Abstract
A laboratory incubation experiment was executed to examine the role of phosphate solubilizing bacteria (with PSB and without PSB) and poultry manure (4, 8 and 12 t PM ha−1) in improving P mobilization/mineralization under four different lime regimes (4.78, 10, 15 and 20% CaCO3 M/M) for 56 days using three factorial complete randomized design (CRD) with triplicates. Phosphorus availability progressively increased over time irrespective of PSB inoculation, PM and lime levels. The PSB and PM (4–12 t ha−1) addition into soil significantly increased Olsen P at all incubation intervals. Post incubation PSB survival increased by 12 and 9% with inoculation and 12 t PM ha−1 over control and 4 t PM ha−1, respectively. Liming ominously reduced P mobilization/mineralization by 1.3, 2.6 and 10.5% and PSB population by 6.6, 7.3 and 16.3% at 10, 15 and 20% (lime), respectively, over control at day 56. However, PSB and PM addition (with increasing rate) into soil significantly counterbalanced these ill effects of lime. Thus, the application of PSB and PM is a promising measure to enhance P availability in calcareous soils and shall be practiced.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phosphorus (P) is 2nd major growth-limiting nutrient after nitrogen (N) in term of its requirements (Salimpour et al. 2010). Contrasting to N, P cannot be made biologically available from atmosphere (Ezawa et al. 2002). On dry weight basis plant contains 0.2–0.8% P (Hao et al. 2002). Approximately, on 30–40% of world cultivable land, crop yield is low due to P deficiency (Fahad et al. 2014, 2015a, b, 2016; Sonmez et al. 2016; Turan et al.2017; Turan et al. 2018). On average, 1.00–2.50% of soil total P (400–1000 mg kg−1) is available to plants (Chen et al. 2008). In soil P is generally found as soluble P, insoluble mineral and organic P. Approximately half of the total soil P is organic (Schutz et al. 2018). Almost 20–80% of the organic P (OP) has been found to be inert (Abdi et al. 2014). Soil mineral P (MP) is obtained by the weathering of rocks and phosphatic fertilizers. Mineral P in soil exist as phosphate anions which either adsorbed to clay surfaces (Halajnia et al. 2009), or make insoluble complexes with cations such as Ca2+ and Mg2+ in alkaline soil or Fe2+ and Al3+ in acidic soils (Yadav et al. 2017). As a consequence, the bio-available P in the soil barely goes beyond 0.1 mg kg−1. Almost 44.12 million tons (MT) of phosphate fertilizers are used every year across the globe, of which 80% is lost (FAO 2017) due to its precipitation, adsorption and immobilization reactions in soil (Gyaneshwar et al. 2002). This not only increases cost of production but also pollute the environment (Tilman et al. 2001).
Khan et al. (2009) estimated that if the accumulated P in agricultural soils is made available by certain means, it will be enough to support optimum plant growth for almost 100 years. Calcareous soils [which are most abundant in Pakistan (Rehim 2016)] due to its high content of calcite may fix substantial amounts of P (Li and Marschner 2019). However, integrated application of P fertilizers with organic substrates (PM, FYM, etc.) into calcareous soil has shown to increase P solubility and availability comparatively for long time than its sole mineral application (Bolan et al. 1994). Among organic manures PM contains greater concentration of nutrients such as N (~ 2%) and P (~ 1.5%), it can be efficiently used as a source of plant nutrients (Selvamani et al. 2019).
Phosphate solubilizing bacteria (PSB) enhance P availability by playing an important role in soil P cycle through involvement in dissolution–precipitation, sorption–desorption, and mineralization–immobilization reactions (Jiang et al. 2018). They produce different types of organic acids such as mono-, di- and tricarboxyclic (Ryan et al. 2001), and mineral acids such as nitric and sulphuric acids (Chen et al. 2006), thus acidifying the soil (Penn and Camberato 2019) and consequently release P from Ca3 (PO4)2 in calcareous soils. These acids can also displace adsorbed phosphate through ligand exchange reactions. Organic acids may also chelate the cations such as Ca2+, Al3+ and Fe3+ and may increase plant available P (Jones 1998). These bacteria may act as a sink for P in the presence of labile carbon by rapidly immobilizing it (Bünemann et al. 2004) and it is released into soil upon their decomposition. Alkaline phosphatases (Rodriguez et al. 2002), H+ protonation (Xiao et al. 2017), anion exchange, chelation, siderophores, hydroxyl ions and CO2 production are other mechanisms by which PSB improve crop growth and soil P nutrition (Sugihara et al. 2010; Iqbal et al. 2019). They may enhance P availability through the liberation of extracellular enzymes (George et al. 2018). The H2S released by PSB when reacts with ferric phosphate make ferrous sulphate with concurrent discharge of phosphate ion. Moreover, phytohormones such as indole acetic acid (Chaiharn and Lumyong 2011), gibberellins and cytokinins (Mehta et al. 2019) produced by PSB are also positively correlated with phosphate solubilization.
