Abstract
The facile and cost-effective chemical bath deposition (CBD) method is used to synthesize highly photoactive facet-controlled bismuth vanadate (BiVO4) thin films on glass and stainless steel (SS) substrates. The facet-controlled BiVO4 thin films are synthesized by variation in anionic precursor with fine tuning of chemical bath pH from alkaline to acidic media. The variation of anionic precursor evolves the morphology of BiVO4 from dispersed nanoparticles to faceted microcrystals. Furthermore, the fine-tuning of chemical bath pH leads to the well-defined octahedral BiVO4 microcrystals. Compared to dispersed nanoparticulate BiVO4 photoanodes, the octahedral BiVO4 photoanodes demonstrated superior photocurrent density of 2.75 mA cm−2 (at 1.23 V vs. RHE), good photostability and charge separation efficiency (45.5%) owing to their excellent PEC reaction kinetics. The present study underscores the usefulness of the CBD method for facet-controlled synthesis of semiconducting thin films for different photo-functional applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In recent years, energy crises and environmental pollution have increased market demands for clean energy storage sources that can replace fossil fuels and provide energy when needed. Sustainable hydrogen (H2) production is a key challenge for developing alternative energy systems that provide an environmentally friendly and inexpensive energy supply [1, 2]. Among the numerous renewable energy sources, solar energy has tremendous potential for energy conversion as it is one of the most abundant, cost-free and clean sources [3]. The conversion of solar energy into H2 via the solar-assisted water-splitting process is one of the most promising technologies because of its potential for green and sustainable H2 production [4]. Photoelectrochemical (PEC) water-splitting technology has emerged as an effective approach for producing H2 using semiconducting photoelectrodes [5]. The PEC water-splitting is an environmentally friendly process, because it uses natural sunlight for water-splitting reactions in the presence of semiconductor photoelectrode.
During the past few decades, ZnO and TiO2 have been extensively studied as photoelectrodes in PEC water splitting due to their high chemical stability and non-toxicity [6, 7]. However, their performance is limited owing to the wide bandgap energy (absorb only ultraviolet radiations; < 4% of solar spectrum). Hence, lots of efforts were put forward on narrow bandgap materials, such as doped-metal oxides, metal sulfides and nitrides for improved PEC water splitting [8,9,10,11,12]. Compared with other photocatalysts, BiVO4 with suitable bandgap energy (~ 2.4 eV), highest photostability, high optical absorption coefficient, least photo-corrosion, low toxicity, high theoretical solar-to-hydrogen conversion efficiency and appropriate band structure for water splitting reaction can be the best choice as a photoelectrode for PEC water splitting application [13, 14].
Considering that the photoactivity of semiconductors is highly dependent on their surface structure, the faceted growth of BiVO4 is highly desirable. Such growth can assist the migration of photogenerated electrons and holes to the particular crystal facets, leading to better charge separation and improved quantum efficiency for PEC water splitting [15,16,17]. From this insight, several reports are available on the faceted growth of BiVO4 by various high-temperature chemical methods, such as hydrothermal, urea hydrolysis and solvothermal [18,19,20,21,22,23,24,25]. Compared to other chemical methods, chemical bath deposition (CBD) is an inexpensive, simple and convenient method for large-area coating carried out in air at room temperature with various substrates. It facilitates better orientation of crystallites with improved grain structure with pinhole-free and uniform deposits. Stoichiometry of deposits is highly maintained as basic building blocks are ions instead of atoms. There are no organo-metallic solvents and no toxic or pyrolyzed gases evolved [26]. Hence, intense research interest has been focused on the faceted growth of BiVO4 by CBD method.
In one instance, the chemically deposited BiVO4 thin films displayed faceted growth of BiVO4 with micrometric and square prism particle morphology [27]. Similarly, the BiVO4 photoanodes with exposed (040) facets were fabricated by the modified CBD method in a neutral bath [28]. Moreover, the CBD method reported the decahedron-shaped duel-faceted BiVO4 microcrystals coated on FTO substrates [29]. Though these all are faceted microstructures, their PEC performance is poor. Compared to other faceted microstructures, an octahedral shape with {121} exposed facets is more effective for photo-functional activity [30].
