Abstract
For most plant species, auxins and cytokinins are added to the culture medium to induce adventitious shoot formation. In ipecac (Carapichea ipecacuanha (Brot.) L. Andersson), however, shoots form on internodal segments without phytohormone treatment. This creates an opportunity to analyze the dynamics of endogenous phytohormones during adventitious shoot formation. Ipecac is a medicinal plant whose root extract is used as an expectorant and emetic. We explored the relationships among adventitious shoot formation and levels of an auxin (indole-3-acetic acid) and four cytokinins in ipecac. Adventitious shoots that formed in the apical region of internodal segments were derived from epidermal cells. One of the shoots grew vigorously with a vascular bundle connected to that of the segment, and the others were suppressed in the outgrowth. When the biggest shoot was removed, another began to grow. During adventitious shoot formation, endogenous auxin accumulated in the basal region of segments, whereas cytokinins accumulated in the middle region. Thus, the distribution of auxin, not cytokinins, determined where adventitious shoots formed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In 1902, Haberlandt proposed the concept of “totipotency,” by which plant cells of any differentiated cell type can divide and differentiate to ultimately regenerate the whole plant (Haberlandt 1902). In tobacco, a high ratio of auxins to cytokinins (CKs) in culture medium induces roots, a low ratio induces shoots, and high concentrations of both induce callus, indicating that plant cells have high plasticity for cell differentiation and organogenesis (Skoog and Miller 1957). The organ regeneration system has since been widely applied for the propagation of economically important crops and for the regeneration of transgenic plants (Ganeshan and others 2002). The effects of exogenously applied auxins and CKs in the formation of adventitious shoots and roots have been extensively studied, and many efforts have been made to determine the optimum concentrations of each in inducing shoots and roots (George and others 2008). Auxins and CKs antagonistically interact to regulate plant growth and development including embryogenesis, shoot and root development, and vascular tissue formation (Schaller and others 2015). However, the optimum concentrations and combinations of phytohormones differ by species and among tissues used as explants (such as leaf, stem, root, and embryo). In addition, little is known about the absorption of exogenous phytohormones in plant tissues and how they affect cell differentiation. To determine the effects of phytohormones in organogenesis, it is necessary to quantify the changes in endogenous phytohormone levels in explants (Djilianov and others 2013; Golovatskaya and Karnachuk 2007; Zhang and others 2012).
Adventitious shoots can be induced on phytohormone-free culture media in some plant species, such as Dianthus caryophyllus L. (Watad and others 1996), Aegle marmelos (L.) Corrêa (Ajithkumar and Seeni 1998), Bacopa monnieri (L.) Pennell (Tiwari and others 2001), Celastrus paniculatus Willd. (Rao and Purohit 2006), Kalanchoë blossfeldiana Poelln. (Sanikhani and others 2006), and Carapichea ipecacuanha (Brot.) L. Andersson (Yoshimatsu and Shimomura 1991), and CK treatment can increase the number of shoots. Changes in endogenous phytohormones within plants can be analyzed during adventitious shoot formation.
Carapichea ipecacuanha (Brot.) L. Andersson (ipecac) is an important medicinal plant whose roots contain alkaloids such as emetine and cephaeline (Teshima and others 1988), which are used as an expectorant, emetic, and an amoebicide (Chatterjee and others 1982). In its native Brazil, ipecac grows as a shrub in tropical rainforests (Yoshimatsu and Shimomura 1993). In Japan, it is cultivated in greenhouses but rarely produces seeds, and the germination rate decreases during seed storage (Yoshimatsu and Shimomura 1993). Therefore, tissue culture methods have been developed to induce shoot formation from the shoot meristem (Ideda and others 1988), adventitious shoot formation on root segments (Yoshimatsu and Shimomura 1994) and internodal segments (Yoshimatsu and Shimomura 1991), and plant regeneration from callus (Rout and others 2000). Adventitious shoot formation on internodal segments induces the most shoots per explant (Yoshimatsu and Shimomura 1991). The method offers two significant clues to the pattern of formation of adventitious shoots: First, adventitious shoots form in the apical region of segments, whereas callus forms in the basal region, indicating polarity (Fig. 1a). Second, one shoot comes to dominate the other shoots that form, indicating an auxin effect (Fig. 1a). Shoots can form on the surface of internodal segments without callusing and can regenerate complete plants (Fig. 1b). In tissue culture, auxins and CKs are generally added to the culture medium to induce adventitious shoots (Skoog and Miller 1957). In ipecac, however, adventitious shoots can form even on phytohormone-free medium (Yoshimatsu and Shimomura 1991). Thus, ipecac culture allows us to analyze the dynamics and effects of endogenous phytohormones during adventitious shoot formation.