As most of Pakistan’s soils are calcareous and hence deficient (90%) in available P (Rehim 2016). Therefore, the use of PSB and PM for improving P availability could be an efficient approach. However, the potential of PSB in enhancing bio-available P has not been fully realized due to their changing behavior under different soils [non calcareous (≤ 5% lime), slightly (≤ 10% lime), moderately (≤ 15% lime) and highly (≤ 20% lime) calcareous]. Thus, this study was designed to evaluate the potential PSB in improving P availability from PM in artificially induced calcareous soil under lab incubation experiment for 56 days.
Materials and methods
Soil description
A surface (0–20 cm) non-calcareous soil (Gulyana soil series) was obtained from Agricultural Research Station (ARS) Swabi, Baja Bamkhel, Distract Swabi, Khyber Pukhtoonkhwa- Pakistan (34°7′12″ North and 72°28′20). The soil used in the experiment was silt loam, non-saline, non-calcareous (4.78% lime), low in organic matter, and deficient in total N, K and Olsen P contents (Table 1).
Inputs used
A non-analytical grade lime in powder form (≤ 2 mm) was obtained from local market. Poultry manure (PM) was acquired from local poultry farm and was analyzed for its NPK concentration. Phosphate solubilizing bacteria (PSB) were obtained from National Agriculture Research Center (NARC) Islamabad and were tested for its microbial composition and population.
Characterization of applied PSB inoculum
The inoculum used was tested for its microbial composition by Bergey’s manual of systematic bacteriology (Krieg and Holt 1984) while using Pikovskaya’s agar medium with Ca3(PO4)2 as insoluble P (Gordon et al. 1973). The inoculum was composed of Pseudomonas (15.3%), Bacillus (12.2%), Rhizobia (16.8%), Burkholderia (11.5%), Micrococcus (5.8%), Flavobacterium (2.9%), Achromobacter (6.6%), Erwinia (10.1%), Agrobacterium (3.9%), and 15% unidentified species (Table 2). The inoculum was also tested for phosphate solubilization (Nautiyal 1999), alkaline phosphatase activity (Eivazi and Tabatabai 1977), siderophores (Alexander and Zuberer 1991), and indole acetic acid (IAA) (Vincet 1970) production. The peat-based PSB inoculum was composed of 1.46 × 107 cfu of PSB g−1. The used PSB showed potential PGPR characteristics (Table 3). They were capable of releasing: axines (4.7 ± 0.51 mg ml−1), indole acetic acids (7.5 ± 0.66 µg ml−1), organic acids (10.6 ± 0.65 g L−1), siderophores (6.2 ± 0.68 diameter of halo in mm) production, and phosphate solubilization (6.7 ± 0.39 diameter of halo in mm).
Experimental procedures
To examine the effect of PSB inoculation on Ca-P mobilization and P mineralization from PM in soil amended with different levels of lime an incubation experiment was conducted. Three factorial CRD with three replications, consisting of (factor A) two inoculation treatments (With PSB and Without PSB) (factor B) three levels of PM (4, 8 and 12 Mg ha−1) and (factor C) four levels of lime (4.78, 10, 15 and 20% powdered CaCO3 M/M) accounting for 24 treatment per replication. A 100, 94.78, 89.78 and 84.78 g soil each was added into 18 plastic incubation pots and amended with 0, 5.22, 10.22 and 15.22 g lime per pot (100 g soil + lime) 30 days before the application of PM and PSB for achieving 4.78, 10, 15 and 20% lime, respectively. The pots were then also treated with 200, 400 and 600 mg PM at the rate of 4, 8 and 12 t PM ha−1. Uniform quantity of N (60 mg kg−1 as urea) and K (30 mg kg−1 as SOP), were applied to all pots as solution form. Peat-based maize PSB (1 mg kg−1soil) was added as 1% (M/V) inoculum water (sterile distilled) suspension. Viable cell count of PSB was 1.42 × 105 cfu ml−1 in prepared suspension (1% M/V) as determined by dilution plate technique (Holt et al. 1994). Pots receiving PSB were treated with 5 ml of this suspension while, without PSB pots were added with 5 ml of sterilized distilled water (Gyaneshwar et al. 1999) followed by proper inversion. The pots were then incubated at 32 ± 2 °C and moisture content of the soil was maintained at about 50% of field capacity throughout the experiment. At day 0, 7, 14, 28 and 56 of incubation 10 g of soil was taken out from all pots each for Olsen extractable P and moisture content. The post incubation PSB population in each pot was also measured at day 56.