Even though there is one report on the deposition of octahedral BiVO4 microcrystals by CBD; a detailed study on the morphology evolution of BiVO4 from nanoparticulate to faceted by precursor variation is not reported yet [31]. In the present work, facet-controlled BiVO4 thin film photoanodes are synthesized by variation in anionic precursor with fine tuning of chemical bath pH from alkaline to neutral to acidic media without buffer solution. The evolution of physicochemical and visible-light-induced PEC properties of BiVO4 are investigated to probe the effect of change in bath composition.
2 Experimental section
2.1 Materials
Bismuth nitrate pentahydrate (Bi(NO3)3·5H2O), sodium orthovanadate (Na3VO4), sodium metavanadate (NaVO3), ethylene diamine tetra acetic acid (EDTA) disodium salt (C10H14N2Na2O8) and sodium hydroxide (NaOH) were purchased from Sigma-Aldrich and used without further purifications. Stainless steel (SS) substrates and glass slides were used to deposit BiVO4 thin films.
2.2 Synthesis of BiVO4 thin films
The glass substrates were cleaned in an ultrasonic bath using labolene detergent, double distilled water (DDW), acetone, ethanol, and again in DDW. The SS substrates were first cleaned with zero-grade polish paper, followed by washing in acetone, ethanol, and again in DDW. Thoroughly cleaned glass and SS substrates were stored in deionized water before use.
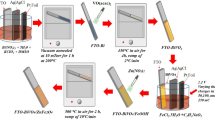
BiVO4 thin films were deposited on well-cleaned glass and SS substrates by the CBD method with a change in anionic precursor and deposition bath pH. A chemical bath for the deposition of BiVO4 thin films was prepared from an aqueous solution of bismuth precursor (Bi(NO3)3·5H2O; 25 mM) complexed with EDTA disodium salt (25 mM) under constant stirring. As another precursor, the aqueous solution of vanadium (Na3VO4 or NaVO3; 25 mM) was prepared. The chemical deposition bath was obtained by dropwise addition of vanadium precursor solution into the bismuth complex under constant stirring. The final pH of the bath was adjusted to alkaline (8.5), neutral (7) and acidic (5.5) with the addition of an aqueous 1 M NaOH solution. After pH maintenance, the bath was heated at 85℃ under constant stirring for 1 h to obtain a transparent pale yellow color solution. Furthermore, the obtained solution was cooled down to room temperature and used as a deposition solution.
The above deposition bath composed of Bi(NO3)3·5H2O and Na3VO4 precursors was named as bath A, and that with Bi(NO3)3·5H2O and NaVO3 precursors was denoted as bath B. The well-cleaned glass and SS substrates were placed in the deposition bath. During the deposition process, the deposition vessel was closed and kept in a water bath at 85 ℃ for 10 h. After the deposition, BiVO4-coated substrates were removed from the bath, washed with DDW and air dried. Afterward, the as-prepared yellowish orange colored BiVO4 thin films were annealed at 400 ℃ for 2 h to get well-crystalline BiVO4 thin films. The schematic representation of the BiVO4 thin film deposition process is shown in Fig. 1. The annealed BiVO4 samples prepared from bath A at pH 5.5, 7 and 8.5 are represented as OAcd, ONut and OAlk, respectively. Similarly, the annealed BiVO4 samples prepared from bath B at pH 5.5, 7 and 8.5 are represented as MAcd, MNut and MAlk, respectively.
2.3 Materials characterizations
The X-ray diffraction (XRD) analysis was used to study the crystal structure and orientation of deposited thin films with the help of Rigaku miniflex 600 Diffractometer (Cu kα radiation: λ = 1.5406 Å). The micro-Raman spectra were collected using a FLEX G spectrometer (Tokyo Instrument, Japan) having an excitation wavelength of 532 nm. The Fourier transform infrared (FT-IR) spectroscopy analysis was used to examine the chemical bonding of BiVO4 (αT Bruker). The oxidation states of elements present on the surface of BiVO4 were investigated with X-ray photoelectron spectroscopy (Thermo VG Scientific Multitab 2000 XPS with Al Kα (1486.6 eV) X-ray source). The morphological features and chemical composition of elements present in the BiVO4 thin films were estimated with scanning electron microscopy (SEM) JEOL JSM-7900 F equipped with energy-dispersive spectroscopy (EDS)-elemental mapping analysis. The ultraviolet–visible diffuse reflectance spectroscopy (UV–Vis DRS) was used to probe the optical properties of BiVO4 thin films using a Jasco spectrometer.
