Abstract
Main conclusion
SL inhibited adventitious shoot formation of ipecac, whereas the SL-related inhibitors promoted adventitious shoot formation. SL-related inhibitors might be useful as new plant growth regulators for plant propagation.
Abstract
In most plant species, phytohormones are required to induce adventitious shoots for propagating economically important crops and regenerating transgenic plants. In ipecac (Carapichea ipecacuanha (Brot.) L. Andersson), however, adventitious shoots can be formed without phytohormone treatment. Here we evaluated the effects of GR24 (a synthetic strigolactone, SL), SL biosynthetic inhibitors, and an SL antagonist on adventitious shoot formation during tissue culture of ipecac. We found that exogenously applied GR24 suppressed indole-3-acetic acid transport in internodal segments and decreased the number of adventitious shoots formed; in addition, the distribution of adventitious shoots changed from the apical to middle region of the internodal segments. In contrast, the SL-related inhibitors promoted adventitious shoot formation on both apical and middle regions of the segments. In particular, SL antagonist treatment increased endogenous cytokinin levels and induced multiple shoot development. These results indicate that SL inhibits adventitious shoot formation in ipecac. In ipecac, one of the shoots in each internodal segment becomes dominant and auxin derived from that shoot suppresses the other shoot growth. Here, this dominance was overcome by application of SL-related inhibitors. Therefore, SL-related inhibitors might be useful as new plant growth regulators to improve the efficiency of plant propagation in vitro.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plant tissue culture is the aseptic culture of cells, tissues, or organs under defined physicochemical conditions, and is an important tool in basic and applied research and commercial applications (Thorpe 2007). Thus, many researchers have attempted to increase propagation efficiency in plant tissue culture (Gahan and George 2008). The wide use of tissue culture has promoted the vegetative propagation of plants. Nowadays, tissue culture techniques are indispensable for production of disease-free plants, propagation of rare plants, genetic transformation in plants, and production of commercially important plants in vitro (Espinosa-Leal et al. 2018). In general, the formation of de novo organs requires exogenous application of phytohormones, such as auxin and cytokinin (CK), which are added to the culture media. The balance of phytohormones applied affects organogenesis: a high ratio of auxin to CK induces roots, whereas a low ratio induces shoots, and a high concentration of both hormones induces callus in tobacco (Nicotiana tabacum L.) (Skoog and Miller 1957). This morphogenic model operates in various plant species (Evans et al. 1981).
Ipecac (Carapichea ipecacuanha (Brot.) L. Andersson) is a medicinal plant that grows in the tropical rainforests of Costa Rica, Nicaragua, Panama, and Brazil. It produces alkaloids, such as emetine and cephaeline, in its roots (Trease and Evans 1989); these compounds are used as expectorants, emetics, and amebicides (Chatterjee et al. 1982). In ipecac, adventitious shoots can be induced on internodal segments without exogenous application of phytohormones (Yoshimatsu and Shimomura 1991). Therefore, it is easy to study the effects of endogenous phytohormones during adventitious shoot formation using tissue culture in this species. In addition, it is possible to evaluate the direct effects of exogenously applied chemicals. Previously, we analyzed the relationship between adventitious shoot formation and the dynamics of endogenous auxin (IAA) and CKs in ipecac (Koike et al. 2017). Adventitious shoots were locally formed on the apical region of internodal segments, whereas IAA accumulated in the basal region and CKs were distributed throughout the segment. These results indicated a negative correlation between the position of adventitious shoot formation and IAA distribution. We hypothesized that the IAA in the apical region of the internodal segments is maintained at a low concentration appropriate for adventitious shoot formation by auxin efflux. To explore this hypothesis, we evaluated the effects of polar auxin transport inhibitors on the position of adventitious shoot formation (Koike et al. 2020). The inhibitors changed the main sites of shoot formation from the apical region to the middle region of the internodal segments, and decreased the levels of endogenous IAA accumulated in the basal region of the internodal segments, suggesting that polar auxin transport from the apical to basal region, at least in part, determines the position of adventitious shoot formation on internodal segments.
Apical dominance is another phenomenon controlled by polar auxin transport in the stem (Ongaro and Leyser 2008; Domagalska and Leyser 2011). The main shoot apex clearly exerts an inhibitory effect on the outgrowth of axillary buds because removing the apex leads to activation of the axillary buds. Auxin is produced in the shoot apex and moves into the stem basipetally (Snow 1929). When auxin is applied to the top of decapitated plants, it mimics the effect of the presence of the shoot apex (Thimann and Skoog 1933). In ipecac, one adventitious shoot becomes dominant and removal of this shoot triggers other shoot growth (Koike et al. 2017). Application of auxin to the cut surface after removal of the dominant shoot strongly inhibits this shoot outgrowth (Koike et al. 2020). These results indicate that auxin flow from a dominant shoot suppresses other shoot growth, as is the case in apical dominance. Auxin interacts with other phytohormones and controls the production of the phytohormones involved in the regulation of shoot branching. For instance, auxin decreases expression of isopentenyl transferase, inhibiting the biosynthesis of CKs that promote axillary bud outgrowth, and auxin stimulates strigolactone (SL) biosynthesis (Beveridge et al. 2000; Foo et al. 2005; Tanaka et al. 2006; Ferguson and Beveridge 2009). In 2008, SLs were discovered as a class of phytohormones that inhibit shoot branching (Gomez-Roldan et al. 2008; Umehara et al. 2008). SLs have multiple functions in plant development and growth, controlling secondary growth of the stem, root architecture, internode elongation, and leaf senescence (Agusti et al. 2011; Kapulnik et al. 2011; Ruyter-Spira et al. 2011; de Saint Germain et al. 2013; Yamada et al. 2014). Although SLs are known to suppress adventitious root formation in Arabidopsis and pea (Rasmussen et al. 2012) and adventitious shoot formation in Zantedeschia (Manandhar et al. 2018), the effect of SLs on de novo organogenesis is poorly understood. Furthermore, the biological activities of SLs in plant tissue culture have not been explored.