Soil analysis
A suspension (1:2) of soil and water was prepared and analyzed for pH (Thomas 1996) and electrical conductivity (Rhoades 1996). Nitrogen content in soil was measured by the method described by Bremner and Breitenbeck (1983) and K the method of Ryan et al. (2001). Phosphorus in soil was determined by the NaHCO3 method (Olsen et al. 1954). Lime content in soil was measured by the acid neutralization method (Loeppert and Suarez 1996), soil texture by hydrometer method (Gee and Bauder 1986) and organic matter content by the Walkley and Black (Nelson and Sommers 1996). The PSB population in soil at day 56 of incubation was determined by suspension dilution plate techniques in fresh soil samples using Pikovskaya’s medium 81 (Holt et al. 1994).
Statistical analysis
The replicated data obtained for P mineralization at each incubation interval and PSB population at day 56 was subjected to analysis of variance (ANOVA) according to three factorial CRD (Steel and Torrie 1996) using statistical package Statistix 8.1. For any significant variation data were further subjected to least significant difference (LSD) test. Results on PGPR characterizations of PSB were processed by descriptive statistics.
Results
Phosphorus mineralization
Results presented in Table 4 show P release from PM by PSB under varying levels of lime over 56 days incubation. The PSB inoculation noticeably increased P availability over un-inoculated control at each incubation interval except day zero. Inoculation (with PSB) increased P availability by 3.7, 3.6, 1.6 and 2.8% over control at day 7, 14, 28 and 56, respectively. Phosphorus release significantly varied among different PM rates at all incubation intervals during 56 days (Table 4). Generally, P availability increased with increasing application of PM at each data interval over 56 days. Maximum P mineralization was observed at 12 t PM ha−1 followed by 8 t PM ha−1 while minimum Olsen P was recorded at 4 t PM ha−1 application at all data intervals. Furthermore, P availably also increased with time at all PM rates. Net P mineralization of 6.37, 11.27 and 14.99 mg kg−1 was observed at 4, 8 and 12 t PM ha−1, respectively, at day 56. Phosphorus release showed inverse relationship to liming (Table 4). Phosphorus release declined with increasing application of lime, however, it response was comparable up to 10% lime at all incubation interval except day 28. Highest Olsen P was noticed for 4.78% (control) lime while the lowest were observed in soil amended with 20% lime at each data interval. Phosphorus release decreased over control lime by 0.8, 6, 12% at day zero, 0.8, 10, 15% at day 7, 1.5, 5.6, 18% at day 14, 2, 7, 13% at day 28 and 1, 3, 10% at day 56 with 10, 15 and 20% lime, respectively. Phosphorus availability potentially increased with time by 11.39, 11.28, 11.19 and 10.30 mg kg−1 at 4.78, 10, 15 and 20% lime, respectively, during 56 days incubation time.
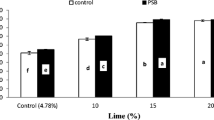
Interactively, PSB inoculation and liming significantly affected P availability at day 7 (Fig. 1) while at rest of data intervals its effect was non-significant. At day 7, PSB inoculated soil released significantly higher P than un-inoculated (without PSB) treatments at each soil lime contents. Liming did not affect soil P content up to 10% both with and without PSB inoculation but its application beyond 10% potentially decreased soil P availability. Highest P release was observed in soil amended with no (4.78%) and 10% lime with inoculation while lowest was recorded at 20% lime without inoculation. Furthermore, 20% lime with inoculation released similar P to 15% lime without inoculation. Similarly, associative effect of PM rates and soil calcification was also significant for P availability at day 0 (Fig. 2), 7 (Fig. 3), 14 (Fig. 4) and 28 (Fig. 5) of incubation. These significant interactions demonstrated that, P availability increased with increasing application of PM from 4 to 12 t ha−1 and reduced with increasing lime level. However, PM was capable of minimizing the hostile effect of liming on P availability. At zero day of incubation, liming exceeding 10% reduced soil Olsen extractable P. The P availability increased with increasing PM regardless of lime level, however, the performance of 12 and 8 t PM ha−1 was comparable at 4.78 and 10% lime. There was no significant effect of liming on P availability at 10% lime at corresponding PM rates. Soil treated with 12 and 8 t PM ha−1 at 20% lime released at par P to that of 8 and 4 t PM ha−1 with 15% lime, respectively. Similarly, soil having 12 t PM ha−1 at 15% lime produced comparable P to 8 t PM ha−1 at 4.78 and 10% lime, which were significantly higher than that P released at 4 t PM ha−1 with 4.78 and 10% lime (Fig. 2).
