Abstract
Direct tunable diode laser absorption spectroscopy (dTDLAS) is a powerful diagnostic technique for absolute and accurate gas analysis with highest chemical specificity. Due to its first principles approach, dTDLAS is often claimed to be “calibration-free”, but this and the absolute accuracy has not been rigorously validated with respect to a high-accuracy reference. This work describes the first rigorous, side-by-side comparison of a dTDLAS hygrometer—called SEALDH—with a highly accurate, internationally validated, primary reference humidity generator (PHG), which also serves as the German national H2O-standard. This PHG provides a humidified air stream with dew points between −30 °C and +60 °C with an uncertainty of 0.035 K (2σ) (equivalent relative H2O mixing ratio uncertainty: 0.4 %). Without any previous calibration, SEALDH was found to accurately reproduce the PHG reference values over the full range from 600 to 20,000 ppmv investigated in the 1-week lab study. Over this range, the SEALDH–PHG relative deviation was in average −1.45 %, the worst case being −2.5 % at 1,000 ppmv, the best −0.2 % at 600 ppmv. As SEALDH’s relative uncertainty was metrologically determined to be 4.3 % (k = 2), these deviations are for all concentration steps in full compliance with the PHG reference. Systematic contributions to the relative deviation could be correlated with line shape deviations between the measured line profile and the fitted Voigt line shape. Using this information, SEALDHs absolute accuracy can be improved further to down to an average relative deviation to the PHG of +0.21 %.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Accuracy is one of the most important features of every analytical instrument. Typically, a well-controllable effect, strongly—preferably linearly—correlated with the measurand, is used as basic sensing principle. But it is also required to have an accurate and precise knowledge of this basic behavior in combination with a sufficient study of all statistical and systematic disturbances and parasitic influences. In most cases, the sensor response is validated via a calibration, i.e., by a comparison of the sensor response with a well-defined and stable, absolute reference. The reference could be another sensor which measures the same quantity or a generator which provides a highly stable, well-characterized reference sample of the quantity to be measured. This calibration procedure allows to “transfer” the knowledge and control over the absolute reference scale to the sensor which has to be validated. The achievable calibration accuracy and precision are strongly influenced (a) by the quality of the reference, (b) by possible imperfections in the transfer of the reference to the sensor, e.g., by losses/errors in the transfer medium such as wires or pipes, (c) by the detection threshold for parasitic effects on the sensor, and finally (d) by the amplitude and velocity of the sensor drift. The latter in combination with the desired calibration accuracy also determine the required repetition frequency of the calibration. Sensor calibration can contribute significantly to the total cost of ownership of the sensor as it requires a specific infrastructure, specifically trained personal, a fair amount of work time and consumables like reference gases. Calibration performance and accuracy can become particularly critical and tedious under “field-conditions” [1] where the stability of both, the sensor and the reference, might deteriorate, thus affecting the calibration accuracy of the sensor.
Especially in the case of hygrometry, i.e., the measurement of the gas water vapor content, all issues mentioned above need to be controlled simultaneously. The accurate generation, transport, and analysis of a well-defined water vapor sample in a gas stream are quite difficult, due strong adsorption of the water molecules to almost any surface and the risk of phase changes, i.e., condensation problems. Calibration procedures therefore get particularly tedious and time consuming at low-water mixture fractions, where the influence of the adsorption/desorption equilibrium (A/D-Eq) at any surface increases drastically. If high calibration accuracy is desired, long waiting times are required for equilibration after each (H2O) step change. In addition, the A/D-Eq temperature dependence makes the entire system more and more sensitive to temperature changes. The time needed for a calibration, the accuracy required, the temporal stability of the sensor calibration, and hence the required calibration frequency are counteracting driving forces which determine the possible overall accuracy, the usable operating life or the maximum continuous measurement time of a given combination of sensor and calibration infrastructure.
2 TDLAS-Hygrometry
The complexity of many field-based hygrometer calibration approaches explains the high interest of the environmental community for absolute measurement measuring principles. One of the most prominent, hygrometry approaches is direct tunable diode laser absorption spectroscopy (dTDLAS) [2, 3]. The term “direct” describes in this context TDLAS systems working with DC-coupled, non-filtered or if at all low-pass filtered data evaluation, which allow a direct evaluation of the unaltered absorption profile, contrary to the very common double wavelength modulation schemes (e.g., 2f WMS [4, 5]), which analyses higher-order derivatives of the absorption profile. dTDLAS is a variant of TDLAS which allows in combination with a special data evaluation [6], a direct extraction of absolute species concentrations without a requirement for a calibration of the sensor response. dTDLAS offers like other TDLAS variants many additional benefits such as high chemical selectivity, excellent sensitivity, high temporal resolution, robustness as well as compactness and low energy consumption.
Over the last decades, a large number of commercial and research TDLAS instruments were developed and described in literature. A complete coverage is due to space restrictions impossible. But a coarse classification and typical examples of the most important TDL-hygrometer types are given below.
A first TDLAS classification relates to the wavelength modulation scheme and covers single modulation frequency (direct TDLAS [6, 7] ), as well as double frequency modulation schemes like wavelength modulation spectroscopy (WMS [4] ) and others. WMS offers with low bit resolution AD converters a precision advantage, which explains somewhat the preference of the WMS techniques [8–14]. WMS also offers better sensitivity with low-cost data acquisition cards and thus is interesting for low-cost systems. However, recently, it has been demonstrated that this advantage has vanished for laboratory applications where high bit resolution data acquisition hardware [15] is ubiquitous. A second classification concerns the absorption path topology, covering single path [16] versus multipath cells [17], as well as cavity-enhanced (CE) techniques such as cavity-ring-down-spectroscopy (CRDS) [18] or off-axis cavity-enhanced-absorption-spectroscopy (CEAS) [19]. Subclasses hereof are discerned via sample gas transport to the optical path measurement region: Closed-path or so-called “extractive” sensors encase the gas in a closed gas cell into which the sample gas is injected and analyzed. Open-path sensors (OPS) avoid gas transport (hence also minimize sampling artifacts) and analyze the gas by directly transilluminating the “process volume”. Analyzing the gas “in place,” they are frequently called in situ systems.Footnote 1 OPS have to be able to cope with pressure and temperature variations in the process volume and may suffer problems with spatial inhomogeneities. Closed-path, extractive configurations trade the advantage of well-defined boundary conditions in the measurement region for possible disadvantages from sampling artifacts, as well as questions related to the sample representativeness and response time limitations due to gas flow restrictions. A last possible classification concerns the calibration frequency. Basically, this leads to in-process calibration, long-term calibration, or potentially calibration-free hygrometers, sometimes also called self-calibrating. The vast majority of the instruments have to be calibrated. “First-principles” or “calibration-free” spectrometers are receiving increasing interest [20, 21], but a rigorous validation of the absolute accuracy possible with such approaches is missing. Here, we present and discuss the first side-by-side comparison of such a “cal-free” sensor with a highly accurate, metrological primary water vapor standard.
3 Hygrometer intercomparisons
Sensor intercomparisons are a well-proven metrological methodology to evaluate the performance of sensors as well as generators. Despite long-lasting discussions on the absolute accuracy of atmospheric and in particular airborne hygrometers [22], there are relatively few, large field hygrometer intercomparisons documented. Many comparisons cover only a small number of instruments [23, 24] thus often generating discussions about representativeness or transferability. “Blind” intercomparisons, which require that the sensor results have to be submitted without any chance to compare with the other instruments or the generator operator, are even less frequent. One of the most comprehensive and largest field hygrometer intercomparisons, termed “AquaVIT” [25], was realized at the AIDA (Aerosol Interaction and Dynamics in the Atmosphere) [26] cloud and aerosol simulation chamber in Karlsruhe (Germany). AIDA, a unique, 76 m3 large, liquid-nitrogen-cooled vessel to simulate atmospheric conditions in the troposphere and stratosphere, provides well-defined, quasi-static, but also dynamic laboratory boundary conditions for the hygrometers. AquaVIT was organized as a blind and externally refereed intercomparison. It included 22 instruments (from 17 international research groups) such as dew point hygrometers (D/FPH), Lyman-alpha fluorescence hygrometers (LAFH), tunable diode laser sensors (TDL), and other principles. Despite the excellent boundary conditions and the quasi-static intercomparison, the well-validated “core group” of hygrometers agreed in the important 1–150 ppmv water vapor volume content (WVC) range only to within ±10 % of the “virtual” reference, defined through the average over all core instruments. An instrument offset to the true WVC value could therefore not be determined this way. Furthermore, the hygrometers were not traced back to absolute metrological references, like the water scale defined by a set of national primary standards [27–29]. Aquavit clearly demonstrated the need for and possible profits of large field instrument intercomparisons to improve the comparability between field hygrometers. The Aquavit outcome can also be interpreted that comparability requires probably a more standardized and validated approach to instrument calibration and in an ideal case a direct link of all instruments to a common, well-defined absolute water scale, e.g., the metrological humidity scale maintained by the global group of national metrology institutes (NMIs). The strong dependence of the Aquavit instruments on the quality of the calibration on the other hand also generates questions, (a) if absolute detection principles like dTDLAS are suited to minimize this dependence on high-quality water references, e.g., under field conditions, and (b) what accuracy level is possible without a repeated calibration, i.e., a frequent comparison to a water reference instrument or generator.