It is likely that endogenous phytohormone levels change dramatically during adventitious shoot formation, triggering the expression of genes required for shoot formation. To explore the relationships between shoot formation and phytohormone levels in ipecac, we counted the number of adventitious shoots formed and measured the endogenous levels of one auxin and four CKs in internodal segments by liquid chromatography–tandem mass spectrometry (LC–MS/MS). We analyzed indole-3-acetic acid (IAA), the most abundant type of auxin (Ljung and others 2001), and four major active types of CKs: isopentenyl adenine (iP), isopentenyl adenine riboside (iPR), trans-zeatin (tZ), and trans-zeatin riboside (tZR), whose biosynthesis is regulated by auxins (Nordström and others 2004; Stolz and others 2011). Here we showed that the level of IAA was low in the apical region of internodal segments, where adventitious shoots formed, but high in the basal region, where shoots did not form. CKs were distributed throughout the segments; in particular, the production of tZR was high before shoot formation.
Materials and Methods
Induction of Adventitious Shoots
We induced adventitious shoot formation on internodal segments according to a previous method (Yoshimatsu and Shimomura 1991). Axenic ipecac shoots (Fig. S1) were subcultured in test tubes (30 mm i.d. × 120 mm) containing 20 mL of phytohormone-free B5 medium (Gamborg and others 1968) solidified with 0.2% Gellan Gum (Wako, Osaka, Japan) at 24 °C under a 14-h light/10-h dark photoperiod (photosynthetic photon flux density, 10–15 µmol m−2 s−1) for every 3 months. To induce adventitious shoots, we put 7- to 8-mm-long internodal segments on 25 mL of the same medium in Petri dishes (90 mm diam. × 20 mm) and cultured them under the same conditions. We used the first (lower) internodes for shoot induction because the number of shoots formed did not differ between the first and second internodes (Fig. S1). Eight to ten segments were used in each experiment, and biologically independent experiments were repeated three times. We counted all shoots longer than 0.3 mm under a digital microscope (DHS1000; Leica Microsystems, Wetzlar, Germany) every week for 10 weeks. To investigate where the shoots formed, we partitioned the segments into four regions (apical to basal region, I–IV) and counted the shoots every week for the first 5 weeks, while their number increased.
Chemicals
IAA and tZR were purchased from Wako Pure Chemical Industry (Osaka, Japan), tZ and iP from Sigma (St. Louis, MO, USA), iPR from Kanto Chemical (Tokyo, Japan), and stable-isotope-labeled compounds used as internal standards (D5-IAA, D5-tZ, D5-tZR, D6-iP, and D6-iPR) from OlchemIm (Olomouc, Czech Republic).
Preparation of Cryo-Sections
Internodal segments were fixed in FAA solution (formalin:acetic acid:50% ethanol = 5:5:90 v/v/v) for 1 day at 4 °C, washed with Milli-Q water to remove the FAA, and placed in 20% sucrose solution for 1 day. They were then embedded in super cryo-embedding medium (Section-Lab, Hiroshima, Japan) and frozen at −100 °C in a UT-2000F freezer (Eyela, Tokyo, Japan). The frozen segments were stuck on Cryofilm type 2C(9) adhesive film (Section-Lab) and sliced at 5 µm on a CM3050 S Research Cryostat (Leica Microsystems) at −20 °C in a cryo-chamber (Kawamoto 2003). The frozen sections were stained with 0.05% toluidine blue O, mounted on a glass slide, and covered with super cryo-mounting medium type R3 (Section-Lab), which was solidified with a UV Quick Cryosection Mounter (Leica Microsystems). The frozen sections were observed under an optical microscope (BX63; Olympus, Tokyo, Japan).