At day 7 (Fig. 3), with increasing application of PM soil P availability significantly increased, however, there was no difference in P at 4.78 and 10% lime at corresponding PM rates. Addition of lime beyond 10% significantly rendered P availability at each PM levels except 12 t PM ha−1 which produced similar P as at 15 and 20% lime. The PM applied at the rate of 12 t ha−1 proved to be as effective as 4 t PM ha−1 at 4.78 and 10% lime. In addition, at 20% lime soil having 8 t PM ha−1 released comparable amount of as at 4 t PM ha−1 with 15% lime. After 14 days of incubation, PM rates varied in their potential for P mineralization at each lime level except 20% lime where 8 and 4 t PM ha−1 released comparable P. Liming did not affect P mineralization from PM (at corresponding rates) at 4.78 and 10% lime, beyond which (10%) liming significantly antagonized soil P availability. The 12 t PM ha−1 with 15% lime was as effective as 12 t PM ha−1 with 10% lime and they were significantly better than that at 8 and 4 t PM ha−1 co-applied with 4.78 and 10% lime. Furthermore, 12 t PM ha−1 at 20% lime resealed comparable P as at 8 t PM ha−1 with 10 and 15% lime and this was better than that at 4 t PM ha−1 with 10 and 15% lime (Fig. 4). The lowest P mineralization was recorded for 20% lime at 8 and 4 t PM ha−1. Twenty-eight days after incubation P mineralization increased with increasing application of PM but addition of lime depressed it (Fig. 5). Highest P was mineralized from 12 t PM ha−1at 4.78% lime while the lowest from 4 t PM ha−1 at 20% lime. The PM application at the rate of 12 t ha−1 mineralized similar amount of P both at 10% and 15% lime. This was significantly higher than P released from 4 and 8 t PM ha−1applied to pots amended with 4.78 and 10% lime. In addition, P mineralized from 8 t PM ha−1with 20% lime was significantly higher than that at 4 t PM ha−1 applied with 15% lime.
Post incubation PSB survival
Results concerning post incubation PSB population in 56 incubated soil as affected by PSB inoculation, PM rates, liming and their interactions are shown in Table 4. Analysis of variance showed that none of the interactions significantly affected post incubation PSB survival. Mainly, PSB inoculation, liming and PM rates demonstrated considerable effect over PSB population. The PSB were significantly more viable in PSB incubated soil than un-inoculated control. Moreover, PSB population was 11.8% greater in inoculated soil (with PSB) than un-inoculated (without PSB). Similarly, PSB survival also increased with increasing application of PM but its population was statistically at par as at 8 and 12 t PM ha−1. Addition of lime adversely affected PSB survival. The PSB viability significantly dropped off with increasing lime content from 4.78 to 20%. Highest PSB survival of 9.22 × 105 cfu g−1 was observed at 4.78% lime while the lowest of 7.72 × 105 cfu g−1 was observed at 20%. The PSB population declined by 6.6, 7.3 and 16.3% over control at 10, 15 and 20% lime, respectively.
Discussion
Large quantity of P applied as chemical fertilizer goes out of soil plant system through complexation and precipitation reaction with highly reactive Ca2+ and Mg2+ in calcareous soils (Hao et al. 2002; Tisdale et al. 2002). Lindsay et al. (1989) reported that, available P anions are very unstable and rapidly forming metal anion complexes with cations such as Ca2+ and Mg2+ in calcareous soil and Al3+ and Fe2+ in acidic soil (Barrow, 2017) due to which approximately 80% of applied P become un-available to plant (Salvagiotti 2017). We observed that lime addition and its increasing rate resulted a gradual decrease in Olsen P which is in validation to the findings of Shen et al. (2016) who described 3–7 mg kg−1 decrease in Olsen P per unit increase in pH. Liming induces P immobilization in the soil by increasing precipitation reactions of P with basic cations (Curtin and Syers 2001). Liming causes Ca2+ toxicity thus enhances the precipitation of P as Ca–P, consequently, reduce P availability in soil.
According to Alvarez et al. (2004) PSB and PM reduce soil pH by releasing H+ ions and enhances the availability of P both from applied and indigenous sources in soil. We noticed that, P availability increased over time; however, this increase was more prominent in inoculated soil compared to un-inoculated. This could be attributed to release of unavailable P into the mobile pool by rapid mineralization of organic P and solubilisation of Ca-P through acidifying and chelating mechanisms (Khan and Sharif 2012). Our results confirm those documented by Satyaprakash et al. (2017) and Khan et al. (2006), who conveyed that PSB produces organic acids, acidify surrounding soil and thus, solubilize Ca-P in alkaline soil. The PSB also produce phosphatase enzyme which plays a significant part in the solubilisation of P (Alori et al. 2017). According to Wu et al. (2005) chelating compounds, siderophores and mineral acids produce by PSB are also accountable for P solubilisation in acidic soils.