A validation of a dTDLAS instrument at the highest accuracy humidity generator—a national primary standard—is a good opportunity to provide further insight in these questions, as the primary standards have been extensively validated via international intercomparisons at the highest level, so that any deviation outside the very low uncertainty range of primary standard certainly belongs to the instrument under test.
4 Metrological transfer standards in hygrometry
One of the core competences of the metrology community is the development and validation of devices and methods for the generation of highly accurate reference samples or reference analyzers including well-defined, quantified, and standardized procedures to determine the uncertainty of the reference “standard”. Such metrological standards can be realized (a) as analytical standard, which defines the capability to provide a traceable analysis of a (gas) sample, or (b) as a preparative standard, which defines the capability to generate a defined reference (gas sample) material with a traceable metrological quantification of its composition. Preparative gas standards are in many cases reference gas mixtures which can be stored over long time periods in pressurized bottles. The metrological humidity scale, however, is—due to the difficulties in long-term storage of gaseous humidity standards—almost exclusively defined via special, primary humidity generators (PHG). PHGs in different National Metrology Institutes (NMIs) are regularly validated via international metrological intercomparisons, e.g., [28], either on a European basis organized by EURAMET or even as global key comparison under the auspices of the BIPM (Bureau International des Poids et Mesures, Sèvres, Paris, France, http://www.bipm.org). PHGs define the top position of the traceability chain and therefore often are—at the expense of an often complete loss of mobility—one to two orders of magnitude more accurate and precise than a transfer standard or a typical field instrument.
The comparison and validation of PHGs therefore need mobile metrological transfer standards (TS). These are not only interesting for PHG intercomparisons but also (if robust enough) as field reference for field hygrometers to be calibrated. An ideal situation would be, if a transfer standard could be used not only as field reference for sensor calibration but itself as traceable, field qualified, reference instrument for measurements on platforms like airplanes, i.e., as an airborne transfer standard.
The most frequently used mobile analytical transfer standards to compare the performance of different PHGs are dew/frost point mirror hygrometers (DPH/FPH) [27, 28]. But, while FPH instruments can nicely serve as mobile TS for ground-based sensor calibration in the atmospheric sciences, they suffer in field applications from several caveats which (despite special commercial airborne versions like the “Buck CR1” [30]) do limit their use when used on airplanes: FPH need direct contact to the sampling gas, which can cause problems with sampling and contamination. Further, they cannot work very close to zero humidity where they will lose their frost layer, which causes problems when calibrating with zero gas. Additional, observations in general are quite difficult for FPH in the transition phase from a liquid to a frozen ice surface on the mirror. Finally, and most important for airborne measurements on high-speed platforms like airplanes, FPH get inherently slow at low humidities, which can cause significant problems for aircraft-based sensors in particular at UT/LS conditions.
Most of these problems can be solved with optical analyzers, and in particular those based on direct TDLAS. dTDLAS promises much higher time resolution than FPHs, excellent selectivity and sensitivity in combination with high mobility due to low weight, size, and power consumption. dTDLAS can also be operated in a first principles and therefore calibration-free mode which is published in detail in [2, 6] and also described in the following chapter. Reduced requirement or complete avoidance of calibrations can make dTDLAS instruments highly interesting for the atmospheric community as a mobile water vapor transfer standard. But, while TDLAS-based sensors and in particular hygrometers are applied in an increasing number of cases for environmental monitoring, this is in most cases in calibrated and not in calibration-free mode. In addition, the calibration-free approach also lacks a solid validation, which could be done by a comparison with a high-precision PHG. But, to our knowledge, an uncalibrated dTDLAS hygrometer has so far been not compared with a primary national humidity standard. There are very few comparisons of this type documented in the literature. However, all of these were carried out on relatively easy to handle gas species like CO2 [31, 32], which allows the reference mixtures to be stored in bottles.
In this paper, we describe the first validation of a cal-free dTDLAS hygrometer at a primary H2O standard. This side-by-side comparison covered the (H2O) range from 600 ppmv up to 200,000 ppmv and was intended to deliver insight in the performance of cal-free TDLAS hygrometers and to allow a detailed analysis of the causes for systematic deviations between dTDLAS system and a PHG.
5 Calibration-free TDLAS hygrometers
Tunable diode laser absorption spectroscopy (TDLAS) in general has been described in numerous cases in the past, e.g., [11, 12, 25, 33–35] so that a general discussion of TDLAS is not in the focus of this paper. This paper focusses exclusively on the goal to evaluate the accuracy of uncalibrated dTDLAS, which is also described in detail in [2, 6] and already used in several applications, e.g., [36–41]. For the sake of clarity, we discuss calibration-free TDLAS here briefly and hint directly to the requirements for that evaluation method.
5.1 Basic principle of dTDLAS
A simplified, schematic setup of a dTDLAS hygrometer is depicted in Fig. 1: The function generator provides a triangle-shaped voltage signal with a typical frequency in the range between 100 Hz and 10 kHz. The laser current driver, which also controls the laser temperature, transforms the voltage signal into a laser current modulation of typically 1–150 mA. This current modulation causes (a) the desired dynamic tuning of the laser wavelength (typically <3 cm−1 with a DFB laser) to scan over an absorption transition, but also (b) an (unwanted) amplitude modulation of the laser intensity. The laser light, with typical power in the mW range, is shaped into a collimated beam using an aspheric lens and then transmitted through the measurement volume. The molecule-specific absorption causes spectrally narrowband losses which are captured using a detector. The detector signal is amplified and digitized by a data acquisition card and saved to the computer hard disk.
The molecule-specific attenuation can be described by the Lambert–Beer equation (Eq. 1) connects the initial intensity I 0(λ) with the transmitted intensity I(λ) thereby also taking into account, the molecular line strength S(T) (depending on the gas temperature T), the line shape function g, the absorber number density N, and the absorption path length L. We typically extend the simple Lambert–Beer equation by including possible background radiation E(t) hitting the detector, as well as and spectrally broadband transmission losses Tr(t), which may be caused, e.g., by the windows contamination. This extended Lambert–beer relation can then be written as
The molecular concentration is derived from the area of the absorption line which can be determined using Eq. (1) taking into account that the area of the line shape function is unity and the ideal gas law to form Eq. 2.
The individual terms in Eq. (2) encompass five different contributions (A–E):
(A) Constants, such as the Boltzmann constant k B. (B) Measurands, that are stable over time, such as the optical absorption path length L, the dynamic tuning coefficient of the laser \(\frac{{{\text{d}}\nu }}{{{\text{d}}t}}\) which is derived experimentally using the Airy-signal of the laser light passing through a planar air-spaced etalon [6, 42]. For the lasers used here, we found \(\frac{{{\text{d}}\nu }}{{{\text{d}}t}}\) to be stable within an uncertainty of 1.8 % over time spans of years. The tuning characterization is therefore long-term stable and treated as a characteristic laser constant, which is determined once with a stable etalon and thus linked to a metrological determination of a length. (C) Includes dynamic measurands that have to be determined with sufficiently high temporal resolution such as gas temperature T, gas pressure p. (D) Encompasses intensity I(ν) related measurands, such as the initial light intensity I 0(ν), the intensity of the transmitted light I(ν), the background emission E(t), and the transmission losses Tr(t), that all are determined for each individual absorption profile with the same detector and with maximum time resolution. (E) Finally, the last group is formed by molecular parameters, like line strength S(T). Aside from their temperature dependence, which comes into play at a rather low level as the gas temperature is kept in the cell within relatively narrow limits, they can be interpreted as internal standards which do not change over time, space or depend on any calibration factors. Such molecular parameters can be taken from literature. We use HITRAN08 [43] data for most parameters, including the parameters describing its temperature dependence, but have determined some broadening related parameters with higher resolution from own measurements [44].
The important statement that should be kept in mind for the further discussion is the fact that this equation describes completely the data evaluation. No further degrees of freedom, calibration, or adjustment factors are introduced during our data evaluation. No calibration is being used to tune the response of the spectrometer during the initial setup or on a regular base during its use. Of course, e.g., pressure and temperature sensors have to be calibrated, but only in their “units”. We do not scale the spectrometer response by a comparison with a known “calibration” gas, which is a widespread procedure in gas analysis and also in TDLAS devices [45]. To describe this fact and to discern the approach from other TDLAS approaches which employ response “tuning”, we use the term “calibration-free”. Throughout the literature, similar terms are sometime used like “self-calibrating”, or “first principle technique,” but usually we find that the boundary conditions are often not well described, which is why we think that using those terms is inappropriate for our work. Of course, it needs to be investigated which accuracy such a cal-free spectrometer can deliver which is the focus of this work. A long-term study to derive stability of the property over at least months if not years is underway and out of the scope of this work presented here.