GR24, a synthetic SL analog (Mangnus et al. 1992), is widely used to analyze the physiological roles of SLs. Inhibitors of SL biosynthesis and signaling are useful tools for analysis of SL functions. Two SL biosynthetic inhibitors, TIS108 and KK5, suppress SL production in roots and increase the number of branches (Ito et al. 2011, 2013; Kawada et al. 2019) in Arabidopsis and rice. In addition, an SL antagonist, KK094, binds to an SL receptor and enhances tiller bud outgrowth in rice (Nakamura et al. 2019). Here, to evaluate the effects of SLs on adventitious shoot formation of ipecac, we treated internodal segments with GR24, TIS108, KK5, or KK094 and counted the number of shoots formed. We also noted the positions of the shoots on each internodal segment (apical region, basal region, or in between).
Materials and methods
Plant materials and culture conditions
The ipecac culture system was established by Prof. Shimomura (Yoshimatsu and Shimomura 1991) and has been maintained at Toyo University. Sterile plants were propagated from shoot tips, nodes, and internodes. To induce adventitious shoots, internodal segments (5 mm) were cut and placed horizontally on 25 ml of phytohormone-free B5 culture medium (Gamborg et al. 1968) solidified with 0.2% Gelrite in a Petri dish (internal diameter, 90 mm; height, 20 mm) and cultured at 24 °C under a 14-h light/10-h dark photoperiod (13–17 μmol photons m−2 s−1). The number of adventitious shoots with length greater than 0.3 mm formed on each internodal segment was counted under a digital microscope (DHS1000; Leica Microsystems, Wetzlar, Germany).
Chemical treatment
GR24 was purchased from Chiralix (Nijmegen, Netherlands). TIS108, KK5, and KK094 were synthesized as described in Ito et al. (2011), Kawada et al. (2019), and Nakamura et al. (2019), respectively. GR24, TIS108, KK5, and KK094 were dissolved in acetone to prepare 100 mM stocks and stored at – 30 °C. To estimate the effective concentrations of TIS108 and KK094, each chemical was preliminarily tested at 0.1, 1, 10, 20, 50, and 100 µM (data not shown). KK5 was also tested at 0.1, 1, 10, 20, and 100 µM (data not shown). The chemicals were mixed with autoclaved B5 culture medium to treat the internodal segments.
IAA and trans-zeatin riboside (tZR) were purchased from Wako Pure Chemical Industry (Osaka, Japan). 2iP and trans-zeatin (tZ) were purchased from Sigma (St. Louis, MO, USA). Kinetin and isopentenyl adenine riboside (iPR) were purchased from Kanto Chemical (Tokyo, Japan). Deuterium-labeled phytohormones (D5-IAA, D5-tZ, D5-tZR, D6-2iP, and D6-iPR) were purchased from OlChemIm (Olomouc, Czech Republic) for use as internal standards. Kinetin was dissolved in alkaline water to prepare 10 mM stock and stored at 4 °C. Other phytohormones were dissolved in acetonitrile to prepare 100 pg µl−1 stock solutions, which were used to generate the standard curves in the LC–MS/MS analysis.
Extraction and purification of IAA and CKs
Internodal segments were cultured on phytohormone-free B5 medium (control) or on B5 medium containing 10 µM GR24 for 1 week. In SL-related inhibitor treatments, internodal segments were cultured on B5 medium or on B5 medium containing 10 µM TIS108 or 10 µM KK094 for 1, 3, or 5 weeks. Cultured segments were cut into four sections (apical to basal, I to IV). Each section (ca. 5–16 mg) was placed in a 2.0-ml tube with a zirconia bead (diameter, 5 mm) and frozen in liquid nitrogen. Frozen samples were crushed by a TissueLyser II (Qiagen, Hilden, Germany) and suspended in 1 ml acetonitrile containing 1% acetic acid; 500 pg D5-IAA, D5-tZR, D6-2iP, and D6-iPR; and 1.5 ng D5-tZ. The samples were incubated for 1 h at 4 °C in darkness and centrifuged at 3500×g for 5 min at room temperature. The precipitate was washed with 80% (v/v) acetonitrile containing 1% (v/v) acetic acid and centrifuged again. Both supernatants were combined and 600 µl water containing 1% (v/v) acetic acid was added. After acetonitrile in the sample was evaporated by nitrogen gas flow, the aqueous sample was loaded onto an equilibrated Oasis MCX cartridge column (Waters, Milford, MA, USA). After the cartridge was washed with 1% (v/v) acetic acid, IAA was eluted with 2 ml of 30% (v/v) acetonitrile containing 1% (v/v) acetic acid. The column was washed with 2 ml of 80% (v/v) acetonitrile containing 1% (v/v) acetic acid. CKs were eluted with 2 ml of 60% (v/v) acetonitrile containing 5% (v/v) NH3 in water. All IAA and CK fractions were evaporated and stored at − 30 °C until LC–MS/MS analysis.