Afif et al. (1995) documented that addition of OM to the soil reduces P-insolubilisation. Poultry manure acidify soil by releasing H+ ions into the soil (Alvarez et al. 2004) as a result P solubility/mineralization of both exogenously applied and soil indigenous P increases (Qin et al. 2019). Addition of organic manures into soil favour the formation of soluble monetite and brushite compared to most stable Ca–P such as hydroxyapatite (Sato et al. 2005) due to the presence of organic anions (i.e. humic, fulvic, tannic and citric acids) that delay the crystallization and transformations of stable Ca–P (Delgado et al. 2002), and thus increase P availability in soil.
Our results reflected that PSB can survive and flourishes in soil for up to 56 days, which agrees with Pahari and Mishra (2017). They documented that PSBs can stay viable in soil almost for 6 months. Additionally, Hameeda et al. (2008) observed that PSB cannot grow in un-inoculated soil. We observed improved PSB survival with the application of PM which is an agreement to Chen et al. (2006) and Chakraborty et al. (2019) who reported that soil enrichment with organic carbon increase soil microbial biomass, consequently enhances P availability by the process of mineralization–immobilization. The PSB viability increased with increasing application of PM. This could also be ascribed to the release of nutrients such as C and N during decomposition which flourishes soil microbes (Nardi et al. 2017; Bais et al. 2001). Liming induces soil alkalinity thus disturb soil microbial activity (Six 2001).
These results summarise that, in calcareous soils P availability decreases but PM and PSB has the potential to minimize such hostile effects of liming on P availability. PSB inoculation and PM fertilization improve also improve microbial population while liming of an alkaline is injurious for PSB viability. Calcareous soils must be treated with PM and PSB for better soil P nutrition. Furthermore, PM has also potential to improve P availability both in calcareous and non-calcareous soils.
Conclusions
The PSB and PM were effective in mobilizing/solubalizing P under non calcareous and artificially induced slightly, moderately and highly calcareous soils. The PSB were more viable in soil amended with PM and/or inoculated with PSB. Phosphorus availability/release significantly decreased with increasing lime level but PSB and PM were effective in nullifying such harmful effects over P release/availability. Phosphate solubilizing bacteria and PM could play a significant role in P mobilization/solubilization both under calcareous and non-calcareous soils and shall be practiced. However, further experimentation is needed under field conditions for verifications of these findings in variety of agro-climatic conditions.
References
Abdi D, Cade-Menun BJ, Ziadiand N (2014) Parent, Long-term impact of tillage practices and phosphorus fertilization on soil phosphorus forms as determined by P nuclear magnetic resonance spectroscopy. J Environ Qual 43:1431–1441
Afif E, Barrow V, Torrent J (1995) Organic matter delays but does not prevent phosphate sorption by Cerrado soils from Brazil. Soil Sci 159:207–211
Alexander DB, Zuberer DA (1991) Use of chrome azurol S reagents to evaluate siderophore production by rhizosphere bacteria. Biol Fertil Soils 12:39–45
Alori ET, Glick BR, Babalola OO (2017) Microbial phosphorus solubilization and its potential for use in sustainable agriculture. Front Microbiol. https://doi.org/10.3389/fmicb.2017.00971
Alvarez R, Evans LA, Milham PJ, Wilson MA (2004) Effects of humic material on the precipitation of calcium phosphate. Geoderma 118:245–260
Bais HP, Loyola-Vargas VM, Flores HE, Vivanco JM (2001) Root-specific metabolism: the biology and biochemistry of underground organs. Vitro Cell Dev Plant Biol 37:30–741
Barrow NJ (2017) The effects of pH on phosphate uptake from the soil. Plant Soil 410:401–410
Bolan NS, Naidu R, Mahimairaja S, Baskaran S (1994) Influence of low-molecular-weight organic acids on the solubilization of phosphates. Biol Fertil Soils 18:311–319