Phytohormone Extraction and Purification
To analyze the dynamics of endogenous phytohormones during shoot formation, we measured phytohormone levels in regions I–IV every week for the first 5 weeks, while the number of shoots increased. IAA, tZ, tZR, iP, and iPR in each region were extracted and purified by solid-phase extraction, as described previously (Yoshimoto and others 2009). We placed 20–50 mg of each internodal segment in a 2.0-mL tube with zirconia beads and froze it in liquid nitrogen. The frozen sample was crushed in a TissueLyser II (Qiagen, Hilden, Germany) and suspended in 1 mL of acetonitrile containing 500 pg of each internal standard. The samples were centrifuged at 11,000×g for 5 min at 4 °C, and the pellet was washed with 80% (v/v) acetonitrile containing 1% (v/v) acetic acid. We added 600 µL of water containing 1% acetic acid to the bulked supernatant, evaporated the acetonitrile, and applied the sample to pre-equilibrated Oasis HLB column cartridges (Waters, Milford, MA, USA). After washing with 1 mL of water containing 1% acetic acid, all hormones were simultaneously eluted with 1 mL of 80% acetonitrile containing 1% acetic acid. The eluate was evaporated to leave the extract in the aqueous fraction and applied to pre-equilibrated Oasis MCX column cartridges (Waters). After the cartridges were washed with 1 mL of water containing 1% acetic acid, IAA was eluted with 2 mL of 30% acetonitrile containing 1% acetic acid. The cartridges were then washed with 2 mL of 80% acetonitrile containing 1% acetic acid, then 1 mL of water, and finally 1 mL of water containing 5% aqueous ammonia. CKs were eluted with 2 mL of 60% methanol containing 5% aqueous ammonia. Each fraction was evaporated and stored at −30 °C until analysis. All phytohormones were measured by LC–MS/MS.
Quantitative Analysis of IAA and CKs
The IAA fraction was resolved in 20 µL of 30% (v/v) acetonitrile, and CK fractions in 20 µL of water containing 1% (v/v) acetic acid. The samples were analyzed by LC–MS/MS using a triple quadrupole MS system (3200 QTRAP; Sciex, Framingham, MA, USA; parameters in Supp. Tables S1, S2) and an HPLC system (Prominence; Shimadzu, Kyoto, Japan) equipped with an Acuity BEH C18 column (ø 2.1 mm × 100 mm; Waters) for IAA analysis and with a Poroshell EC-C18 column (ø 2.1 mm × 50 mm; Agilent Technologies, Santa Clara, CA, USA) for CK analysis, under the control of the Analyst v. 1.5.1 spectrometer software. In IAA analysis, the mobile phase was changed from 5% acetonitrile containing 0.1% acetic acid to 50% over 7 min after injection, at a flow rate of 0.34 mL min−1. In CK analysis, the mobile phase was changed from 2% acetonitrile containing 0.1% acetic acid to 40% over 5 min after injection, at a flow rate of 0.40 mL min−1. The temperature of the column oven was set at 40 °C. IAA and CKs were quantified on a standard curve of the ratio of unlabeled to deuterium-labeled standards using MultiQuant v. 2.0.2 software (Sciex).
Statistical Analysis
To confirm the differences in the number of adventitious shoots between internodal segments with and without a dominant shoot, a pairwise t test was performed. To compare phytohormone levels among different regions (I–IV) in the segments, a multiple comparison was performed by Tukey’s honestly significant difference (HSD) test. These analyses were carried out using SPSS 23.0 (IBM SPSS Inc., Armonk, NY, USA).