LC–MS/MS analysis
The IAA fraction was dissolved in 20 µl of 10% (v/v) acetonitrile containing 0.5% (v/v) acetic acid, and the CK fraction was dissolved in 20 µl of water containing 1% (v/v) acetic acid. For IAA and CK analysis, the samples were analyzed by an LC–MS/MS system consisting of a triple quadrupole mass-spectrometer (3200 QTRAP; Sciex, Framingham, MA, USA) and a high-performance liquid chromatography system (Prominence; Shimazu, Kyoto, Japan) equipped with an Acuity BEH C18 column (diameter, 2.1 mm; height, 50 mm; Waters), under the control of Analyst v.1.5.1 spectrometer software (Sciex). Water containing 0.05% acetic acid and acetonitrile containing 0.05% acetic acid were used as the mobile phases for LC. In IAA analysis, the mobile phase was increased linearly from 10% (v/v) to 55% (v/v) acetonitrile by 6 min after injection at a flow rate of 0.4 ml min−1. In CK analysis, the gradient was increased linearly from 2% (v/v) to 15% (v/v) acetonitrile over 4 min and to 40% (v/v) acetonitrile by 7 min after injection, at a flow rate of 0.4 ml min−1. The temperature of the column oven was set at 40 °C. The ion source parameters for IAA and CK analyses were as described previously (Koike et al. 2018). IAA and CKs were quantified on a curve of the ratio of each unlabeled to deuterium-labeled standard using MultiQuant v.2.0.2 software (Sciex).
Statistical analysis
Statistical analysis was carried out with IBM SPSS Statistics 26.0 software (IBM SPSS Inc., Armonk, NY, USA). Following assessment of the equality of variances by F-test, groups were compared by Student’s t-test. Following assessment of the equality of variances by ANOVA, groups were compared by Tukey's honestly significant difference. P values less than 0.05 were considered statistically significant. All experiments were carried out as a completely randomized design.
Results
Effects of exogenously applied SL on adventitious shoot formation
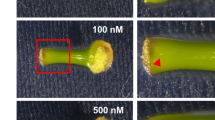
To evaluate the effect of exogenously applied SL, we added the SL synthetic analog GR24 to the culture medium of ipecac internodal segments. After 5 weeks of culture, adventitious shoots were mainly formed in the apical region of the internodal segments in the control samples (no treatment), whereas adventitious shoots were formed in the middle region when GR24 was applied (Fig. 1a). Adventitious shoot formation was suppressed in accordance with GR24 concentration (Fig. 1a, b). The average total number of shoots was 11.2 in the control samples; this number decreased by 74% to 2.9 in the 10 µM GR24–treated samples (Fig. 1b). To confirm where adventitious shoots were formed on the internodal segments, we partitioned the segments into four regions (apical to basal regions, I–IV) and counted the shoots in each region after 5 weeks of culture (Fig. 1c). There were on average 7.9 shoots in region I, 3.3 in region II, and 0.5 in region III in the control samples, whereas there were 0.3 in region I, 1.6 in region II, and 0.9 in region III in the 10 µM GR24–treated samples; there were no shoots in region IV in control or treated samples. Thus, 10 µM GR24 treatment decreased the shoot number by about 95% in region I, and almost doubled the shoot number in region III compared with the control. Simultaneous application of GR24 and TIS108 suppressed the stimulatory effects of TIS108 on adventitious shoot formation: the number of shoots formed decreased to the number in GR24 treatment alone (Fig. S1).
Effect of GR24 on adventitious shoot formation. a Representative images of adventitious shoots (arrowheads) on internodal segments after 5 weeks of culture under the indicated conditions. Bar, 2 mm. b Number of adventitious shoots formed on internodal segments treated with GR24. Data are means ± SE (n = 3). Eight to ten segments were used in each experiment. *, †, and # indicate P < 0.05 for 0.1, 1 μM, and 10 μM GR24, respectively, versus control (0 μM GR24) (Student’s t-test). c Number of adventitious shoots in each region (I to IV) of the internodal segments after 5 weeks of culture. Data are means ± SE (n = 3). Eight segments were used in each experiment. *P < 0.05 versus control for total number of shoots formed (Student’s t-test)
In our previous study, endogenous IAA and CK levels in internodal segments of ipecac transiently increased after 1 week of culture (Koike et al. 2017). Here, to investigate the effect of SL on endogenous auxin and CKs, we measured the endogenous levels of IAA and CKs (tZ, tZR, 2iP, and iPR) in regions I–IV after 1 week of culture in the control or 10 µM GR24 condition. IAA accumulated predominantly in region IV (I–III, ~ 4.9 pg/mg FW; IV, 16.7 pg/mg FW) in the control samples. Treatment with 10 µM GR24 significantly decreased the IAA level in region IV by about 60% (I–III, ~ 3.3 pg/mg FW; IV, 6.4 pg/mg FW) compared with the control (Fig. 2). The levels of tZ, tZR, and iPR did not differ significantly between the control and GR24-treated samples. However, when we treated the internodal segments with 10 µM GR24, 2iP levels significantly decreased in region I compared with the control (control, 0.66 pg/mg FW; 10 µM GR24, 0.1 pg/mg FW) and significantly increased in region III compared with the control (control, 0.08 pg/mg FW; 10 µM GR24, 0.38 pg/mg FW), and iPR levels were slightly but significantly increased in regions II and III compared with the control (Fig. 2).