Bremner JM, Breitenbeck G (1983) A simple method for determination of ammonium in semi-micro Kjeldahl analysis of soil and plant material using a block digestor. Commun Soil Sci Plant Anal 14:905–913
Bünemann EK, Bossio DA, Smithson PC, Frossard E, Oberson A (2004) Microbial community composition and substrate use in a highly weathered soil as affected by crop rotation and P fertilization. Soil Biol Biochem 36:889–901
Chaiharn M, Lumyong S (2011) Screening and optimization of indole-3-acetic acid production and phosphate solubilization from rhizobacteria aimed at improving plant growth. Curr Microbiol 62(1):173–181
Chakraborty P, Ranojit KS, Rupsa R, Abhrajyoti G, Debasish M, Prosun T (2019) Bioaugmentation of soil with Enterobacter cloacae AKS7 enhances soil nitrogen content and boosts soil microbial functional-diversity. 3 Biotech 9(7):253
Chen YP, Rekha PD, Arun AB, Shen FT, Lai WA, Young CC (2006) Phosphate solubilizing bacteria from subtropical soil and their tricalcium phosphate solubilizing abilities. Appl Soil Ecol 34:33–41
Chen Z, Ma S, Liu LL (2008) Studies on phosphorus solubilizing activity of a strain of phosphobacteria isolated from chestnut type soil in China. Bioresour Technol 99:6702–6707
Curtin D, Syers JK (2001) Lime-induced changes in indices of soil phosphate availability. Soil Sci Soc Am J 65:147–152
Delgado A, Madrid A, Kassem S, Andreu L, Campillo MC (2002) Phosphorus fertilizer recovery from calcareous soils amended with humic and fulvic acids. Plant Soil 245:277–286
Eivazi F, Tabatabai MA (1977) Phosphatases in soils. Soil Biol Biochem 9:167–172
Ezawa T, Smith SE, Smith FA (2002) P metabolism and transport in AM fungi. Plant Soil 244:221–230
Fahad S, Hussain S, Bano A, Saud S, Hassan S, Shan D, Khan FA, Khan F, Chen Y, Wu C, Tabassum MA, Chun MX, Afzal M, Jan A, Jan MT, Huang J (2014) Potential role of phytohormones and plant growth-promoting rhizobacteria in abiotic stresses: consequences for changing environment. Environ Sci Pollut Res 22(7):4907–4921. https://doi.org/10.1007/s11356-014-3754-2
Fahad S, Hussain S, Saud S, Tanveer M, Bajwa AA, Hassan S, Shah AN, Ullah A, Wu C, Khan FA, Shah F, Ullah S, Chen Y, Huang J (2015a) A biochar application protects rice pollen from high-temperature stress. Plant Physiol Biochem 96:281–287
Fahad S, Nie L, Chen Y, Wu C, Xiong D, Saud S, Hongyan L, Cui K, Huang J (2015b) Crop plant hormones and environmental stress. Sustain Agric Rev 15:371–400
Fahad S, Hussain S, Saud S, Hassan S, Tanveer M, Ihsan MZ, Shah AN, Ullah A, Nasrullah KF, Ullah S, AlharbyH NW, Wu C, Huang J (2016) A combined application of biochar and phosphorus alleviates heat-induced adversities on physiological, agronomical and quality attributes of rice. Plant Physiol Biochem 103:191–198
Food and Agriculture Organization of the united nations—Rome (2017)
George TS, Giles CD, Menezes-Blackburn D, Condron LM, Gama-Rodrigues AC, Jaisi D, Lang F, Neal AL, Stutter MI, Almeida DS, Bol R (2018) Organic phosphorus in the terrestrial environment: a perspective on the state of the art and future priorities. Plant Soil 427(1–2):191–208
Gee GW, Bauder JW (1986) Particle-size analysis. In: Klute A (ed) Methods of soil analysis, Part 1. 2nd ed. Agronomy Monographs 9. ASA and SSSA, Madison, WI, pp 383–411
Gordon RE, Haynes WC, Pang CN (1973) The Genus Bacillus. Agricultural Handbook. No. 427.U.S. Department of Agriculture, Washington D.C
Gyaneshwar P et al (1999) Involvement of a phosphate starvation inducible glucose dehydrogenase in soil phosphate solubilization by Enterobacter asburiae. FEMS Microbiol Lett 171:223–229
Gyaneshwar P, Naresh KG, Poole PSP (2002) Role of soil microorganisms in improving P nutrition of plants. Plant Soil 245:83–93
Halajnia A, Fotovat Haghnia G H, Khorasani R (2009) Phosphorus fractions in calcareous soils amended with P fertilizer and cattle manure. Geoderma 150:209–213
Hameeda B, Harini G, Rupela OP, Wani SP, Reddy G (2008) Growth promotion of maize by phosphatesolubilizing bacteria isolated from composts and macrofauna. Microbiol Res 163:234–242