Results
Time Course of Adventitious Shoot Formation in Ipecac
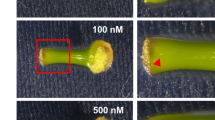
To investigate when adventitious shoots are formed during internode culture, we observed their formation for 10 weeks. Small shoots formed on internodal segments by 2 weeks of culture (Fig. 2a). By 6 weeks, the number increased to approximately 15 and plateaued (Fig. 2b). By 7 weeks, one shoot became dominant, whereas the others seemed almost to stop growing, indicating that the dominant shoot might suppress the others via apical dominance (Fig. 2a). To confirm the effect of the dominant shoot, we removed it and found that another shoot started to enlarge, which resembles a release from apical dominance (Fig. 3). The number of shoots did not increase further in intact segments, but when the biggest shoot was removed, the number increased to approximately 20 after another 3 weeks, and adventitious roots elongated from callus that formed from the basal region of the segments (Fig. 3).
Time course of adventitious shoot formation in ipecac. a Adventitious shoots were observed every week. Photographs show morphological changes in a representative segment; bar 5 mm. b Number of adventitious shoots (>0.3 mm) formed. Values are means ± SEM (n = 3). Ten segments were used in each experiment
Removal of the dominant adventitious shoot (dotted line). a The dominant shoot was removed after 7 weeks of culture and the remained segment cultured for further 3 weeks. Photographs show morphological changes in a representative segment; bar 5 mm. b Total number of adventitious shoots including newly developed shoots. Values are means ± SEM. Eight segments were used in each experiment (*P < 0.05 by t test)
Internal Structure of Adventitious Shoots and Internodal Segments
To find which tissues formed adventitious shoots in internodal segments, we prepared frozen sections of these segments and examined their internal structure. Very small adventitious shoots were visible by 2 weeks of culture (Fig. 4b). In segments in which adventitious shoots were not observed on the surface, epidermal cells started to divide (Fig. 4b). Shoots were clear on the surface of segments by 3 weeks (Fig. 4a). Cell division activity increased in the cortex and endodermis; a vascular bundle started to differentiate in shoots by 4 weeks and connected to that of the segment by 5 weeks (Fig. 4b).
Distribution of adventitious shoots and endogenous phytohormone levels in internodal segments
To confirm which region of an internodal segment formed adventitious shoots, we partitioned the segments into four regions (apical to basal regions, I–IV) and counted the shoots formed in each of them every week for the first 5 weeks. Adventitious shoots were mainly formed in the apical regions (I and II; Fig. 5). By 5 weeks of culture, there were approximately seven shoots in region I, 5.3 in region II, 0.4 in region III, and none in the basal region (IV) (Fig. 5).
To explore the relationships between the number of shoots formed and phytohormone dynamics in the internode during adventitious shoot formation, we measured the endogenous levels of IAA and four CKs in each region using LC–MS/MS. Before culture, the IAA level was slightly higher in the apical region (I, 4.1 pg/mg FW) than in the other regions (II–IV, ~2.5 pg/mg FW) (Fig. 6a). After 1 week of culture, it had increased to 11.4 pg/mg FW in region IV, but had decreased to 1.5–2.2 pg/mg FW in regions I–III. After 2 weeks of culture, it had decreased to approximately 4.4 pg/mg FW in region IV, indicating that IAA accumulation was transient. Between 3 and 5 weeks, an IAA concentration gradient between regions I and IV emerged.
Analysis of CK levels in the same samples showed only trace levels of CKs before culture (Fig. 6b). During the culture, they were detected largely in regions II and III. Changes in levels differed between CK types. The tZ-type CKs increased by 1 week and then gradually decreased. In particular, tZR increased to 13.8 pg/mg FW in region II and to 18.1 pg/mg FW in region III. On the other hand, the iP-type CKs gradually increased during the culture from below the limit of quantification; iP increased to 1.8–2.2 pg/mg FW by 5 weeks in region II and iPR increased to around 2.7 pg/mg FW by 4 weeks in region III.
Discussion
Subepidermal cells of internodal segments of ipecac reportedly initiate cell division before adventitious shoot formation (Yoshimatsu and Shimomura 1991). However, our time-course observations revealed that epidermal cells differentiated adventitious shoots (Fig. 4b), as in Antirrhinum majus and Neolamarckia cadamba (Busse and others 2005; Huang and others 2014). However, it is still unclear why cells differentiate in some parts of the epidermal layer but not others; however, this may result from small-scale variations in phytohormone concentrations due to structural or physiological heterogeneity within a segment.
