wheat rhizosphere and their effect on plant growth promotion. Front Microbiol 6:198–207
Mitra S, Pramanik K, Sarkar A, Ghosh PK, Soren T, Maiti TK (2018) Bioaccumulation of cadmium by Enterobacter sp. and enhancement of rice seedling growth under cadmium stress. Ecotoxicol Environ Saf 156:183–196
Moretti LG, Crusciol CA, Kuramae EE, Bossolani JW, Moreira A, Costa NR, Hungria M (2020) Effects of growth-promoting bacteria on soybean root activity, plant development, and yield. Agron J 112:418–428
Munira S, Farenhorst A, Akinremi W (2018) Phosphate and glyphosate sorption in soils following long-term phosphate applications. Geoderma 313:146–153
Mussa A, Million T, Assefa F (2018) Rhizospheric bacterial isolates of grass pea (Lathyrus sativus L.) endowed with multiple plant growth promoting traits. J Appl Microbiol 125:1786–1801
Naseer I, Ahmad M, Hussain A, Jamil M (2020) Potential of zinc solubilizing Bacillus strains to improve rice growth under axenic conditions. Pak J Agri Sci 57:1057–1071
Ngo NP, Yamada T, Higuma S, Ueno N, Saito K, Kojima K, Maeda M, Yamaya H, Ohkama ON, Kanekatsu M, Yokoyama T (2019) Spore inoculation of Bacillus pumilus TUAT1 strain, a biofertilizer microorganism, enhances seedling growth by promoting root system development in rice. Soil Sci Plant Nutr 2:1–7
Patra PS, Haque S (2011) Effect of seedling age on tillering pattern and yield of rice (Oryza sativa L.) under system of rice intensification. Eur Phys J A 11:55–78
Roca LF, Romero J, Bohórquez JM, Alcántara E, Fernández-Escobar R, Trapero A (2018) Nitrogen status affects growth, chlorophyll content and infection by Fusicladium oleagineum in olive. Crop Prot 109:80–85
Sakoda M, Mizusawa M, Shiotsu F, Sakagami N, Nishizawa T (2019) Azoarcus sp. strain KH32C affects rice plant growth and the root-associated soil bacterial community in low nitrogen input paddy fields. Soil Sci Plant Nutr 65:451–459
Salazar-Cerezo S, Martínez-Montiel N, García-Sánchez J, Pérez-y-Terrónb R, Martínez-Contreras RD (2018) Gibberellin biosynthesis and metabolism: a convergent route for plants, fungi and bacteria. Microbiol Res 208:85–98
Saleemi M, Kiani MZ, Sultan T, Khalid A, Mahmood S (2017) Integrated effect of plant growth-promoting rhizobacteria and phosphate-solubilizing microorganisms on growth of wheat (Triticum aestivum L.) under rainfed condition. Agric Food Secur 6:46–53
Stanojkovic-sebic A, Pivic R, Dinic Z, Ilicic R, Latkovic D, Josic D (2018) Effect of Indigenous Pseudomonas sp. and Bacillus sp. strains on yield and main chemical growth parameters of radicchio. Contemp Agric 67:20–26
Sun T, Liu Y, Wu S, Zhang J, Xu J (2020) Effects of background fertilization followed by co-application of two kinds of bacteria on soil nutrient content and rice yield in northeast china. Int J Agric Biol Eng 13:154–162
Tahir M, Khalid U, Ijaz M, Shah GM, Naeem MA, Shahid M, Mahmood K, Ahmad N, Kareem F (2018) Combined application of bio-organic phosphate and phosphorus solubilizing bacteria (Bacillus strain MWT 14) improve the performance of bread wheat with low fertilizer input under an arid climate. Braz J Microbiol 49:15–24
Thulasi K, Jayakumar A, Pillai AB, Sankaramangalam VKG, Kumarapillai H (2018) Efficient methanol-degrading aerobic bacteria isolated from a wetland ecosystem. Arch Microbiol 200:829–833
Wang J, Zhang Y, Li Y, Wang X, Liu Z, Nan W, Zhao C, Wang F, Ma J, Bi Y (2016) Involvement of polar auxin transport in the inhibition of Arabidopsis seedling growth induced by Stenotrophomonas maltophilia. Biol Plant 60:299–310
Xiao M, Zang H, Ge T, Chen A, Zhu A, Zhou P, Atere CT, Wu J, Su Y, Kuzyakov Y (2019) Effect of nitrogen fertilizer on rice photosynthate allocation and carbon input in paddy soil. Eur J Soil Sci 70:786–795
Xiao Y, Wang X, Chen W, Huang Q (2017) Isolation and identification of three potassium-solubilizing bacteria from rape rhizospheric soil and their effects on ryegrass. Geomicrobiol J 34:873–880
Yu LY, Huang HB, Wang XH, Li S, Feng NX, Zhao HM, Huang XP, Li YW, Li H, Cai QY, Mo CH (2019) Novel phosphate-solubilising bacteria isolated from sewage sludge and the mechanism of phosphate solubilisation. Sci Total Environ 658:474–484
Zhang C, Kong F (2014) Isolation and identification of potassium-solubilizing bacteria from tobacco rhizospheric soil and their effect on tobacco plants. Soil Ecol 82:18–25
Acknowledgements
This work was supported by the Major technological innovation program in Hubei Province of China (2019ABA092 and 2019ABA095).
Author information
Authors and Affiliations
Contributions
ZL, NX, YH, CW, YS and DL designed the study; ZL, XZ, LL and NX performed the research; ZL, NX, YS and DL analyzed the data; and ZL, NX, CW, YS and DL wrote the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research Involving Human and Animal Rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Handling editor: Rhonda Peavy.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, Z., Zhang, X., Li, L. et al. Isolation and Characterization of Three Plant Growth-Promoting Rhizobacteria for Growth Enhancement of Rice Seedling. J Plant Growth Regul 41, 1382–1393 (2022). https://doi.org/10.1007/s00344-021-10393-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-021-10393-4