Hao X, Cho CM, Racz GJ, Chang C (2002) Chemical retardation of phosphate diffusion in an acid soil as affected by liming. Nutr Cycl Agroecosyst 64:213–224
Holt JG, Krieg NR, Sneath PHA, Staley JT, Williams ST (1994) (eds) Bergey’s manual of determinative bacteriology, 9th Edn. The Williams and Wilkin, Baltimore, pp 787
Iqbal A, Arshad M, Karthikeyan R, Gentry TJ, Rashid J, Ahmed I, Schwab AP (2019) Diesel degrading bacterial endophytes with plant growth promoting potential isolated from a petroleum storage facility. 3 Biotech 9(1):35
Jiang H, Qi P, Wang T, Wang M, Chen M, Chen N, Chi X (2018) Isolation and characterization of halotolerant phosphate-solubilizing microorganisms from saline soils. 3 Biotech 8(11):461
Jones DL (1998) Organic acids in the rhizosphere—a critical review. Plant Soil 205:25–44
Khan M, Sharif M (2012) Solubility enhancement of phosphorus from rock phosphate through composting with poultry litter. Sarhad J Agric 28:415–420
Khan MS, Zaidi A, Wani PA (2006) Role of phosphate solubilizing microorganisms in sustainable agriculture—a review. Agron Sustain Dev 26:1–15
Khan AA, Jilani G, Akhter MS, Naqvi SMS, Rasheed M (2009) Phosphorous solubilizing bacteria; occurrence, mechanisms and their role in crop production. J Agric Biol Sci 1:48–58
Krieg NR, Holt JG (1984) Bergey’s manual of systematic bacteriology, vol 1, 9th edn. The Williams and Wilkins, Baltimore, p 984
Li J, Marschner P (2019) Phosphorus pools and plant uptake in manure-amended soil. J Soil Sci Plant Nutr 19(1):175–186
Lindsay WLPLGV, Chien SH (1989) Phosphate minerals, Ch. 22. In: Dixon JB, Weed SB (ed) Minerals in soil environments, 2nd edn. Soil Science Society of America, Madison, WI, USA, pp 1089–1130
Loeppert RH, Suarez DL (1996) Carbonate and gypsum. In: Sparks D, Page A, Helmke P et al (ed) Methods of soil analysis: part 3, chemical methods. Madison: Soil Sci Soc Am J, pp 437–474
Mehta P, Sharma R, Putatunda C, Walia A (2019) Endophytic fungi: role in phosphate solubilization. In: Advances in endophytic fungal research. Springer, Cham. pp 183–209
Nardi S, Pizzeghello D, Ertani A (2017) Hormone-like activity of the soil organic matter. Appl Soil Ecol. https://doi.org/10.1016/j.apsoil.2017.04.020
Nautiyal CS (1999) An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Microbiol Lett 170:265–270
Nelson DW, Sommers LE (1996) Total carbon, organic carbon, and organic matter. In: Methods of soil analysis, part 2, 2nd ed. Soc. of Agron, Inc. Madison, WI. Agronomy, vol 9, pp 961–1010
Olsen SR, Cole CV, Watanabe FS, Dean LA (1954) Estimation of available phosphorus in soils by extraction with sodium bicarbonate. U.S. Department of Agriculture Circ, pp 939
Pahari A, Mishra BB (2017) Characterization of siderophore producing Rhizobacteria and Its effect on growth performance of different vegetables. Int J Curr Microbiol App Sci 6(5):1398–1405
Penn CJ, Camberato JJ (2019) A critical review on soil chemical processes that control how Soil pH affects P availability to plants. Agriculture 9(6):120
Qin J, Xiong H, Ma H, Li Z (2019) Effects of different fertilizers on residues of oxytetracycline and microbial activity in soil. Environ Sci Pollut Res 26(1):161–170
Rehim A (2016) Band-application of phosphorus with farm manure improves phosphorus use efficiency, productivity, and net returns of wheat on sandy clay loam soil. Turk J Agric For 40:319–326
Rhoades JD (1996) Salinity: electrical conductivity and total dissolved solids. In: Sparks D, Page A, Helmke P et al (ed) Methods of soil analysis: part 3, chemical methods. Madison: Soil Sci Soc Am J, pp 417–435
Rodriguez B, Martinez C, Velazquez E (2002) Effect of inoculation of a phosphate solubilising strain from Pseudomonas jesseniion growth of barley and chickpea plants under growth chamber conditions. In: Proceedings of international congress of bacteriology and applied microbiology, Paris 27 July–1 August. pp 170
Ryan PR, Delhaize E, Jones DL (2001) Function and mechanism of organic anion exudation from plant roots. Annu Rev Plant Physiol Plant Mol Biol 52:527–560