One of the adventitious shoots on each segment came to dominate (Fig. 3). When that shoot was removed, another shoot started to grow. This is similar to the breaking of apical dominance, a phenomenon in which the shoot apex inhibits axillary bud outgrowth through the production of auxins reviewed by Cline (1991) and which can be mimicked by the application of auxins to the top of a decapitated plant (Thimann and Skoog 1933). The auxins produced in shoot tips are transported basipetally in the high-conductance polar auxin transport stream (PATS), suppressing the biosynthesis of CKs that promote axillary bud outgrowth (Blakeslee and others 2005; Prusinkiewicz and others 2009; Shimizu-Sato and others 2009). Auxins suppress local CK biosynthesis by decreasing the expression level of the isopentenyl transferase gene, which encodes a key enzyme in CK biosynthesis (Müller and others 2015; Tanaka and others 2006). We speculate that, in our experiments, the apex of the biggest shoot produced auxins, which were transported along the internodal segments. A vascular bundle started to differentiate in the shoot by 3 weeks of culture and connected to that of the segment by 5 weeks, becoming able to supply nutrients directly to the shoot, strengthening its growth (Fig. 4b). Auxin transport leads to the differentiation of new vascular tissues (Sachs 1981, 1991). In ipecac, auxin is released from the dominant adventitious shoot, which acts as an auxin sink, inducing the connection between its vascular tissues and those of the internodal segment. A recent model suggests that, in addition to PATS, widespread low-conductance auxin transport acts locally to exchange information between PATS and the surrounding tissues in shoot branching regulation (Bennett and others 2016). The widespread complex auxin flow might suppress the outgrowth of shoots surrounding the dominant shoot and stimulate vascular bundle formation in ipecac.
Adventitious shoots were formed at the apical end of the internodal segments (regions I and II), indicating polarity in the segments (Fig. 5). IAA accumulated in the basal region (IV) by 1 week of culture, owing to either active IAA biosynthesis there or polar transport from the apical region, and induced callus formation in the basal region (Fig. 6a). Observing adventitious shoot formation in the apical region of internodal segments of B. monniera and K. blossfeldiana, Sanikhani and others proposed that an auxin gradient from the basal region to the apical region determines the position of shoot regeneration (Sanikhani and others 2006; Tiwari and others 1998). Our results strongly support this proposal.
tZ-type CKs (notably tZR) increased in the middle region of internodal segments after 1 week of culture, but the total amount of CKs in that region decreased by 2 weeks (Fig. 6b). Although the iP-type CKs increased slowly during culture, their levels were much lower (Fig. 6b). Therefore, we presume that tZR-type CKs are mainly involved in the induction of adventitious shoots in ipecac.
Auxins and CKs are normally required for cell dedifferentiation and redifferentiation. Their ratio is important for organogenesis. In ipecac, cutting of the internode triggers adventitious shoot formation. In Arabidopsis, WOUND INDUCED DEDIFFERENTIATION 1 (WIND1), which encodes an AP2/ERF transcription factor, is involved in the control of cell dedifferentiation (Iwase and others 2011). WIND1 is rapidly induced at wound sites, where it stimulates cell dedifferentiation and subsequent cell proliferation to form callus, and cells around wound sites greatly increased responsiveness to CKs. In ipecac, expression of WIND1-like genes might induce callus formation in the high-auxin basal region of internodal segments. On the other hand, CK levels were lower in the apical region than in the middle region, suggesting that sensitivity to CK promoted by a WIND1-like gene, not increased CK levels, induces adventitious shoot formation in the apical region. Furthermore, a wooden leg-3 (wol-3) mutant an allele of the ARABIDOPSIS HISTIDINE KINASE 4 (AHK4) gene that encodes a CK receptor (Kuroha and others 2006). Analysis of wol-3 suggests that CK receptors have an important role in the formation of auxin transporting vascular tissues in the hypocotyl.