Effect of exogenously applied SL-related inhibitors on adventitious shoot formation
To suppress endogenous SL biosynthesis in the internodal segments, we added an SL biosynthetic inhibitor, TIS108, at various concentrations (1 to 20 µM) to the culture medium. At 8 weeks after treatment with TIS108 at 5, 10, or 20 µM, many small shoots were observed on the internodal segments (Fig. 3a). When we applied TIS108 at more than 5 µM, the total number of adventitious shoots formed was significantly increased compared with the control after 6 weeks of culture, and the number of shoots was further increased to about twice that of the control samples after 8 weeks of culture (Fig. 3b). Next, we added another SL biosynthetic inhibitor, KK5, at 1 to 20 µM to the culture medium and evaluated the effect on adventitious shoot formation (Fig. 4a). The total number of shoots in 20 µM KK5–treated samples was significantly increased compared with the control after 5 weeks of culture (Fig. 4b). After 8 weeks of culture, the shoot number reached about three times that of the control samples (Fig. 4b). These results indicate that both TIS108 and KK5 promoted adventitious shoot formation in ipecac internodal segments.
Effect of TIS108 on adventitious shoot formation. a Representative images of adventitious shoots on internodal segments after 8 weeks of culture under the indicated conditions. Bar, 2 mm. b Number of adventitious shoots formed on internodal segments treated with TIS108. Data are means ± SE (n = 3). Eight to ten segments were used in each experiment. *, †, and # indicate P < 0.05 for 5 μM, 10 μM, and 20 μM TIS108, respectively, versus control (0 μM TIS108) (Student’s t-test)
Effect of KK5 on adventitious shoot formation. a Representative images of adventitious shoots on internodal segments after 8 weeks of culture under the indicated conditions. Bar, 2 mm. b Number of adventitious shoots formed on internodal segments treated with KK5. Data are means ± SE (n = 3). Eight to ten segments were used in each experiment. *P < 0.05 for 20 μM KK5 versus control (0 μM KK5) (Student’s t-test)
To suppress SL signaling in internodal segments, we added an SL antagonist, KK094, at various concentrations (1 to 20 µM) to the culture medium (Fig. 5a). When we treated with 1 µM KK094, the total number of shoots was significantly higher than in the control samples after 4 weeks of culture (Fig. 5b), suggesting that shoot proliferation was one week earlier than when either of the SL biosynthetic inhibitors was applied (Figs. 3b, 4b). The number of shoots in the 10 µM KK094–treated samples was significantly higher than that in the control samples after 6 weeks of culture, and reached 1.7 times that of control samples after 8 weeks of culture (Fig. 5b). The number of shoots formed following 20 µM KK094 treatment also tended to be higher than that in the control after 8 weeks of culture but the difference was not statistically significant (Student’s t-test, P = 0.051). Overall, the results indicate that KK094 treatment promoted adventitious shoot formation.
Effect of KK094 on adventitious shoot formation. a Representative images of adventitious shoots after 8 weeks of culture under the indicated conditions. Bar, 2 mm. b Number of adventitious shoots formed on internodal segments treated with KK094. Data are means ± SE (n = 3). Eight to ten segments were used in each experiment. *, †, and # indicate P < 0.05 for 1 μM, 5 μM, and 10 μM KK094, respectively, versus control (0 μM TIS108) (Student’s t-test)
Because GR24 treatment changed the main position of adventitious shoot formation from regions I and II to regions II and III (Fig. 1), we evaluated the shoot positions after treatment with SL-related inhibitors. Following TIS108, KK5, or KK094 treatment, promotion of adventitious shoot formation was observed in regions I, II, and III of the internodal segments (Fig. 6), suggesting that the main shoot position did not change.
Effects of three SL-related inhibitors, TIS108 (a), KK5 (b), and KK094 (c), on adventitious shoot formation in four regions (apical to basal, I to IV) of internodal segments. The numbers of adventitious shoots formed on each region of the internodal segments after 8 weeks of culture are shown. Data are means ± SE (n = 3). Eight to ten segments were used in each experiment. *P < 0.05 versus corresponding control for total number of shoots formed (0 μM inhibitor) (Student’s t-test). n.s., not significant
Changes in IAA and CK levels after treatment with SL-related inhibitors
To understand how auxin and CK levels changed during adventitious shoot formation after treatment with SL-related inhibitors, we measured endogenous IAA, tZ, tZR, 2iP, and iPR levels by LC–MS/MS after 1, 3, and 5 weeks of culture with 10 µM TIS108 or KK094. After 1 week of culture, endogenous IAA accumulated predominantly in region IV in the control and in the TIS108- and KK094-treated samples (Fig. 7). In all regions of the internodal segments, there were no significant differences between the levels in the control and SL-related inhibitor–treated samples. After 3 weeks of culture, the IAA level in region IV of the TIS108-treated samples was significantly higher than that in the control samples, and after 5 weeks of culture, the IAA level in region I of the KK094-treated samples was slightly but significantly higher than that in control samples. However, overall we could not find large effects of SL-related inhibitors on IAA levels.
IAA and CK levels in internodal segments treated with SL-related inhibitors (TIS108 and KK094). Internodal segments were collected after 1, 3, and 5 weeks of culture. Data are means ± SE (n = 4). Four segments were used in each experiment. *P < 0.05 versus control (0 μM inhibitor) (Student’s t-test). n.d., not detected
The tZ level in region III decreased significantly in the KK094-treated segments after 1 week of culture and in the TIS108-treated segments after 5 weeks of culture (Fig. 7). However, it was difficult to analyze tZ in the TIS108 and KK094-treated segments after 3 or 5 weeks of culture because of the low levels. There were no large changes in tZR levels between the control and TIS108 or KK094 treatment after 1, 3, or 5 weeks of culture. In contrast, 2iP and iPR levels tended to increase in the apical region of internodal segments following TIS108 or KK094 treatment (Fig. 7). In TIS108-treated segments, 2iP levels significantly increased in region I after 3 weeks of culture, and iPR levels significantly increased in regions I and II after 1 week of culture. In KK094-treated segments, 2iP levels significantly increased in region I after 1 week of culture, regions I and II after 3 weeks of culture, and regions I to III after 5 weeks of culture, and iPR levels increased in region I after 1 or 5 weeks of culture. However, iPR levels also increased significantly in region IV after 1 or 5 weeks of culture.