2.4 Electrochemical and photoelectrochemical measurements
The band structure of BiVO4 was estimated from the cyclic voltammetry (CV) measurements performed in sodium perchlorate (NaClO4) electrolyte using CHI650D electrochemical workstation. In three electrode configuration, BiVO4 thin film deposited on SS substrate, platinum plate and Ag/AgCl were utilized as working, counter and reference electrodes, respectively.
The PEC characteristics of BiVO4 photoanodes were studied with linear sweep voltammetry (LSV) and chronoamperometry measurements. All the PEC measurements were carried out with CHI650D electrochemical workstation and standard three-electrode configuration. The three-electrode assembly was composed of BiVO4 thin film deposited on SS substrate, saturated calomel electrode (SCE) and platinum plate as working, reference and counter electrode, respectively. 0.1 M potassium phosphate buffer solution (pH 7) was used as an electrolyte. In addition, during the PEC measurements, 0.2 M sodium sulfite (Na2SO3) was added to the electrolyte solution, which served as a hole scavenger that minimized the rate of recombination of charge carriers and hence increased the PEC performance [32].
The photocurrent of BiVO4 photoanodes was measured under front illumination with a light intensity of 100 mW cm−2 simulated by the AM 1.5 G and 420 nm optical cutoff filters from 35 W xenon lamp. During the PEC experiments, photocurrent was measured by either applying a fixed potential or sweeping the potential to the positive direction with a scan rate of 10 mV s−1. Samples were tested in the chopped illumination arrangement to measure the transient photocurrents. All the electrochemical results were stated against the reversible hydrogen electrode (RHE) scale. Following equations represents the interconversion between potentials vs. RHE and vs. SCE scales [33]:
Applied Bias Photon to Current Efficiency (ABPE):
The applied bias photon to current efficiency (ABPE) was estimated from photocurrent density vs. potential (J–V) measurements assuming 100% Faradaic efficiency with the help of the following formula:
where J represents the photocurrent density, Vbias is the applied potential and Pin is the incident illumination power density at 1 Sun illumination (AM 1.5 G, 100 mW cm−2).
Furthermore, the charge separation efficiency (ƞbulk) of BiVO4 photoanodes was estimated using the following equation [34]:
where \({J}_{{{\text{Na}}}_{2}{{\text{SO}}}_{3}}\) and \({J}_{{\text{abs}}}\) (7.5 mA cm−2) represents the measured photocurrent density in the presence of a hole scavenger and the theoretical photocurrent density of BiVO4, respectively.
3 Results and discussion
3.1 Reaction mechanism for the deposition of BiVO4
Deposition of the thin film via the CBD method is based on the principle of controlled precipitation of desired material over the heterogeneous substrate surface. During the deposition of BiVO4 thin films, the aqueous supersaturated solution of [EDTA–Bi]+1 complex and Na3VO4 or NaVO3 transforms into a saturated state via precipitation of BiVO4.
Initially, the reaction of Bi(NO3)3 with EDTA disodium salt gives [EDTA–Bi]+1 complex (represented in the following reaction), which avoids the spontaneous precipitation in the bath [31]:
On the other hand, the aqueous solution of vanadium (Na3VO4 or NaVO3) was prepared as another precursor. The hydrolysis reaction of vanadium leading various polyoxovanadate (POV) anions, such as layered oxides [V2O5], chain metavanadates [VO3−]n and compact polyanions [V10O28]6− as represented in the following reaction [35, 36]:
The structure of POV anions is concentration and pH dependent [36, 37]. In the above reaction, the hydrolysis ratio (h) increases with the pH, forming aquo, hydroxo, or oxo vanadium species [35, 36]. In previous reports, the structure and stability of POV anions are reported [36, 37].
The chemical deposition bath for BiVO4 was obtained by adding vanadium precursor solution (Na3VO4 or NaVO3) into a [EDTA–Bi]+1 complex solution. As a result, the milky white color of the bismuth solution was changed to a yellowish-orange color and the solution became supersaturated. The final pH of the chemical bath was adjusted to alkaline (8.5), neutral (7) and acidic (5.5) with the addition of aqueous 1 M NaOH solution. At pH 5.5, [V10O27(OH)]5− prominent vanadium species are present in the chemical bath, while [V3O9]3− species are present at pH 7 and 8.5 [36].