In conclusion, we found that adventitious shoots were formed in the apical region of internodal segments. IAA levels gradually increased from the apical region to the basal region, but CKs accumulated in the middle region (Fig. 7). CKs probably induce adventitious shoot formation in ipecac, but the distribution of the shoots is determined by an auxin gradient. It is hypothesized that the root meristem of vascular plants has evolved from the shoot meristem as an adaptation to land habitats (Gensel and Edwards 2001). Although the shoot and root meristems are initiated at the opposite poles of the embryo, similar regulons act in both meristems (Stahl and Simon 2010). Adventitious shoot formation on the stem might be similar to primitive root generation. In the fern Azolla, which has the most basal euphyllophyte root, auxins restrict root meristem development, whereas CK promotes it (de Vries and others 2016). This is opposite to what is observed in Arabidopsis. Our results support the idea that adventitious shoot formation is inhibited by IAA, indicating that adventitious shoot formation in ipecac has intermediate characteristics in shoot and lateral root development and uses an evolutionarily old system of meristem differentiation.
Large changes in endogenous phytohormone levels likely trigger the expression of genes required for adventitious shoot formation or inactivate genes that block dedifferentiation in epidermal cells by 1 week of culture. Identification of these genes and finding ways to control their expression will enable clonal propagation of plant species that are difficult to propagate by tissue culture.
References
Ajithkumar D, Seeni S (1998) Rapid clonal multiplication through in vitro axillary shoot proliferation of Aegle marmelos (L.) Corr., a medicinal tree. Plant Cell Rep 17(5):422–426. doi:10.1007/s002990050418
Bennett T, Hines G, van Rongen M, Waldie T, Sawchuk MG, Scarpella E, Ljung K, Leyser O (2016) Connective auxin transport in the shoot facilitates communication between shoot apices. PLoS Biol 14(4):e1002446. doi:10.1371/journal.pbio.1002446
Blakeslee JJ, Peer WA, Murphy AS (2005) Auxin transport. Curr Opin Plant Biol 8(5):494–500. doi:10.1016/j.pbi.2005.07.014
Busse JS, Figueroa-Cabanas M, Stimart DP (2005) Developmental anatomy of adventitious shoot formation on snapdragon (Antirrhinum majus L.) hypocotyls in vitro. J Am Soc Hortic Sci 130(2):147–151
Chatterjee SK, Nandi RP, Ghosh NC (1982) Cultivation and utilization of ipecac in West Bengal. In: Atal CK, Kapur BM (eds) Cultivation and utilization of medicinal plants, vol 5. Regional Research Laboratory, Council of Scientific and Industrial Research, Jammu-Tawi, , pp 295–301
Cline MG (1991) Apical dominance. Bot Rev 57:318–358
de Vries J, Fischer AM, Roettger M, Rommel S, Schluepmann H, Brautigam A, Carlsbecker A, Gould SB (2016) Cytokinin-induced promotion of root meristem size in the fern Azolla supports a shoot-like origin of euphyllophyte roots. New Phytol 209(2):705–720. doi:10.1111/nph.13630
Djilianov DL, Dobrev PI, Moyankova DP, Vankova R, Georgieva DT, Gajdošová S, Motyka V (2013) Dynamics of endogenous phytohormones during desiccation and recovery of the resurrection plant species Haberlea rhodopensis. J Plant Growth Regul 32(3):564–574. doi:10.1007/s00344-013-9323-y
Gamborg OL, Miller RA, Ojima K (1968) Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res 50(1):151–158
Ganeshan S, Caswell KL, Kartha KK, Chibbar RN (2002) Shoot regeneration and proliferation. In: Khachatourians GG, McHughen A, Scorza R, Nip WK (eds) Transgenic plants and crops. Marcel Dekker, Inc., New York, pp 69–84