5.2 Absorption line selection
Our application-specific selection of a suitable absorption line has been done following the criteria defined in [46, 47] and lead to the 1,370-nm line which we and other groups have also used in numerous other publications [17, 25, 34, 35, 48–54] and even have made own measurements to improve knowledge of the spectral parameters [44]: Maximization of the line strength ensures the highest possible sensitivity in the hygrometer but should not get the highest priority. Other important parameters include the suppression of the cross-sensitivity to other gases in ambient air. For our setup, nearby CO2 lines and other species were carefully checked using literature data. The basic idea of the data evaluation is to minimize the degrees of freedom during the fitting process. To simplify and stabilize the fitting process, the selected absorption should be well isolated from foreign species and even from other water lines nearby. The temperature dependency of the line strength should also be kept low (0.5 %/K for our 1,370 nm line) as it minimizes the influence of the uncertainties in gas temperature measurements, but this parameter is more important for open-path sensors and of minor influence for temperature stabilized extractive configurations. Finally and sometimes, the primary constraint represents the availability of suitable laser diodes and their additional accessories such as fibers and optic components. For the many of our TDLAS humidity sensors, the 1,370-nm line used here has been a quite good choice in particular due to its excellent isolation from neighboring absorption lines. Other lines might be more suitable for other applications.
6 Instrument description
This paper describes the side-by-side comparison of a primary metrological water standard with a new calibration-free laser hygrometer (SEALDH). This instrument is initially developed for airborne water vapor detection on research airplanes applications such as [55–57]. Of course, it should be noted that the instrument used in this work is outside an airborne application scenario, i.e., without an airborne inlet system and in a well-controlled and stabilized lab environment. The intention of the work presented here is to investigate the capabilities of our dTDLAS concept with regard to accuracy and not the specific airborne configuration and features of SEALDH; the latter has to be done in an airborne scenario.
6.1 SEALDH setup
The setup and basic principle of the selective extractive airborne laser diode hygrometer (SEALDH) is depicted in Fig. 1. The extractive (closed path) multi-path absorption cell is of the white-type and fiber-coupled without any transfer optics thereby minimizing any parasitic absorption effects due the water vapor in the ambient air. This cell permits an optical path length of about 1.1 m (ca. 7 cm base length, 8 rounds) at a volume of about 300 ccm and allows in this configuration a concentration range of sub 50 ppmv to about 25,000 ppmv. The typical sample gas flow of 4 std l/min from the primary standard leads with a plug flow approximation and one cell volume exchange to a response time of about 4 s. The used distributed feedback laser (DFB, 1,370 nm) is directly packaged from (NEL) with a built-in Peltier element and thermistor in a butterfly package and was controlled by a LDC 8002 laser driver and TES 8020 Peltier driver from Thorlabs. The triangle laser current modulation from 4 mA to 88 mA with a frequency of 140 Hz allows a wavelength scan of 1.5 cm−1 across the 110–211 H2O absorption line in the ro-vibrational combination band near 1,370 nm (Fig. 2). The current of the detector, standard type with 1-mm active zone, was amplified with a trans-impedance amplifier and acquired with a NI PCI 6251 (16 bit, M-serie, 1 MS/s) card.
The main focus of the intercomparison at the primary generator was a validation of the absolute accuracy of the dTDLAS instrument SEALDH under well-controlled quasi-static conditions; the dynamic behavior was not studied. Due to the static conditions, SEALDH could be operated in a reduced data throughput mode for slow concentration variations, i.e., around 10 % of the available 140 scans per second were sampled and 20 successive scans were directly averaged which led to a data point separation of 1.4 s. These averaged raw scans were evaluated by Levenberg–Marquart-based approach fitting a model function consisting of a 3rd-order background polynomial, and Voigt line shapes to extract the line area while synchronously correcting for transmission changes and background offsets (Eq. 1). The evaluation method is largely unchanged to our previous work and has been frequently published in detail elsewhere, e.g., [2, 6]. The line strength S(T) is taken from HITRAN08 [43], broadening coefficient and temperature coefficient from own measurements [44]. Further, input quantities that are taken into account are measured gas temperature/pressure as well as previously measured path lengths and laser tuning (described in more detail the next paragraph).
6.2 Uncertainty of SEALDH
A rigorous comparison of the absolute performance of the dTDLAS hygrometer versus the PHG requires a detailed discussion of the individual uncertainty contributions. SEALDH’s gas pressure sensor (MKS Baratron, 1,000 hPa) with its uncertainty of 0.5 hPa was calibrated with a precision absolute pressure transmitter (MKS Baratron, 0–1,300 hPa, uncertainty 0.07 hPa) which again was directly calibrated at the national primary pressure standard at PTB. Meaningful uncertainties always are derived (and stated) including the digital voltage conversion electronics of the pressure sensor. Our overall relative pressure uncertainty is estimated to be 1 hPa, i.e., 10−3 of the full scale. The thermocouple temperature sensor (type E) with its digitization electronics (RedLab TC with internal cold-junction compensation) was calibrated with an absolute temperature transmitter (Rosemount platinum resistance thermometer type 162CE (PRT-25), uncertainty 1.5 mK) again directly at the national primary temperature standard at PTB. P/T-sensor accuracy in SEALDH is only one contribution to the gas temperature determination in the gas cell. Spatial inhomogeneities in the temperature field in the cell are experimentally minimized by use of an additional heat exchanger in close thermal contact with and placed before the multipath cell. A measurement with two thermocouples could not measure within their uncertainty of 0.5 K any T–inhomogeneity in the cell. Despite this extra measure, we use 0.5 % (or 1.4 K) as a conservative estimate of the overall uncertainty of the gas temperature, including spatial inhomogeneity. The optical path length was calculated by the mirror measured separation. In addition, we validated it with a Zemax-based ray tracing simulation. Furthermore, L was validated via a test measurement with a known amount of CH4 gas in the cell. The maximum deviation between all three values for L was 11 mm (1.9 %), which we therefore use as a conservative uncertainty estimate. The currently best uncertainty for the used H2O absorption line strength is 3.5 % [44]. Finally, overall uncertainty of the fitting process is estimated to be 1 %. This leads to an overall uncertainty for SEALDH of 4.3 %. The largest relative influences to this total uncertainty budget results from the line strength (65 %), followed by the uncertainty of the path length (19 %), as well as pressure and temperature measurements (5 % each).
7 PTB National primary humidity standard
PTB as the NMI of Germany holds the national primary standard for humidity. The extreme wide range of the humidity scale is realized with two different generators. The trace humidity region, from a water volume content of about 5 ppbv to 650 ppmv, is realized with a coulometric trace humidity generator [58] using nitrogen as carrier gas. The upper range, from a water volume content of 600 ppmv up to 50 vol%, is covered with a two-pressure humidity generator. The latter served as the humidity reference in the comparison with the SEALDH instrument.
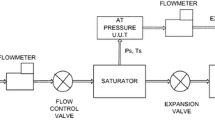
The basic idea of the two-pressure generator is to completely saturate air with water vapor under increased pressures in a special designed saturator (Fig. 3) and then expand it to the desired pressure level of about 1,013 hPa. Compressed air from an external compressor is first saturated with water in a pre-saturator at a temperature about 10 K higher than the temperature in the following thermostatic bath t s. This procedure ensures a water vapor excess in the air flowing through the metallic saturator block immersed in the thermostatic bath. The connection tube and all following stainless-steel tubes are heated to prevent the humid air from condensing, which would result in a loss of gas phase water. In the saturator block, the water vapor excess in the gas phase condenses. Important design features of the saturator block to ensure an efficient condensation are a large inner wall surface and an excellent thermal contact of the gas with the walls. The latter is realized by the construction details shown in the figure. (The condensate has to be released from time to time) The air leaving the saturator block is therefore saturated to exactly 100 % relative humidity regarding the thermostatic bath temperature t s. Consequently, the water vapor partial pressure e w in the saturator outflow is defined by the saturator temperature and can be calculated using the water vapor pressure equation. Therefore, we use the equation by Sonntag (Eq. 3) [59], which is the commonly used standard formula in the metrology community. The meteorology communities, however, often use alternative equations such as Murphy and Koop [60], Marti and Mauersberger [61], Wexler [62, 63], or Hyland and Wexler [64] which can show small deviations in the single-digit percent range to Sonntag [59], depending on the specific parameter set, the assumptions on the boundary conditions. These deviations are studied in detail, e.g., in [60].
This equation is only valid in the pure phase (only water molecules in the system). Humidified air, however, is a multi-component system (O2, N2, H2O, and small amounts of other gases) so that a correction factor for e W is needed. This correction factor is called enhancement factor f W according to Greenspan [65] and depends on the temperature t s and the pressure p s in the saturator. After the saturation process at the increased pressure p s, the air is expanded via a needle valve to the target pressure p t needed at the device to be tested with the PHG. The resulting water vapor partial pressure e’ after the expansion is calculated with the Eq. 4.
The water vapor volume fraction φ (Eq. 5) is defined as the ratio of the water vapor partial pressure e′ (Eq. 4) to the total gas pressure (=target pressure p t) in the measuring cell, i.e., after the expansion. Equation 5 for the water vapor volume fraction φ shows that p t is reduced from the fraction and therefore φ is independent of the cell pressure p t and only depends on the saturator temperature t s and the saturator pressure p s which has to be measured as accurately as possible.