Effect of SL-related inhibitors on growth of adventitious shoots
A single dominant shoot was observed on each internodal segment after 8 weeks of culture under control conditions, whereas many small shoots were formed on the segments treated with TIS108, KK5, or KK094 at various concentrations (Figs. 3a, 4a, 5a). To confirm whether the small shoots on these internodal segments can grow normally, the segments cultured on an SL-related inhibitor (TIS108, KK5, or KK094) were further cultured for 2 weeks on medium containing the same inhibitor at 10 μM. In the control samples, only the dominant shoot continued to grow vigorously, whereas following TIS108, KK5, or KK094 treatment, multiple shoots grew (Fig. S2). To investigate whether the small shoots could grow after removal from the internodal segments, the segments were first cultured under control conditions or in medium containing 10 µM TIS108, KK5, or KK094 for 8 weeks, and then the adventitious shoots were removed and cultured on fresh control culture medium for a further 8 weeks. The removed shoots grew normally (Fig. S3).
Effect of exogenously applied kinetin on adventitious shoot formation
We then explored whether kinetin, a type of CK, promotes adventitious shoot formation. When we applied 1 µM kinetin, the total number of shoots significantly increased compared with the control after 3 weeks of culture, and gradually increased until 8 weeks of culture (Fig. 8a, b), at which time the average total number of shoots in the 1 µM kinetin–treated samples (14.6 shoots) became about 1.5 times that in the control samples (8.9 shoots) (Fig. 8b). When we counted the shoot number in each region of the internodal segments, there were on average 3.5 in region I, 4.3 in region II, and 1.0 in region III in the control samples, compared with 9.6 in region I, 3.7 in region II, and 1.3 in region III in the 1 µM kinetin–treated samples (Fig. 8c); neither control nor treated samples produced shoots in region IV. Thus, promotion of shoot formation in the 1 µM kinetin–treated samples occurred mainly in region I of the internodal segments.
Effect of kinetin on adventitious shoot formation. a Representative images of adventitious shoots after 8 weeks of culture under the indicated conditions. Bar, 2 mm. b Number of adventitious shoots formed on internodal segments treated with kinetin. Data are means ± SE (n = 3). Ten segments were used in each experiment. *P < 0.05 for 1 μM kinetin versus control (0 μM kinetin) (Student’s t-test). c Number of adventitious shoots in each region of the internodal segments. Data are means ± SE (n = 3). Ten segments were used in each experiment. *P < 0.05 versus control for total number of shoots formed (0 µM kinetin) (Student’s t-test). n.s., not significant
Discussion
We can directly evaluate the effects of exogenously applied chemicals on adventitious shoot formation in the ipecac tissue culture system, in which adventitious shoots can be induced without exogenous application of phytohormones (Yoshimatsu and Shimomura 1991). Here, we evaluated the effects of SL on adventitious shoot formation. We found that an exogenously applied SL analog (GR24) suppressed adventitious shoot formation (Fig. 1), whereas the number of shoots was increased by treatment with an SL biosynthetic inhibitor (TIS108 or KK5) or SL antagonist (KK094) (Figs. 3, 4, 5). These findings indicate that SL has an inhibitory effect on adventitious shoot formation in ipecac.
We demonstrated that GR24 treatment also changed the main position of adventitious shoot formation from the apical region (I and II) to the middle region (II and III) of the internodal segments in ipecac (Fig. 1). We previously showed that, after 1 week of culture, endogenous IAA transiently accumulated in the basal region of internodal segments of ipecac (Koike et al. 2017). Here, the accumulation of endogenous IAA in the basal region was inhibited by GR24 treatment (Fig. 2). SLs can reduce basipetal auxin flow by suppressing the expression of PIN-FORMED1 (PIN1), an auxin efflux protein, located on the basal side of the plasma membrane in Arabidopsis (Crawford et al. 2010). SLs interfere with auxin effects on PIN polar targeting, constitutive PIN trafficking, and clathrin-mediated endocytosis in stem cells of pea and Arabidopsis (Zhang et al. 2020). In our previous work, treatment with two auxin transport inhibitors, TIBA (2,3,5-triiodobenzoic acid and NPA [N-1-naphthylphthalamic acid]), moved the main position of adventitious shoot formation from the apical region to the middle region of ipecac internodal segments, and suppressed IAA accumulation in the basal region of the segments (Koike et al. 2020). These findings indicate that disturbance of polar auxin transport changes the distribution pattern of adventitious shoot formation.
In ipecac, one of the shoots becomes dominant on each internodal segment because auxin derived from this shoot suppresses the growth of other shoots (Koike et al. 2018, 2020). Here, treatment with all the SL-related inhibitors increased the number of adventitious shoots compared with the control (Figs. 3, 4, 5). However, there are some differences among the shoot phenotypes produced by the SL biosynthetic inhibitors and SL antagonist (Figs. 3, 4, 5). In the TIS108- and KK5-treated internodal segments, individual shoot development was slow, with most shoots were rounded with no leaves during the 8-week culture period (Fig. 3a and 4a); in contrast; many shoots grew at the normal rate under the KK094 treatment (Fig. 5a). In addition, the patterns of the 2iP and iPR levels were different between 10 µM TIS108 and 10 µM KK094 treatment: (1) 2iP gradually increased in cultures under KK094 treatment but not under TIS108 treatment during the 5-week culture period, and (2) iPR levels increased after 1 week of culture under TIS108 treatment, but the changes in levels were smaller than those under KK094 treatment (Fig. 7). Development of multiple shoots with leaves following KK094 treatment might be due to the observed increase of 2iP in internodal segments. In ipecac, 2iP might have an important role in the stimulation of adventitious shoot formation.