Gensel PG, Edwards D (2001) Plants invade the land. Columbia University Press, New York
George EF, Hall MA, De Klerk GJ (2008) Adventitious regeneration. In: George EF, Hall MA, De Klerk GJ (eds) Plant propagation by tissue Culture, vol 1. 3rd edn. Springer, Dordrecht, pp 355–401
Golovatskaya IF, Karnachuk RA (2007) Dynamics of growth and the content of endogenous phytohormones during kidney bean scoto-and photomorphogenesis. Russ J Plant Physiol 54(3):407–413. doi:10.1134/s102144370703017x
Haberlandt G (1902) Kulturversuche mit isolierten Pflanzenzellen. Sitzungsber Math-Naturwiss Kl Akad Wiss Wien 111:69–92
Huang H, Li J, OuYang K, Zhao X, Li P, Liao B, Chen X (2014) Direct adventitious shoot organogenesis and plant regeneration from cotyledon explants in Neolamarckia cadamba. Plant Biotechnol 31(2):115–121. doi:10.5511/plantbiotechnology.14.0125a
Ideda K, Teshima D, Aoyama T, Satake M, Shimomura K (1988) Clonal propagation of Cephaelis ipecacuanha. Plant Cell Rep 7(4):288–291. doi:10.1007/bf00272545
Iwase A, Mitsuda N, Koyama T, Hiratsu K, Kojima M, Arai T, Inoue Y, Seki M, Sakakibara H, Sugimoto K, Ohme-Takagi M (2011) The AP2/ERF transcription factor WIND1 controls cell dedifferentiation in Arabidopsis. Curr Biol 21(6):508–514. doi:10.1016/j.cub.2011.02.020
Kawamoto T (2003) Use of a new adhesive film for the preparation of multi-purpose fresh-frozen sections from hard tissues, whole-animals, insects and plants. Arch Histol Cytol 66(2):123–143
Kuroha T, Ueguchi C, Sakakibara H, Satoh S (2006) Cytokinin receptors are required for normal development of auxin-transporting vascular tissues in the hypocotyl but not in adventitious roots. Plant Cell Physiol 47(2):234–243. doi:10.1093/pcp/pci240
Ljung K, Bhalerao RP, Sandberg G (2001) Sites and homeostatic control of auxin biosynthesis in Arabidopsis during vegetative growth. Plant J 28(4):465–474
Müller D, Waldie T, Miyawaki K, To JP, Melnyk CW, Kieber JJ, Kakimoto T, Leyser O (2015) Cytokinin is required for escape but not release from auxin mediated apical dominance. Plant J 82(5):874–886. doi:10.1111/tpj.12862
Nordström A, Tarkowski P, Tarkowska D, Norbaek R, Åstot C, Dolezal K, Sandberg G (2004) Auxin regulation of cytokinin biosynthesis in Arabidopsis thaliana: a factor of potential importance for auxin-cytokinin-regulated development. Proc Natl Acad Sci USA 101(21):8039–8044. doi:10.1073/pnas.0402504101
Prusinkiewicz P, Crawford S, Smith RS, Ljung K, Bennett T, Ongaro V, Leyser O (2009) Control of bud activation by an auxin transport switch. Proc Natl Acad Sci USA 106(41):17431–17436. doi:10.1073/pnas.0906696106
Rao MS, Purohit SD (2006) In vitro shoot bud differentiation and plantlet regeneration in Celastrus paniculatus Willd. Biol Plant 50(4):501–506. doi:10.1007/s10535-006-0079-0
Rout GR, Samantaray S, Das P (2000) In vitro somatic embryogenesis from callus cultures of Cephaelis ipecacuanha A. Richard. Scientia Hortic 86 (1):71–79. doi:10.1016/S0304-4238(00)00130-8
Sachs T (1981) The control of the patterned differentiation of vascular tissues. Adv Bot Res 9:151–262
Sachs T (1991) Cell polarity and tissue patterning in plants. Development 113(Supplement 1):83–93
Sanikhani M, Frello S, Serek M (2006) TDZ induces shoot regeneration in various Kalanchoë blossfeldiana Poelln. cultivars in the absence of auxin. Plant Cell Tiss Org Cult 85(1):75–82. doi:10.1007/s11240-005-9050-6
Schaller GE, Bishopp A, Kieber JJ (2015) The yin-yang of hormones: cytokinin and auxin interactions in plant development. Plant Cell 27(1):44–63. doi:10.1105/tpc.114.133595
Shimizu-Sato S, Tanaka M, Mori H (2009) Auxin-cytokinin interactions in the control of shoot branching. Plant Mol Biol 69(4):429–435