The pressure transducer measuring the saturator pressure (MKS Baratron) is directly traced back to the National Standard for pressure, the two Pt-25 standard platin resistance thermometer (SPRT) measuring the saturation temperature (Tinsley Inc., USA) are traced back to the National Standard for temperature at the fixed points of the mercury solidification point, the water and indium triple point. The measurement uncertainties (k = 2) for these calibrations are 0.8 hPa for the pressure transducer and 1.5 mK for the SPRT.
Measured TDLAS raw spectra using a triangular laser current modulation. Clearly visible is the 1,370 nm absorption line, showed for five different water vapor concentrations. The total transmission of the measurement path is influenced by broadband scattering, thermal and pressure effects in detector, fibers and opto-mechanical distortion, but is corrected at each individual absorption profile during the fitting process
The total uncertainty of the generated water volume fraction φ (Eq. 5) in air can be calculated with the GUM [66, 67] workbench software based on the uncertainties of p s and t s, plus additional information about the temperature inhomogeneity in the thermostat bath of 2 mK, the resolution of the p and T sensors (0.1 hPa, 0.1 mK), the estimated uncertainties of the Sonntag equation (Eq. 3) and the enhancement factor in air.
The performance of this PTB-PHG is regularly validated via periodic, international intercomparisons using a highly stable dew point hygrometer as a transfer standard. Recent comparisons on a European scale were realized in 2004 a comparison called EURAMET.T-K6 covering frost/dew points between −50 and 20 °C [27], in 2005 EURAMET P746 (−20 to 60 °C FP/DP) [29] and in 2010 EURAMET.T-K8 (DP 30–95 °C)(in preparation). A global key comparison, CCT-K8, in parallel to EURAMET.T-K8, is currently under preparation. The results of such metrological comparisons are reviewed and refereed by the so called calibration and measurement capabilities (CMC) review group, within the international metrology organization [Bureau International des Poids et Mesures (BIPM), Sèvres, Paris, France). Once accepted, these comparisons lead to an entry in the key comparison database (KCDB) of the BIPM, Paris, which lists on an international scale the proven capabilities of the national metrological institutes. The total DP/FP uncertainty for the generation of humid air using the German PHG described above in the dew point range from −25 to 60 °C is 35 mK, which corresponds to a relative uncertainty of 0.35 % at a generated humidity of 600 ppmv, 0.3 % at 2,000 ppmv and 0.22 % at 20,000 ppmv.
8 Results and discussion
It is important to keep in mind, that SEALDH has a rather generic setup and does not contain any particularly “special” or expensive components compared with our previous 1,370 nm hygrometers. This intercomparison therefore can be seen as a validation of our general configuration and data evaluation procedure. Further, our previous campaigns such as [25] or [48] may also be some indication for the longtime stability of the method and setup. Due to the almost permanent utilization of PTB primary generators for calibration purposes, it was up to now not possible to carry out a permanent long-term validation of SEALDH for weeks or months. Of course, this could be done on other non-metrological water vapor sources, but those are significantly less stable and accurate than the PHG, which would reduce the quality of the produced gas stream and increase the measurement uncertainty compared with a PHG intercomparison.
8.1 Optical signal analysis with regard to sensor precision
Typical absorption signals of the dTDLAS instrument during this intercomparison (pre-averaged over 20 individual scans, Δt = 1.4 s) and the spectroscopic model fitted to them are shown for the lowest [in Fig. 4(left, top)] and highest water vapor mixture fractions [in Fig. 4(right, top)]. Depicted below, the absorption profiles are also the residuals between the measured data and the model function [Fig. 4(bottom)]. One obvious difference in the two residuals is the systematic, w-shaped structure for high concentrations (right side) versus the noise-like residual for low concentrations (left) which will be discussed later in the text. The first immediate use of these individual scan profiles and residuals is to derive an estimate on the optical precision of the spectrometer on short time scales of seconds. For this, we compare the local residuals [Fig. 4(bottom)] with the peak absorptions (ODepeak) on the optical density (ODe = −ln(I/I 0)) scale for a very high and a very low H2O concentration. The residual can quantified by the 1σ SD (=ODenoise) averaged over the full scan width GLOBAL. From this, we derive the signal-to-noise ratio (SNR) where the signal is defined as the ODepeak value and noise is defined by the statistical standard deviation of the residual and thus equals to ODnoise. At 600/19,950 ppmv, this results in a SNR of 140 respectively 4,133 (see Table 1). This treatment is based on the assumption that changes in the line ODepeak which are smaller than the ODnoise can be clearly detected. Dividing the concentration measured with this scan by the SNR, we can derive the noise equivalent concentration (NEC) at the respective concentration level, which is for the above cases 4.3 and 4.8 ppm at 600/19,950 ppm. In order to allow a performance comparison with other spectrometers with other path lengths and/or measurement bandwidths, it is common to normalize the noise equivalent concentration (NEC) with respect to the path length and the square root of the temporal bandwidth, which yields in our case 5.6 ppmv m Hz−½, respectively 6.6 ppmv m Hz−½ at 600/19,950 ppmv. This approach assumes that the spectrometer is quite linear and the residuum structure is dominated by white noise (which might not be the case for high concentrations, see later discussion).
Typical preprocessed absorption signals (after baseline, offset and transmission correction) for the lowest (left) and highest (right) water vapor ratio during the validation at national primary standard. Twenty individual raw scans were pre-averaged for each absorption profile. The laser modulation frequency was 140 Hz at 100 mA current tuning of the 1.4-μm DFB Laser, resulting in a time per data point of 1.4 s. The maximum residuum deviation (MRD) for characterizing the line shape deficits and absorption signal deviations is defined as shown (bottom, right). The global residual variance 1σ global is defined as the SD of the residual over the entire fit range. The local residual variance 1σ local is defined in the small shaded box on the bottom right panel. It should be noted that the four vertical axes have different scales; all parameters are discussed in the text
Since the structure of the residual at least for the low 600 ppmv scan is quite stable on the wavenumber scale and not purely statistical, i.e., noise and mainly consists of small, stable fringes, the above assumption leads to conservatively estimated lower limits for SNR and NEC.
Although high temporal resolution and high precision of the sensor were not necessarily required for the quasi-static comparison at the high-accuracy primary generator, it is nevertheless useful for the evaluation of the sensor stability to investigate the temporal sensor behavior on longer time scales and to extract the best possible precision which is achievable via pure scan averaging. To investigate these parameters, it is common to derive the Allan variance of the spectrometer [68, 69], which we did using a 45 min “constant” concentration time series [48]. From this data, we derived an optimal precision of 0.160 ppmv when 41 pre-averaged scans are further averaged (i.e., 820 single scans in total). In this configuration, this is equivalent to a time resolution of 58 s and to a normalized precision of: 2.4 ppmv m Hz−½. If we would remove the data throughput limitations of the spectrometer (caused by the sample frequency limit of 140 kS/s of this DAQ-version) and capture not only 10 % of all scans but 100 %, we would expect to see an improvement of (10½), i.e., about a factor of three to 0.75 ppmv m Hz−½. Although this would have been possible, we kept this DAQ configuration (despite an available improved solution) in order to maintain the comparability with the hygrometer used in the same configuration in the previous blind intercomparison [48] at the research center of Jülich. Thereby, we can also link our results from the comparison with the less well-characterized Lyman-alpha reference used in [48] with deviations to the highly defined values of the primary standard by using SEALDH as a quasi-transverse standard.
8.2 Side-by-side comparison: SEALDH versus. PTB-PHG
The direct comparison between SEALDH and PTB-PHG was realized with the same sensor configuration as before. The SEALDH instrument was connected to the primary standard via stainless-steel pipes and compared at nine different concentration levels. In order to minimize sampling artifacts related to a possible H2O adsorption onto the piping and the cell, which could falsify the absolute values in a static cell arrangement, the comparison had to be done in a steady flow configuration. The gas flow was kept constant at a rather high value of about 3.5 standard liters per minute (std l/min). All pressure and temperature sensors in the PHG and in SEALDH were calibrated with respect to primary references. All raw data were measured in a consecutive fashion over a continuous 4-day period without repeating any measurements and any immediate data analysis. The data therefore already indicate the instrument stability over at least several days to a week.
The applied post-evaluation strategy generated a situation similar to a blind intercomparison, in the sense that no information from the generator could be “fed into” the TDLAS data analysis.
The typical performance and short-term stability of the dTDLAS setup are depicted for a 20-min time slice (Fig. 5) at a water vapor concentration of about 7,500 ppm. Also depicted is the associated temporal evolution of the measured pressure and temperature in the cell and the water vapor molar fraction determined with the dTDLAS SEALDH instrument. The signal-to-noise levels of the gas temperature and gas pressure in SEALDH’s cell (defined as measurand average divided by the 1 sigma standard deviation of the measurand over the time slice), which are both used in the data evaluation of the TDLAS signal, were 7,970 for T and 4,220 for p. The dTDLAS concentration signal shows a similarly high signal-to-noise level of 3,140. The histogram of the dTDLAS signal [Fig. 5(bottom)] fits quite well into a Gaussian distribution with a standard deviation of 2.35 ppmv and justifies therefore the reduction to a simple mean value with its standard deviation. The same procedure was used at all other concentration levels to determine average and SD (note: no drift removal like for instance in the AQUAVIT comparison [25] was neither needed nor used here).