In our previous work, we hypothesized that endogenous tZ-type CKs might be mainly involved in induction of adventitious shoot formation because endogenous tZR levels are highly elevated after 1 week of culture (Koike et al. 2017). 2iP-type CKs are converted to tZ-type CKs through hydroxylation of the prenyl side chain by the cytochrome P450 CYP735A in Arabidopsis (Takei et al. 2004). Deficiency of CYP735A activity retards shoot growth without affecting total CK content; this retardation is overcome by application of tZ but not 2iP (Kiba et al. 2013). Thus, we expected that tZ-type CKs would be important for adventitious shoot formation in ipecac. However, in most cases the distribution and levels of tZ and tZR did not differ significantly between control internodal segments and those treated with GR24 or SL-related inhibitor (Figs. 2 and 7). In contrast, the distribution and levels of 2iP and iPR were often significantly altered by treatment with GR24 or SL-related inhibitor (Figs. 2 and 7). After 10 µM GR24 treatment, adventitious shoots were mainly formed in regions II and III, 2iP levels were decreased in region I and increased in region III, and iPR levels were increased in regions II and III (Fig. 2). SLs promote CK degradation by activating the expression of CYTOKININ OXIDASE9, thereby inhibiting shoot branching in rice (Duan et al. 2019). We therefore consider that reduction of 2iP levels in region I might be caused by CK degradation as well as reduction of CK biosynthesis. The distribution pattern of 2iP was associated with the position of adventitious shoot formation on internodal segments (Figs. 1, 2, 6, and 7). These results are consistent with the notion that 2iP might have an important role in the stimulation of adventitious shoot formation.
To determine whether the effects of SL-related inhibitors on adventitious shoot formation were caused by the increased 2iP in the internodal segments, we compared the effects of these treatments to that of the synthetic CK kinetin. We found that kinetin stimulated adventitious shoot formation (Fig. 8), as previously found for another synthetic CK, 6-benzylaminopurine (Yoshimatsu and Shimomura 1991). However, the effects of SL-related inhibitors and kinetin differed in the following ways. The small shoots induced by kinetin had young leaves, whereas those induced by SL-related inhibitors were round, with no leaves (Figs. 3, 4, 5, 8). In addition, kinetin stimulated adventitious shoot formation in region I alone of the internodal segments, whereas SL-related inhibitors stimulated it in regions I to III (Figs. 6, 8).
The effect of SL on adventitious shoot formation is similar to that on axillary bud outgrowth, but has a different mechanism. Shoot branching consists of two steps: the formation of axillary meristems and the subsequent outgrowth of axillary buds in the leaf axil (Beveridge and Kyozuka 2010). SL signaling is thought to be involved in the control of the latter step. SL regulates whether axillary buds continue to develop or become dormant (Minakuchi et al. 2010). In ipecac, plant regeneration in vitro takes place through formation of new shoot meristems from somatic cells. Interestingly, in the current study, SL-related inhibitors promoted adventitious shoot formation on internodal segments, indicating that SL inhibits the formation of adventitious meristems.
Many small shoots were induced after 8 weeks of culture with TIS108, KK5, and KK094, and they could grow normally after a further 2 weeks of culture (Fig. S2). In addition, when small shoots after 8 weeks of culture were removed from internodal segments and cultured on control culture medium, the shoots could grow normally (Fig. S3): the small shoots were not malformed and could proceed to normal plant regeneration.
Auxin and CKs have been widely used as plant growth regulators in plant regeneration. Here we found that SL-related inhibitors promote adventitious shoot formation in ipecac. We anticipate that SL-related inhibitors will be useful as new plant growth regulators for efficient propagation of plants in vitro.
Author contribution statement
KO and MU conceived and designed the research. KS provided sterile ipecac plants. KO, SW, and IK conducted the ipecac tissue culture experiments. SI and TA synthesized TIS108. KK and SI synthesized KK5. HN and TA synthesized KK094. KO and MU analyzed the data. KO, KS, and MU wrote the manuscript. All authors read and approved the manuscript.