Skoog F, Miller CO (1957) Chemical regulation of growth and organ formation in plant tissues cultured in vitro. Symp Soc Exp Biol 11:118–130
Stahl Y, Simon R (2010) Plant primary meristems: shared functions and regulatory mechanisms. Curr Opin Plant Biol 13(1):53–58. doi:10.1016/j.pbi.2009.09.008
Stolz A, Riefler M, Lomin SN, Achazi K, Romanov GA, Schmulling T (2011) The specificity of cytokinin signalling in Arabidopsis thaliana is mediated by differing ligand affinities and expression profiles of the receptors. Plant J 67(1):157–168. doi:10.1111/j.1365-313X.2011.04584.x
Tanaka M, Takei K, Kojima M, Sakakibara H, Mori H (2006) Auxin controls local cytokinin biosynthesis in the nodal stem in apical dominance. Plant J 45(6):1028–1036. doi:10.1111/j.1365-313X.2006.02656.x
Teshima D, Ikeda K, Satake M, Aoyama T, Shimomura K (1988) Production of emetic alkaloid by in vitro culture of Cephaelis ipecacuanha A. Richard. Plant Cell Rep 7(4):278–280. doi:10.1007/bf00272542
Thimann KV, Skoog F (1933) Studies on the growth hormone of plants: III. The inhibiting action of the growth substance on bud development. Proc Natl Acad Sci USA 19(7):714–716
Tiwari V, Deo Singh B, Nath Tiwari K (1998) Shoot regeneration and somatic embryogenesis from different explants of Brahmi [Bacopa monniera (L.) Wettst.]. Plant Cell Rep 17(6):538–543. doi:10.1007/s002990050438
Tiwari V, Tiwari KN, Singh BD (2001) Comparative studies of cytokinins on in vitro propagation of Bacopa monniera. Plant Cell Tiss Org Cult 66(1):9–16. doi:10.1023/a:1010652006417
Watad AA, Ahroni A, Zuker A, Shejtman H, Nissim A, Vainstein A (1996) Adventitious shoot formation from carnation stem segments: a comparison of different culture procedures. Scientia Hortic 65 (4):313–320. doi:10.1016/0304-4238(96)00874-6
Yoshimatsu K, Shimomura K (1991) Efficient shoot formation on internodal segments and alkaloid formation in the regenerates of Cephaelis ipecacuanha A. Richard. Plant Cell Rep 9(10):567–570. doi:10.1007/bf00232333
Yoshimatsu K, Shimomura K (1993) Cephaelis ipecacuanha A. Richard (Brazilian Ipecac): Micropropagation and the production of emetine and cephaeline. In: Bajaj YPS (ed) Biotechnology in agriculture and forestry, vol 21. Medicinal and aromatic plants IV. Springer, Berlin, pp 87–103
Yoshimatsu K, Shimomura K (1994) Plant regeneration on cultured root segments of Cephaelis ipecacuanha A. Richard. Plant Cell Rep 14(2–3):98–101. doi:10.1007/bf00233769
Yoshimoto K, Jikumaru Y, Kamiya Y, Kusano M, Consonni C, Panstruga R, Ohsumi Y, Shirasu K (2009) Autophagy negatively regulates cell death by controlling NPR1-dependent salicylic acid signaling during senescence and the innate immune response in Arabidopsis. Plant Cell 21(9):2914–2927. doi:10.1105/tpc.109.068635
Zhang L, Wang Y, Zhang X, Zhang M, Han D, Qiu C, Han Z (2012) Dynamics of phytohormone and DNA methylation patterns changes during dormancy induction in strawberry (Fragaria × ananassa Duch.). Plant Cell Rep 31(1):155–165. doi:10.1007/s00299-011-1149-0
Acknowledgements
This study was supported in part by Research Center for Life and Environmental Sciences, Toyo University. We thank Akira Murakami for his technical support, and Shosaku Kashiwada and Uma Maheswari Rajagopalan for their constructive comments on this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Koike, I., Taniguchi, K., Shimomura, K. et al. Dynamics of Endogenous Indole-3-acetic Acid and Cytokinins During Adventitious Shoot Formation in Ipecac. J Plant Growth Regul 36, 805–813 (2017). https://doi.org/10.1007/s00344-017-9684-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-017-9684-8