Temporal behavior of the SEALDH pressure and temperature (top panel) as well as the laser-derived H2O concentration mid panel) at a constant generator level near 7,500 ppmv. The temperature is measured directly in the white-cell using a built-in thermocouple, the pressure directly at the outlet of the cell. The histogram of the laser-derived H2O signals (bottom) shows the distribution of the individual measured SEALDH sample points (Δt = 1.4 s); the Gaussian fit matched to the distribution indicating a signal to noise of over 3,000
The entire data set of the comparison and all preparatory experiments was taken within a week. Instability effects of the instruments over periods of up to a week are therefore already contained in the intercomparison results, even though they are difficult to extract. As we work with a primary reference, i.e., the PHG of PTB, it is obvious that there are no superior validated devices available (only other—comparable—national primary reference generators which have already compared with the PHG during metrological key comparisons). Hence, there is no possibility to measure and absolutely quantify the stability and accuracy of the primary generator. From the comparison of SEALDH to the PHG, we can only derive the total uncertainty/drift of both devices and not the uncertainty/drift of one of them alone. To determine the stability over longer time spans (weeks, months, years) was outside the scope and possibilities of this study as this would require not only a very high workload but also almost unlimited access to the PHG. This is impossible at any national PHG as it is, e.g., is needed for legal metrology (i.e., tracing back other instruments to the national humidity scale). Longer-term stability studies therefore require either permanent access to the PHG (not possible), a dedicated PHG to stability studies (too expensive), or a new analytical or preparative gold standard (unavailable). The stability analysis numbers generated here therefore always have to be seen under the premise that a certain difficult to determine amount of noise and drift comes from the generator itself and not purely from the dTDLAS device.
To get some indication on the longer-term stability, we therefore used the longest consecutive data set under constant conditions at the generator. This was a 10-h long record taken overnight at: 3,921.35 ± 0.65 ppmv (1σ) water vapor, 1,011.0 ± 0.3 hPa gas pressure, and 24.66 ± 0.1 °C (1σ) gas temperature. All distributions were Gaussian shape, but had small long-term variations (drifts) due to changing laboratory and PHG conditions. After removing these variations for temperature (0.5 K) and pressure (1.2 hPa), the 1-sigma deviations of the remaining noise-like fluctuations were reduced to ±0.010 K and ±0.009 hPa, respectively. The SEALDH concentration was measured with a temporal resolution of 7 ms and (to save disk space) pre-averaged to a response time of about 3.5 s. Using this data, we computed the Allan variance of SEALDH over 10 h: The noise leg of the Allan deviation improved until 0.01 h = 36 s maximum averaging time and delivered an optimal variance of 120 ppbv (or a signal to noise of 3,921/0.12 = 32,700). After this, the “drift leg” rose and reached a maximum of 380 ppbv at 0.2 h (=12 min) of averaging. Even later, the Allan deviation kept oscillating around an average value of 380 ppbv (or a signal to noise of 3,920/0.38 = 10,300) due to small regulation cycles (2 ppmv) of the PHG for up to 10 h of averaging .
For the comparison exercise, we selected only those “late” time slices, where the dynamic behavior of the PHG or SEALDH wasn’t visible, so that the concentration measured with SEALDH was assumed to be stable. A waiting period of at least 15 min was applied after each concentration change in order to avoid the typical drifts toward the new equilibrium level after each level change in the generator. Such concentration—“instabilities” can be caused by adsorption/desorption or other sampling effects in the PHG, the transfer pipe, or in the SEALDH instrument (Fig. 6). During all measurement, SEALDHs cell and pipes were not actively heated, so that the dew point at room temperature (about 20 °C) defined the upper limit for a reliable sample gas transfer to about 20,000 ppmv at 950 hPa, corresponding to a dew point of 16 °C. Higher values than a DP of 16 °C were not studied in order to avoid the risk to cause possible errors by condensation in the gas transfer system.
Equilibration behavior of the SEALDH–PTB-PHG system: Relative SEALDH concentration levels normalized to the reference value given by the PTB-PHG (=100 %). The primary standard is set to a desired (H2O) value by changing pressure and temperature in the saturator. Generator, piping, and SEALDH instrument require a settling time after each level change. Only the flat, constant areas were used for comparison to avoid any dynamic artifacts. All the measurements are done at one 4 days PHG operation time slot, with an evaluation several weeks later—no testing, adjustments and in particular calibration was done during the PHG operation time in order to operate the instrument in the same way as it is used in a field application and at a blind intercomparison, such as [42]. One of the comparison targets was to validate the actual state of methodology for calibration-free TDLAS, and thereby no special activities were done compared with our other campaigns
The typical equilibration dynamics of the dTDLAS measurands for some selected (H2O)-levels are plotted in Fig. 6. To allow an easy intercomparison between the individual concentration levels, we plotted the SEALDH concentration on a relative scale, i.e., the respective value preset by the generator is shown as the 100 % level. The red horizontal bars indicate the section of the TDLAS data used for further comparison with the PHG.
Figure 7 shows the SEALDH versus PTB-PHG correlation plot over the full range of this intercomparison. All individual data points (about 150–250)—selected according to the procedure described above—are plotted, but are difficult to discern due to the low scatter in the SEALDH data. Uncertainty bars of 4.3 % (see discussion further above) are indicated on each concentration level. The insets show enlarged sections of the scatter plot for two individual H2O levels in order to depict the distribution of the individual measurement points. It has to be noted that the black line in Fig. 7 does not show a linear fit to the correlation but only indicates the ideal 1:1 correlation. The 1:1 line (within the range of the uncertainty bars) coincides well with the measured data over the entire accessible range of the national primary standard from 600 ppmv up to 20,000 ppmv. A linear fit to the SEALDH–PTB-PHG correlation data yields a slope of 0.99554, an intercept of −54 ppmv and a Pearson correlation coefficient of r = 0.99998, which nicely shows the absolute accuracy and excellent linearity of the SEALDH sensor, taking into account that SEALDH has never been calibrated by comparison with another humidity reference.
SEALDH’s linearity over the accessible range of the national primary standard from 600 ppmv up to 20,000 ppmv. The upper limit, corresponding to 16 °C dew point, results from the condensation prevention in SEALDHs measurement cell. SEALDH’s relative uncertainty of 4.3 % is plotted as error bars. The drawn line shows the 1:1 bisector, a linear fit results in a slope of 0.99554, an intersection of −54 ppmv with a Pearson correlation coefficient r = 0.99998. (Signal-to-noise ratios at each concentration level are shown in Fig. 8)
In Fig. 8, we plot for each individual concentration level the relative deviation between SEALDH and the PHG in order to investigate the systematics behind the local concentration-dependent deviations of SEALDH with respect to the PHG. As in Fig. 7, all individual data points are plotted thereby depicting the little scatter and the high precision of the SEALDH data. The maximum relative deviation occurs at 1,000 ppmv (−2.5 %), the lowest at 600 ppmv (−0.2 %). Linearly averaging all individual “local” deviations, we derive an average deviation of −1.45 % over all concentration levels. The scatter of the individual data points at each H2O level is obviously much smaller than the deviation to the reference, which is an indication for systematic deviations in the data. On a first glance, one might explain the deviation at 600 ppmv with a “measurement error” and claim for the rest a quite linear concentration-dependent effect. But such a “simple explanation”, we think, falls short.
Overall compilation of the relative deviations between SEALDH and primary standard. All values agree within SEALDH’s uncertainty range of 4.3 %. The average deviation to the reference is −1.45 %. Triangle in red show the mean value, bars in blue represent the range of scatter on this relative scale. The trend of the deviation in dependence to the concentration level is discussed in the text in detail. Also stated in the figure are at each concentration level the 1σ signal-to-noise ratio (SNc 1σ ) of the concentration values, which range from 984 (at 600 ppmv) to 3,140 (at 6,000 ppmv)
Instead, we have a closer look at the shape of the fit residuals of the individual scans, e.g., like the one depicted in Fig. 4(bottom, right), and define the three following quantities:
(A) 1σ global, the global optical noise, as the standard deviation over the entire fit residual in the fitting range;
(B) 1σ local, the local optical noise, as the standard deviation in a segment of the residual chosen in a way, so that the obvious “w-shaped” deviations related to the absorption line (shape) are minimized. And
(C) MRD, the m aximum r esiduum d eviation, as the peak to peak deviation [see Fig. 4(bottom, right)] in the “W” shaped structure, which is obviously strongly correlated with the position and strength of the absorption line.
All quantities are correlated in different ways with the slight imperfections in the fitted absorption profiles in Fig. 6 and thus presumably also with the imperfections of the correlation plot in Figs. 7 and 8. Looking at Fig. 4: 1σ global, includes all disturbances (optical baseline, optical noise, electrical noise, line shape, etc.) visible in the individual fit residual and therefore is not a good choice to investigate the cause of the systematic effects in Fig. 8. In contrast, 1σ local represents solely the optical and electrical noise. It includes optical baseline noise but is supposed to have no contributions from the absorption line shape.