Abbreviations
- CK:
-
Cytokinin
- iPR:
-
Isopentenyl adenine riboside
- SL:
-
Strigolactone
- tZ:
-
Trans-zeatin
- tZR:
-
Trans-zeatin riboside
References
Agusti J, Herold S, Schwarz M, Sanchez P, Ljung K, Dun EA, Brewer PB, Beveridge CA, Sieberer T, Sehr EM, Greb T (2011) Strigolactone signaling is required for auxin-dependent stimulation of secondary growth in plants. Proc Natl Acad Sci USA 108(50):20242–20247. https://doi.org/10.1073/pnas.1111902108
Beveridge CA, Kyozuka J (2010) New genes in the strigolactone-related shoot branching pathway. Curr Opin Plant Biol 13(1):34–39. https://doi.org/10.1016/j.pbi.2009.10.003
Beveridge CA, Symons GM, Turnbull CG (2000) Auxin inhibition of decapitation-induced branching is dependent on graft-transmissible signals regulated by genes Rms1 and Rms2. Plant Physiol 123(2):689–698
Chatterjee SK, Nandi RP, Ghosh NC (1982) Cultivation and utilization of ipecac in west Bengal. In: Atal CK, Kapur BM (eds) Cultivation and utilization of medicinal plants. Regional Research Laboratory, Council of Scientific and Industrial Research, Jammu-Tawi, pp 295–301
Crawford S, Shinohara N, Sieberer T, Williamson L, George G, Hepworth J, Muller D, Domagalska MA, Leyser O (2010) Strigolactones enhance competition between shoot branches by dampening auxin transport. Development 137(17):2905–2913. https://doi.org/10.1242/dev.051987
de Saint GA, Ligerot Y, Dun EA, Pillot JP, Ross JJ, Beveridge CA, Rameau C (2013) Strigolactones stimulate internode elongation independently of gibberellins. Plant Physiol 163(2):1012–1025. https://doi.org/10.1104/pp.113.220541
Domagalska MA, Leyser O (2011) Signal integration in the control of shoot branching. Nat Rev Mol Cell Biol 12(4):211–221. https://doi.org/10.1038/nrm3088
Duan J, Yu H, Yuan K, Liao Z, Meng X, Jing Y, Liu G, Chu J, Li J (2019) Strigolactone promotes cytokinin degradation through transcriptional activation of CYTOKININ OXIDASE/DEHYDROGENASE 9 in rice. Proc Natl Acad Sci USA 116(28):14319–14324. https://doi.org/10.1073/pnas.1810980116
Espinosa-Leal CA, Puente-Garza CA, García-Lara S (2018) In vitro plant tissue culture: means for production of biological active compounds. Planta 248(1):1–18. https://doi.org/10.1007/s00425-018-2910-1
Evans DA, Sharp WR, Flick CE (1981) Growth and behavior of cell cultures: Embryogenesis and organogenesis. In: Thorpe TA (ed) Plant tissue culture: methods and applications in agriculture. Academic Press, New York, pp 45–113
Ferguson BJ, Beveridge CA (2009) Roles for auxin, cytokinin, and strigolactone in regulating shoot branching. Plant Physiol 149(4):1929–1944
Foo E, Bullier E, Goussot M, Foucher F, Rameau C, Beveridge CA (2005) The branching gene RAMOSUS1 mediates interactions among two novel signals and auxin in pea. Plant Cell 17(2):464–474
Gahan PB, George EF (2008) Adventitious regeneration. In: George EF, Hall MA, De Klerk GJ (eds) Plant propagation by tissue culture, vol 1, 3 edn. Springer, Dordrecht, pp 355–401
Gamborg OL, Miller RA, Ojima K (1968) Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res 50(1):151–158. https://doi.org/10.1016/0014-4827(68)90403-5
Gomez-Roldan V, Fermas S, Brewer PB, Puech-Pages V, Dun EA, Pillot JP, Letisse F, Matusova R, Danoun S, Portais JC, Bouwmeester H, Becard G, Beveridge CA, Rameau C, Rochange SF (2008) Strigolactone inhibition of shoot branching. Nature 455(7210):189–194. https://doi.org/10.1038/nature07271
Ito S, Umehara M, Hanada A, Kitahata N, Hayase H, Yamaguchi S, Asami T (2011) Effects of triazole derivatives on strigolactone levels and growth retardation in rice. PLoS ONE 6(7):e21723. https://doi.org/10.1371/journal.pone.0021723
Ito S, Umehara M, Hanada A, Yamaguchi S, Asami T (2013) Effects of strigolactone-biosynthesis inhibitor TIS108 on Arabidopsis. Plant Signal Behav. 8:e24193
Kapulnik Y, Delaux PM, Resnick N, Mayzlish-Gati E, Wininger S, Bhattacharya C, Sejalon-Delmas N, Combier JP, Becard G, Belausov E, Beeckman T, Dor E, Hershenhorn J, Koltai H (2011) Strigolactones affect lateral root formation and root-hair elongation in Arabidopsis. Planta 233(1):209–216. https://doi.org/10.1007/s00425-010-1310-y
Kawada K, Takahashi I, Arai M, Sasaki Y, Asami T, Yajima S, Ito S (2019) Synthesis and biological evaluation of novel triazole derivatives as strigolactone biosynthesis inhibitors. J Agric Food Chem 67(22):6143–6149. https://doi.org/10.1021/acs.jafc.9b01276
Kiba T, Takei K, Kojima M, Sakakibara H (2013) Side-chain modification of cytokinins controls shoot growth in Arabidopsis. Dev Cell 27(4):452–461. https://doi.org/10.1016/j.devcel.2013.10.004
Koike I, Taniguchi K, Shimomura K, Umehara M (2017) Dynamics of endogenous indole-3-acetic acid and cytokinins during adventitious shoot formation in ipecac. J Plant Growth Regul 36(4):805–813. https://doi.org/10.1007/s00344-017-9684-8
Koike I, Shimomura K, Umehara M (2018) Quantification of endogenous auxin and cytokinin during internode culture of ipecac. J vis Exp 133:e56902. https://doi.org/10.3791/56902