Salimpour S, Khavazi K, Nadian H, Besharati H, Miransari M (2010) Enhancing phosphorous availability to canola (‘Brassica napus’ L.) using P solubilizing and sulfur oxidizing bacteria. Austral J of Crop Sci 4(5):330
Salvagiotti F (2017) S stoichiometry in grains and physiological attributes associated with grain yield in maize as affected by phosphorus and sulfur nutrition. Field Crops Res 203:128–138
Sato S, Solomon D, Hyl QM, Ketterings Lehmann J (2005) Phosphorus speciation in manure and manure-amended soils using XANES spectroscopy. Environ Sci Technol 39:7485–7491
Satyaprakash M, Nikitha T, Reddi EUB, Sadhana B, Vani SS (2017) Phosphorous and phosphate solubilising bacteria and their role in plant nutrition. Int J Curr Microbiol App Sci 6:2133–2144
Schutz L, Gattinger A, Meier M, Müller A, Boller T, Mäder P, Mathimaran N (2018) Improving crop yield and nutrient use efficiency via bio-fertilization—a global meta analysis. Front Plant Sci 8:2204
Selvamani K, Annadurai V, Soundarapandian S (2019) Improved co-composting of poultry manure with complementary consortium of indigenous Bacillus spp. 3 Biotech 9(6):215
Shen Q, Camps Hedley M, Arbestain M, Kirschbaum MUF (2016) Can biochar increase the bioavailability of phosphorus? J Soil Sci Plant Nutr 16:268–286
Six J (2001) Sources and composition of soil organic matter fractions between and within soil aggregates. Eur J Soil Sci 52:607–618
Sonmez O, Turan V, Kaya C (2016) The effects of sulfur, cattle, and poultry manure addition on soil phosphorus. Turk J Agric For 40(4):536–541. https://doi.org/10.3906/tar-1601-41
Steel RGD, Torrie JH (1996) Principles and procedures of statistics: a biometrical approach. Mc Graw-Hill, New York
Sugihara S, Funakawa S, Kilasara M, Kosaki T (2010) Dynamics of microbial biomass nitrogen in relation to plant nitrogen uptake during the crop growth period in a dry tropical cropland in Tanzania. Soil Sci Plant Nutr 56:105–114
Thomas GW (1996) Soil pH and soil acidity. Methods of soil analysis: part 3, chemical methods. Madison: Soil Sci Soc Am, pp 475–490
Tilman D, Fargione J, Wolff BD, Antonio C, Dobson A, Howarth R, Schindler D, Schlesinger WH, Simberloff D, Wackhamer D (2001) Forecasting agriculturally driven global environmental change. Science 292:281–284
Tisdale SL, James WLN, Beaton D, John L (2002) Soil fertility and fertilizers, 5th edn. Prentice-Hall, New Delhi
Turan V, Ramzani PMA, Ali Q, Irum A, Khan W-U-D (2017) Alleviation of nickel toxicity and an improvement in zinc bioavailability in sunflower seed with chitosan and biochar application in pH adjusted nickel contaminated soil. Arch Agron Soil Sci 64(8):1053–1067
Turan V, Khan SA, Iqbal M, Ramzani PM, Fatima M (2018) Promoting the productivity and quality of brinjal aligned with heavy metals immobilization in a wastewater irrigated heavy metal polluted soil with biochar and chitosan. Ecotoxicol Environ Saf 161:409–419. https://doi.org/10.1016/j.ecoenv.2018.05.082
Vincet JMA (1970) Manual for the practical study of the root-nodule bacteria. IBPH and Book No. 15. Blackwell Scientific Publication, Oxford
Wu SC, Cao ZH, Li ZG, Cheung KC, Wong MH (2005) Effects of biofertilizer containing N-fixer, P and K solubilizers and AM fungi on maize growth: a greenhouse trial. Geoderma 125:155–166
Xiao Y, Wang X, Chen W, Huang Q (2017) Isolation and identification of three potassium-solubilizing bacteria from rape rhizospheric soil and their effects on ryegrass. Geomicrobiol J 34(10):1–8
Yadav H, Fatima R, Sharma A, Mathur S (2017) Enhancement of applicability of rock phosphate in alkaline soils by organic compost. Appl Soil Ecol 113:80–87
Acknowledgements
This work was supported by the National Key R&D Program of China (Grant no. 2017YFD0300100).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Adnan, M., Fahad, S., Khan, I.A. et al. Integration of poultry manure and phosphate solubilizing bacteria improved availability of Ca bound P in calcareous soils. 3 Biotech 9, 368 (2019). https://doi.org/10.1007/s13205-019-1894-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-019-1894-2