The H2O-concentration dependence of the three σ-quantities is depicted (Fig. 9): 1σ local remains relatively constant with the rise in H2O, while the two other indicators are strongly correlated with (H2O). As expected, MRD and in weaker extent also 1σ global show the anticipated rise with the concentration (Fig. 9). 1σ global mixes the MRD with other effects contained in 1σ local and therefore increases similarly but slower. 1σ local, however, which contains a small fringe structure and uncorrelated “noise” [Fig. 4(bottom)] has no linkage to (H2O) and remains constant, with a negligible increase from the minimum 4.17 × 10−4 OD at 600 ppmv to its maximum 4.21 × 10−4OD at 19,950 ppmv.
Analysis of the contribution to the fit residuals (details and definitions see text and Fig. 4). The defined signal analysis parameter shows that the local 1σ residual variance is concentration independent, the maximum residuum deviation (MRD) is rising due line shape deviations, detector nonlinearity, etc. The global 1σ residual variance includes the MRD as small influence and therefore increases slower
Since all measurements were carried out at near identical pressures, the peak absorbance of the absorption line (ODepeak) is linearly correlated with the concentration. MRD is dominated by the contributions from the absorption line, but has the local noise contributions still included. Therefore, we subtract 1 − σ local from the MRD with the intention to remove this noise/fringe contribution. In order to derive the relative strength of the line profile “deficits,” we reference the difference (MRD − 1σ local) to the peak optical density (ODepeak), which then yields a new derived quantity: MRDrelative.
This allows—as shown in Fig. 10—to directly compare the line shape related deviations in the absorbance space with the deviations in concentration space. Comparing MRDrelative from Fig. 10 with the relative H2O concentration deviations (Fig. 8) shows a very similar shape and amplitude. And going one step further by plotting the relative concentration deviation versus the MRDrelative (Fig. 11) reveals indeed a quite linear correlation.
Concentration dependence of the “relative MRD”. The ratio between the MRD corrected by the 1σ local residual and the peak absorbance (ODe) shows a very similar trend as the relative deviation between SEALDH and primary standard (Fig. 8)
It is important to keep in mind that the contributions to the uncertainty budget from, e.g., line strength and path length accuracy could already explain the absolute value deviations, but those contributions cannot explain the concentration dependence in the “shape” of the deviations.
The good correlation between Figs. 8 and 10 supports the assumption that the (systematic) deviations between SEALDH and the PTB-PHG are dominantly caused by a spectrometric effect, which we think are line shape problems.
An obvious next step, to remove these systematic deviations, would be therefore be, to include higher-order line shape (hoLS) models like Galatry profiles [70] in the fitting model. However, this hoLS models also introduce additional degrees of freedom (e.g., a diffusion coefficient) which, if fitted freely, can significantly reduce the stability of the fit and cause increased scatter in the (H2O) data. Our dTDLAS concept and approach, however, requires in order to yield the demonstrated performance (A) a clear physical explanation for all fitting parameters and (B) that any additional fitting parameters are to be tied via an analytical function to a measurement parameter (e.g., pressure and/or temperature). These two conditions are currently not possible to fulfil due to missing hoLS spectral line data. hoLS therefore first require detailed studies to determine spectral line parameters (which are currently underway see: www.eumetrispec.org) as well as the design of new fitting procedures. All this was out of the scope of this work. In addition, the use of hoLS models would compensate the “w-shaped” deviations (Fig. 4) which may be related to Dicke narrowing. In the near future, more detailed spectra analysis can be done with these improved line parameters to separate and clarify the different contributions to this shape deviation. Furthermore, the high complexity of the hoLS fits is expected to reduce data evaluation speed significantly. This would be acceptable for a PHG intercomparison but quite likely not for a transfer into a field application like high-speed measurements on airplanes.
Finally, we intend to transfer the dTDLAS concept in the future also to field and airborne applications, for which a higher degree of robustness and rapid data evaluation capabilities is essential. An increase of the absolute accuracy should (in our philosophy) thus not come at the expense of stability and robustness. The current average accuracy of −1.45 % would be an excellent value for the above-mentioned applications, in particular taking into account that the instrument is uncalibrated and that the instrument is—via the comparison with the PHG—to our knowledge—the first hygrometer whose absolute accuracy can be linked to a metrological primary humidity standard.
Certainly, it is possible to use the correlation in Fig. 11 to correct the described deviations. (Alternatively we could always improve the performance by “calibrating” SEALDH, as we already described in [48]). By use of the correlation in Fig. 11 to correct the deviations, we can improve the absolute accuracy of SEALDH leveraging on the higher accuracy of the primary standard and reduce the local maximum deviation from the PHG reference value to 0.41 % and the average SD (1σ) over all measuring points to only 0.21 %. However, we also have to note that such a procedure would contradict our calibration-free approach as it ties the SEALDH performance to the PHG, which might then require not only regular but also possibly frequent comparisons of SELADH with the PHG. Such a referencing process would be impossible to achieve under field conditions, so that we would take it into account only for lab studies and not for a field application.
9 Conclusion and outlook
Tunable diode laser absorption spectroscopy (TDLAS) is often used in applications where a fast, accurate, and precise gas analysis in combination with a compact and robust setup is needed. A particularly interesting question for such applications, but also in a metrological sense when talking about the transfer standard capabilities of dTDLAS, is, what accuracy dTDLAS can achieve if initial and regular calibrations using gas standards are omitted and a calibration-free evaluation as described above is used. dTDLAS thereby might lose some accuracy, possible via a gas calibration, but in exchange it would allow to avoid the typical dependencies caused by calibration processes, which are particularly for water vapor quite cumbersome and complex due to the caveats of handling water vapor standards. These complexities can also limit the accuracy of calibrated hygrometers or at least cause significant work load, and/or significant costs, as well as sensor down time.
We present in this paper the first successful side-by-side comparison of an absolute, non-calibrated dTDLAS hygrometer—the SEALDH instrument—with a primary reference water vapor generator (PHG). By analyzing the extremely accurately humidified air samples provided by the PHG with a dTDLAS instrument, we investigated the deviation between SEALDH and the PHG. The maximum relative deviation was found to be −2.5 % at 1,000 ppmv, the lowest deviation was −0.2 % at 600 ppmv. The average relative deviation over all concentration levels in a concentration range from 600 to 19,900 ppmv was −1.45 %. This performance was achieved without any previous calibration of SEALDH and in a situation similar to a blind intercomparison, i.e., without any testing before and during the intercomparison campaign.
We also showed that the SEALDH deviation from the PHG can be consistently related to certain structures in the fit residuals caused by the imperfections of the Voigt line shape model used to evaluate the measured absorption profiles. This results in the promising perspective of increasing SEALDH’s absolute accuracy even further into the sub-percent range, either by using the quantified deviations of the Voigt shape as a correction factor, or by including a fully characterized higher-order line shape model in the fitting process. Requirements for the latter, however, are that the hoLS models needed for the removal of the residual can be integrated in the software without increasing the number of the degrees of freedom and that the needed spectral line data and their pressure and temperature dependence can be measured with sufficient accuracy.
A perspective target of a fully traceable, cal-free dTDLAS hygrometer with further significant uncertainty reduction would resemble at least an excellent transfer standard and if suitable for field applications a very interesting hygrometer concept. To enable that, this would require in the future a traceable determination of the dynamic tuning, which basically could be realized by characterizing the diode lasers tuning behavior via comparison with a frequency comb referenced to an atomic clock. Further, the performance of future dTDLAS transfer standards would require the availability of highly accurate, traceable spectral data, which are targeted in the recently started “EUMETRISPEC” (www.eumetrispec.org) initiative at PTB, which also targets the generation of the traceable H2O line data using a metrological qualified FTIR facility.
Certainly, it still possible to directly calibrate a dTDLAS sensor like SEALDH (if needed or desired) or to validated it, e.g., by an on-site calibration setup like [71]. Making use of the above data at the PHG for a calibration of SEALDH, in the calibrated case further increased the absolute accuracy of SEALDH by a factor of almost seven, well into the sub-percent range (0.21 % relative average deviation to the PHG). However, despite this excellent value, we think that the uncalibrated performance is good enough for numerous filed applications. The concept of using uncalibrated, highly stable dTDLAS sensors with a we call it “calibration-free” or first-principles evaluation is certainly not only attractive for metrological purposes, serving as a transfer standard, but also for many applications in industry or, e.g., the environmental sciences, where the substantial savings in cost, man power, complexity, or sensor down time—possible by using our uncalibrated dTDLAS configuration—could allow many innovative approaches. Of course, the long-term stability of such uncalibrated sensors would have to be validated in the future in time scales of many months to years in order to foster such applications. A first step with a very rigorous validation at one of the highest quality water vapor standards has been successfully described in this paper.
Notes
Note: Some community often uses the term “in situ” for airborne sensors, to indicate that the sensor is “brought” to the atmospheric compartment to be analyzed and to discern this situation from remote sensing instruments like lidar, satellites or ground-based FTIR and from air sampler based instruments, whose collected gases are subsequently analyzed in the laboratory. Nevertheless, in most cases these “in situ” sensors use extractive sampling and behave quite different to an open-path in situ sensor.