Koike I, Watanabe S, Okazaki K, Hayashi KI, Kasahara H, Shimomura K, Umehara M (2020) Endogenous auxin determines the pattern of adventitious shoot formation on internodal segments of ipecac. Planta 251(3):73. https://doi.org/10.1007/s00425-020-03367-5
Manandhar S, Funnell K, Woolley D, Cooney J (2018) Interaction between strigolactone and cytokinin on axillary and adventitious bud development in Zantedeschia. J Plant Physiol Pathol 6(1):1000172. https://doi.org/10.4172/2329-955X.1000172
Mangnus EM, Dommerholt FJ, Dejong RLP, Zwanenburg B (1992) Improved synthesis of strigol analog GR24 and evaluation of the biological-activity of its diastereomers. J Agric Food Chem 40(7):1230–1235
Minakuchi K, Kameoka H, Yasuno N, Umehara M, Luo L, Kobayashi K, Hanada A, Ueno K, Asami T, Yamaguchi S, Kyozuka J (2010) FINE CULM1 (FC1) works downstream of strigolactones to inhibit the outgrowth of axillary buds in rice. Plant Cell Physiol 51(7):1127–1135. https://doi.org/10.1093/pcp/pcq083
Nakamura H, Hirabayashi K, Miyakawa T, Kikuzato K, Hu W, Xu Y, Jiang K, Takahashi I, Niiyama R, Dohmae N, Tanokura M, Asami T (2019) Triazole ureas covalently bind to strigolactone receptor and antagonize strigolactone responses. Mol Plant 12(1):44–58. https://doi.org/10.1016/j.molp.2018.10.006
Ongaro V, Leyser O (2008) Hormonal control of shoot branching. J Exp Bot 59(1):67–74
Rasmussen A, Mason M, De Cuyper C, Brewer PB, Herold S, Agusti J, Geelen DN, Greb T, Goormachtig S, Beeckman T, Beveridge CA (2012) Strigolactones suppress adventitious rooting in Arabidopsis and pea. Plant Physiol 158:1976–1987. https://doi.org/10.1104/pp.111.187104
Ruyter-Spira C, Kohlen W, Charnikhova T, van Zeijl A, van Bezouwen L, de Ruijter N, Cardoso C, Lopez-Raez JA, Matusova R, Bours R, Verstappen F, Bouwmeester H (2011) Physiological effects of the synthetic strigolactone analog GR24 on root system architecture in Arabidopsis: another belowground role for strigolactones? Plant Physiol 155(2):721–734. https://doi.org/10.1104/pp.110.166645
Skoog F, Miller CO (1957) Chemical regulation of growth and organ formation in plant tissues cultured in vitro. Symp Soc Exp Biol 11:118–130
Snow R (1929) The young leaf as the inhibiting organ. New Phytol 28(5):345–358. https://doi.org/10.1111/j.1469-8137.1929.tb06765.x
Takei K, Yamaya T, Sakakibara H (2004) Arabidopsis CYP735A1 and CYP735A2 encode cytokinin hydroxylases that catalyze the biosynthesis of trans-zeatin. J Biol Chem 279(40):41866–41872. https://doi.org/10.1074/jbc.M406337200
Tanaka M, Takei K, Kojima M, Sakakibara H, Mori H (2006) Auxin controls local cytokinin biosynthesis in the nodal stem in apical dominance. Plant J 45(6):1028–1036. https://doi.org/10.1111/j.1365-313X.2006.02656.x
Thimann KV, Skoog F (1933) Studies on the growth hormone of plants: III. The inhibiting action of the growth substance on bud development. Proc Natl Acad Sci USA 19(7):714–716
Thorpe TA (2007) History of plant tissue culture. Mol Biotechnol 37(2):169–180. https://doi.org/10.1007/s12033-007-0031-3
Trease GE, Evans WC (1989) Trease and Evans’ phamacognosy, 13th edn. Bailliere Tindall, London
Umehara M, Hanada A, Yoshida S, Akiyama K, Arite T, Takeda-Kamiya N, Magome H, Kamiya Y, Shirasu K, Yoneyama K, Kyozuka J, Yamaguchi S (2008) Inhibition of shoot branching by new terpenoid plant hormones. Nature 455(7210):195–200. https://doi.org/10.1038/nature07272
Yamada Y, Furusawa S, Nagasaka S, Shimomura K, Yamaguchi S, Umehara M (2014) Strigolactone signaling regulates rice leaf senescence in response to a phosphate deficiency. Planta 240(2):399–408. https://doi.org/10.1007/s00425-014-2096-0
Yoshimatsu K, Shimomura K (1991) Efficient shoot formation on internodal segments and alkaloid formation in the regenerates of Cephaelis ipecacuanha A. Richard. Plant Cell Rep 9(10):567–570. https://doi.org/10.1007/bf00232333
Zhang J, Mazur E, Balla J, Gallei M, Kalousek P, Medveďová Z, Li Y, Wang Y, Prát T, Vasileva M, Reinöhl V, Procházka S, Halouzka R, Tarkowski P, Luschnig C, Brewer PB, Friml J (2020) Strigolactones inhibit auxin feedback on PIN-dependent auxin transport canalization. Nat Commun 11(1):3508. https://doi.org/10.1038/s41467-020-17252-y
Acknowledgements
This study was in part supported by the Inoue Enryo Memorial Foundation for Promoting Science from Toyo University (KO), The 30th Botanical Research Grant of ICHIMURA Foundation for New Technology (MU), and by the Research Center for Life and Environmental Sciences, Toyo University. We thank Shosaku Kashiwada and Hiroki Higashibata (Toyo University) for their constructive comments on this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Dorothea Bartels.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Okazaki, K., Watanabe, S., Koike, I. et al. Strigolactone signaling inhibition increases adventitious shoot formation on internodal segments of ipecac. Planta 253, 123 (2021). https://doi.org/10.1007/s00425-021-03640-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00425-021-03640-1