References
M. Zöger, A. Afchine, N. Eicke, M.-T. Gerhards, E. Klein, D.S. McKenna, U. Mörschel, U. Schmidt, V. Tan, F. Tuitjer, T. Woyke, C. Schiller, Fast in situ stratospheric hygrometers: A new family of balloon-borne and airborne Lyman photofragment fluorescence hygrometers t VUV-A. J. Geophys. Res. 104(D1), 1807–1816 (1999)
C. Schulz, A. Dreizler, V. Ebert, J. Wolfrum, Combustion diagnostics, in Handbook of Experimental Fluid Mechanics, ed. by C. Tropea, A.L. Yarin, J.F. Foss (Springer, Berlin, 2007), pp. 1241–1316
J. Wolfrum, T. Dreier, V. Ebert, C. Schulz, Laser-based combustion diagnostics, in Encyclopedia of Analytical Chemistry, 2nd edn., ed. by R.A. Meyers (Wiley, Chichester, 2011), p. 33. doi:10.1002/9780470027318.a0715.pub2
J.A. Silver, Frequency-modulation spectroscopy for trace species detection: theory and comparison among experimental methods: errata. Appl. Opt. 31(24), 707–717 (1992)
J. Reid, D. Labrie, Second-harmonic detection with tunable diode lasers—comparison of experiment and theory. Appl. Phys. B 26(3), 203–210 (1981)
V. Ebert, J. Wolfrum, Absorption spectroscopy, in Optical Measurements—Techniques and Applications, 2nd edn., ed. by F. Mayinger, O. Feldmann (Springer, München, 2001), pp. 227–265
M.R. Sargent, D.S. Sayres, J.B. Smith, M. Witinski, N.T. Allen, J.N. Demusz, M. Rivero, C. Tuozzolo, J.G. Anderson, A new direct absorption tunable diode laser spectrometer for high precision measurement of water vapor in the upper troposphere and lower stratosphere. Rev. Sci. Instrum. 84(7), 074102 (2013). doi:10.1063/1.4815828
A.K. Vance, A. Woolley, R. Cotton, K. Turnbull, S. Abel, C. Harlow, Final report on the WVSS-II sensors fitted to the FAAM BAe 146. Met Office, no. November, pp. 0–31, 2011
J. Silver, D. Hovde, Near-infrared diode laser airborne hygrometer. Rev. Sci. Instrum. 65(5), 1691–1694 (1994)
C. Webster, G. Flesch, K. Mansour, Mars laser hygrometer. Appl. Opt. 43(22), 4436–4445 (2004)
W. Gurlit, R. Zimmermann, C. Giesemann, T. Fernholz, V. Ebert, J. Wolfrum, U.U. Platt, J.P. Burrows, Lightweight diode laser spectrometer ‘CHILD’ for balloon-borne measurements of water vapor and methane. Appl. Opt. 44(1), 91–102 (2005)
G.S. Diskin, J.R. Podolske, G.W. Sachse, T.A. Slate, Open-path airborne tunable diode laser hygrometer. Proc. SPIE 4817, 196–204 (2002)
M. Zondlo, M.E. Paige, S.M. Massick, J. Silver, Vertical cavity laser hygrometer for the National Science Foundation Gulfstream-V aircraft. J. Geophys. Res. 115(D20) (2010). doi:10.1029/2010JD014445
J. Podolske, G. Sachse, G. Diskin, Calibration and data retrieval algorithms for the NASA Langley/Ames Diode Laser Hygrometer for the NASA transport and chemical evolution over the Pacific (TRACE-P) mission. J. Geophys. Res. 108(D20), 8792 (2003). doi:10.1029/2002JD003156
B. Lins, R. Engelbrecht, B. Schmauss, Software-switching between direct absorption and wavelength modulation spectroscopy for the investigation of ADC resolution requirements. Appl. Phys. B 106(4), 999–1008 (2012). doi:10.1007/s00340-011-4827-2
V. Ebert, T. Fernholz, H. Pitz, In-situ monitoring of water vapour and gas temperature in a coal fired power-plant using near-infrared diode lasers, in Laser Applications to Chemical and Environmental Analysis, pp. 4–6 (2000)
S. Hunsmann, K. Wunderle, S. Wagner, Absolute, high resolution water transpiration rate measurements on single plant leaves via tunable diode laser absorption spectroscopy (TDLAS) at 1.37 μm. Appl. Phys. B 92(3), 393–401 (2008). doi:10.1007/s00340-008-3095-2
B.A. Paldus, A. Kachanov, An historical overview of cavity-enhanced methods. Can. J. Phys. 83(10), 975–999 (2005)
D. Atkinson, Cavity ring-down spectroscopy: techniques and applications. J. Am. Chem. Soc. 132(13), 4972 (2010)
A. Farooq, J. Jeffries, R. Hanson, In situ combustion measurements of H2O and temperature near 2.5 μm using tunable diode laser absorption. Meas. Sci. Technol. 19(7), 075604 (2008). doi:10.1088/0957-0233/19/7/075604
R. Mihalcea, D. Baer, R. Hanson, Diode laser sensor for measurements of CO, CO2, and CH4 in combustion flows. Appl. Opt. 36(33), 8745–8752 (1997)
T. Peter, C. Marcolli, P. Spichtinger, T. Corti, When dry air is too humid. Science 314, December (2006)
A. Mangold et al., Intercomparison of water vapour detectors under field and defined conditions, in EGS-AGU-EUG Joint Assembly, vol. 1 (2003)
A. Hoff, WVSS-II assessment at the DWD Deutscher Wetterdienst/German Meteorological Service Climate Chamber of the Meteorological Observatory Lindenberg, Deutscher Wetterdienst, September 2009
D. Fahey, R. Gao, Summary of the AquaVIT water vapor intercomparison: static experiments. https://aquavit.icg.kfa-juelich.de/WhitePaper/AquaVITWhitePaper_Final_23Oct2009_6MB.pdf, 2009
O. Möhler, O. Stetzer, S. Schaefers, C. Linke, M. Schnaiter, R. Tiede, H. Saathoff, M. Krämer, A. Mangold, P. Budz, P. Zink, J. Schreiner, K. Mauersberger, W. Haag, B. Kärcher, U. Schurath, Experimental investigation of homogeneous freezing of sulphuric acid particles in the aerosol chamber AIDA. Atmos. Chem. Phys. 3(1), 211–223 (2003). doi:10.5194/acp-3-211-2003
M. Heinonen, A comparison of humidity standards at seven European national standards laboratories. Metrologia 39, 303–308 (2002). doi:10.1088/0026-1394/39/3/7
M. Heinonen, M. Anagnostou, S. Bell, M. Stevens, R. Benyon, R. A. Bergerud, J. Bojkovski, R. Bosma, J. Nielsen, N. Böse, P. Cromwell, A. Kartal Dogan, S. Aytekin, A. Uytun, V. Fernicola, K. Flakiewicz, B. Blanquart, D. Hudoklin, P. Jacobson, A. Kentved, I. Lóio, G. Mamontov, A. Masarykova, H. Mitter, R. Mnguni, J. Otych, A. Steiner, N. Szilágyi Zsófia, D. Zvizdic, Investigation of the equivalence of national dew-point temperature realizations in the −50 °C to +20 °C Range. Int. J. Thermophys. 33(8–9), 1422–1437 (2011). doi:10.1007/s10765-011-0950-x
EURAMET Final Report. http://www.euramet.org/fileadmin/docs/projects/746_THERM_final.pdf
R. Busen, A.L. Buck, A high-performance hygrometer for aircraft use: description, installation, and flight data. J. Atmos. Ocean. Technol. 12, 73 (1995)
J.A. Nwaboh, O. Werhahn, P. Ortwein, D. Schiel, V. Ebert, Laser-spectrometric gas analysis: CO2—TDLAS at 2 μm. Meas. Sci. Technol. 24, 015202 (2013). doi:10.1088/0957-0233/24/1/015202
K. Jousten, G. Padilla-Víquez, T. Bock, Investigation of tunable diode laser absorption spectroscopy for its application as primary standard for partial pressure measurements. J. Phys: Conf. Ser. 100(9), 092005 (2008). doi:10.1088/1742-6596/100/9/092005
H.I. Schiff, G.I. Mackay, J. Bechara, The use of tunable diode laser absorption spectroscopy for atmospheric measurements. Res. Chem. Intermed. 20(3), 525–556 (1994). doi:10.1163/156856794X00441
R.D. May, Open-path, near-infrared tunable diode laser spectrometer for atmospheric measurements of H2O. J. Geophys. Res. 103(D15), 19161–19172 (1998)
D. Hovde, J. Hodges, G. Scace, J. Silver, Wavelength-modulation laser hygrometer for ultrasensitive detection of water vapor in semiconductor gases. Appl. Opt. 40(6), 829–839 (2001)
C. Lauer, D. Weber, S. Wagner, V. Ebert, Calibration free measurement of atmospheric methane background via tunable diode laser absorption spectroscopy at 1.6 μm. Laser Applications to Chemical, Security and Environmental Analysis, OSA Technical Digest (CD) (Optical Society of America), vol. LMA2, 2008
O. Witzel, A. Klein, C. Meffert, S. Wagner, C. Meffert, C. Schulz, V. Ebert, VCSEL-based, high-speed, in situ TDLAS for in-cylinder water vapor measurements in IC engines. Opt. Express 21(17), 19951–19965 (2013)
K. Wunderle, S. Wagner, I. Pasti, Distributed feedback diode laser spectrometer at 2.7 μm for sensitive, spatially resolved H2O vapor detection. Appl. Opt. 48(4), B172–B182 (2009)
A.R. Awtry, B.T. Fisher, R.A. Moffatt, V. Ebert, J.W. Fleming, Simultaneous diode laser based in situ quantification of oxygen, carbon monoxide, water vapor, and liquid water in a dense water mist environment. Proc. Combust. Inst. 31, 799–806 (2007). doi:10.1016/j.proci.2006.07.046
V. Ebert, T. Fernholz, C. Giesemann, H. Pitz, H. Teichert, J. Wolfrum, H. Jaritz, Simultaneous diode-laser-based in situ detection of multiple species and temperature in a gas-fired power plant. Proc. Combust. Inst. 28, 423–430 (2000)
V. Ebert, J. Fitzer, I. Gerstenberg, K.-U. Pleban, H. Pitz, J. Wolfrum, M. Jochem, J. Martin, Simultaneous laser-based in situ detection of oxygen and water in a waste incinerator for active combustion control purposes. Proc. Combust. Inst. 27, 1301–1308 (1998). doi:10.1016/S0082-0784(98)80534-1
H.E. Schlosser, T. Fernholz, H. Teichert, V. Ebert, In situ detection of potassium atoms in high-temperature coal-combustion systems using near-infrared-diode lasers. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 58(11), 2347–2359 (2002)
L.S. Rothman, I.E. Gordon, A. Barbe, D.C. Benner, P.F. Bernath, M. Birk, V. Boudon, L.R. Brown, A. Campargue, J.-P. Champion, The HITRAN 2008 molecular spectroscopic database. J. Quantum Spectrosc. Radiat. Transf. 110(9–10), 533–572 (2009). doi:10.1016/j.jqsrt.2009.02.013
S. Hunsmann, S. Wagner, H. Saathoff, O. Möhler, U. Schurath, V. Ebert, Messung der Temperaturabhängigkeit der Linienstärken und Druckverbreiterungskoeffizienten von H2O-Absorptionslinien im 1.4 μm band. VDI Berichte (1959) VDI Verlag, Düsseldorf, pp. 149–164, 2006
R.J. Muecke, B. Scheumann, F. Slemr, P.W. Werle, Calibration procedures for tunable diode laser spectrometers, in SPIE’s International Symposium on Optical Sensing for Environmental Monitoring, pp. 87–98, 1994
K. Wunderle, T. Fernholz, V. Ebert, Selection of optimal absorption lines for tunable laser absorption spectrometers. VDI Berichte 1959, 137–148 (2006)
S. Wagner, M. Klein, T. Kathrotia, U. Riedel, Absolute, spatially resolved, in situ CO profiles in atmospheric laminar counter-flow diffusion flames using 2.3 μm TDLAS. Appl. Phys. B 109(3), 533–540 (2012). doi:10.1007/s00340-012-5242-z
B. Buchholz, B. Kühnreich, H.G.J. Smit, V. Ebert, Validation of an extractive, airborne, compact TDL spectrometer for atmospheric humidity sensing by blind intercomparison. Appl. Phys. B 110(2), 249–262 (2013). doi:10.1007/s00340-012-5143-1
B. Buchholz, N. Böse, S. Wagner, V. Ebert, Entwicklung eines rückführbaren, selbstkalibrierenden, absoluten TDLAS-Hygrometers in kompakter 19 “Bauweise”, in AMA-Science, 16. GMA/ITG-Fachtagung Sensoren und Messsysteme 2012, pp. 315–323, 2012. doi:10.5162/sensoren2012/3.2.3
V. Ebert, H. Teichert, C. Giesemann, H. Saathoff, U. Schurath, Fasergekoppeltes In-situ-Laserspektrometer für den selektiven Nachweis von Wasserdampfspuren bis in den ppb-Bereich. tm—Tech. Mess. 72(1), 23–30 (2004). doi:10.1524/teme.72.1.23.56689
A. Seidel, S. Wagner, V. Ebert, TDLAS-based open-path laser hygrometer using simple reflective foils as scattering targets. Appl. Phys. B 109(3), 497–504 (2012). doi:10.1007/s00340-012-5228-x
O. Witzel, A. Klein, S. Wagner, C. Meffert, C. Schulz, V. Ebert, High-speed tunable diode laser absorption spectroscopy for sampling-free in-cylinder water vapor concentration measurements in an optical IC engine. Appl. Phys. B 109(3), 521–532 (2012). doi:10.1007/s00340-012-5225-0
V. Ebert, In situ absorption spectrometers using near-IR diode lasers and rugged multi-path-optics for environmental field measurements, in Laser Applications to Chemical, Security and Environmental Analysis, Technical Digest (Optical Society of America), vol. WB1, 2006
K. Wunderle, B. Al-Zaitone, I. Pašti, S. Wagner, S. Hunsmann, C. Tropea, V. Ebert, TDLAS-Spektrometer zur räumlich aufgelösten absoluten Wasserdampfbestimmung um akustisch levitierte Einzeltröpfchen. VDI-Berichte 2047, 103–112 (2008)
A. Marenco, V. Thouret, P. Nédélec, Measurement of ozone and water vapor by Airbus in-service aircraft: The MOZAIC airborne program. An overview. J. Geophys. Res. 103(D19), 25631–25725 (1998)
High Altitude and LOng range research aircraft. http://www.halo.dlr.de/, 2012
R. Petersen, L. Cronce, W. Feltz, E. Olson, D. Helms, WVSS-II moisture observations: a tool for validating and monitoring satellite moisture data. EUMETSAT Meteorological Satellite Conference, vol. 22, pp. 67–77, (2010)
P. Mackrodt, A new attempt on a coulometric trace humidity generator. Int. J. Thermophys. 33(8–9), 1520–1535 (2012). doi:10.1007/s10765-012-1348-0
D. Sonntag, Important new values of the physical constants of 1968, vapour pressure formulations based on the ITS-90, and psychrometer formulae. Z Meteorol 40, 340–344 (1990)
D.M. Murphy, T. Koop, Review of the vapour pressures of ice and supercooled water for atmospheric applications. Q. J. R. Meteorolog. Soc. 131(608), 1539–1565 (2005). doi:10.1256/qj.04.94
J. Marti, K. Mauersberger, A survey and new measurements of ice vapor pressure at temperatures between 170 and 250 K. Geophys. Res. Lett. 20(5), 363–366 (1993)
A. Wexler, Vapor pressure formulation for water in range 0 to 100 C. A revision. J. Res. Natl. Bur. Stand. Sect. A: Phys. Chem. 80A(5–6), 775 (1976). doi:10.6028/jres.080A.071
A. Wexler, Vapor pressure formulation for ice. J. Res. Natl. Bur. Stand. Sect. A: Phys. Chem. 81(1), 5–19 (1977)
R.W. Hyland, A. Wexter, Formulations for the thermodynamic properties of the saturated phases of H2O from 173.15 K to 473.15 K. ASHRAE Trans. 89(2A), 500–519 (1983)
L. Greenspan, Functional equations for the enhancement factors for CO2-free moist air. J. Res. NBS: A Phys. Chem. 80A(1), 41–44 (1976)
Evaluation of measurement data—an introduction to the ‘Guide to the expression of uncertainty in measurement’ and related documents. Joint Committee for Guides in Metrology, JCGM no. 104, 2009
BIPM, Evaluation of measurement data—guide to the expression of uncertainty in measurement. Joint Committee for Guides in Metrology, JCGM no. 100, 2008. http://www.bipm.org
D.W. Allan, Statistics of atomic frequency standards. Proc. IEEE 54(2), 221–230 (1966). doi:10.1109/PROC.1966.4634
P. Werle, R. Mücke, F. Slemr, The limits of signal averaging in atmospheric trace-gas monitoring by tunable diode-laser absorption spectroscopy (TDLAS). Appl. Phys. B 57(2), 131–139 (1993)
P. Maddaloni, P. Malara, P. De Natale, Simulation of Dicke-narrowed molecular spectra recorded by off-axis high-finesse optical cavities. Mol. Phys. 06, 749–755 (2010)
A.W. Rollins, T.D. Thornberry, R.-S. Gao, B.D. Hall, D.W. Fahey, Catalytic oxidation of H2 on platinum: a robust method for generating low mixing ratio H2O standards. Atmos. Meas. Tech. 4(10), 2059–2064 (2011). doi:10.5194/amt-4-2059-2011
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Buchholz, B., Böse, N. & Ebert, V. Absolute validation of a diode laser hygrometer via intercomparison with the German national primary water vapor standard. Appl. Phys. B 116, 883–899 (2014). https://doi.org/10.1007/s00340-014-5775-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00340-014-5775-4