Afterward, the chemical bath was kept at an elevated temperature of 85 ℃ with vertically immersed substrates. During this process, continuous deprotonation occurred that led to the transformation of POV anions to oxovanadium anions VO43− [37]. Simultaneously, [EDTA–Bi]+1 complex dissociated by releasing free Bi3+ cations, which then react with VO43− anions present in the chemical bath [31]. Consequently, the solution became saturated, leading to precipitation through the heterogeneous (on the substrate surface) and homogeneous growth of the BiVO4, as represented by the following reaction.
The above reaction is susceptible to the release rate of free Bi+3 cations and deprotonation of POV anions that led to various BiVO4 growth rates from baths A and B. These growth rate variations can cause the microstructural evolution of BiVO4. Typically, ‘bath A’ composed of Na3VO4 and [EDTA–Bi]+1 complex, release faster free Bi+3 cations and deprotonated VO43− anions, due to easily available VO43− anions (\({{\text{Na}}}_{3}{{\text{VO}}}_{4}\to 3{{\text{Na}}}^{+}+{{\text{VO}}}_{4}^{3-}\)), which leads to the rapid formation of BiVO4 [36]. In this process, the nucleation centers and activation sites were maximum; consequently, BiVO4 particle size became smaller and nanoparticulate BiVO4 formed. Conversely, ‘bath B’ composed of [EDTA–Bi]+1 complex and NaVO3, released slow Bi+3 cations and deprotonated VO43− anions, which led to the slow growth of BiVO4. The slow growth of BiVO4 can be attributed to the absence of VO43− anions in the initial state. As a result, it requires time for the formation of VO43− anions through the following reactions: \({{\text{NaVO}}}_{3}+2{\text{NaOH}}\to {{\text{Na}}}_{3}{{\text{VO}}}_{4}+{{\text{H}}}_{2}{\text{O}}\) and \({{\text{Na}}}_{3}{{\text{VO}}}_{4}\to 3{{\text{Na}}}^{+}+{{\text{VO}}}_{4}^{3-}\). This ultimately hinders the growth of BiVO4 [38]. In this process, nucleation centers and activation sites were low, resulting in faceted octahedral growth of BiVO4. Moreover, maintaining the acidic pH of ‘bath B’ further slowed the release of Bi+3 cations and deprotonated VO43− anions, which led to the slow growth of BiVO4. Thus, well-defined octahedral-shaped BiVO4 microcrystal morphology was evolved for the acidic pH of ‘bath B’. Thus, by adjusting the chemical bath composition, one can effectively tune the BiVO4 crystal morphology from nanoparticulate to faceted octahedral morphologies.
3.2 Characterizations of BiVO4
3.2.1 Structural analysis
The crystallographic properties of deposited BiVO4 thin films were probed with the XRD technique, as shown in Fig. 2. All BiVO4 thin films display typical Braggs reflections that match the monoclinic (I2/a) BiVO4 phase (JCPDS no.: 14-0688). Interestingly apparent peak splitting is observed at 19°, 35° and 46° indicating the formation of a monoclinic scheelite BiVO4 phase [39]. In the monoclinic scheelite BiVO4 phase, each bismuth and vanadium ion are co-ordinally bonded to eight and four oxygen atoms, respectively [40]. As represented in Fig. 2c, each bismuth ion is bound to eight VO4 tetrahedral units (Crystallography Open Database card no. 9013437). The estimated lattice parameters a = 5.27 Å, b = 11.60 Å and c = 5.087 Å of BiVO4 are in good agreement with the monoclinic scheelite BiVO4 phase.
Moreover, all the BiVO4 thin films deposited from baths A and B display significant variation in the relative intensities of the XRD peaks. This variation in the relative intensities of the XRD peaks with the highest intensity (121) plane indicates the preferential growth of the BiVO4 crystals. The intensity ratio of (121)/(040) characteristic XRD peaks is calculated and denoted as R, which varies with the synthesis conditions of BiVO4 thin films. The intensity ratio R for the BiVO4 thin films deposited from bath A is 1.63, 1.57 and 3.25 for OAcd, ONut and OAlk samples, respectively. Similarly, R for the BiVO4 thin films deposited from baths B is 6.44, 9.75 and 3.52 for MAcd, MNut and MAlk samples, respectively. This change in R is characteristic of the preferential growth of the BiVO4 crystals. According to variation in R, the BiVO4 thin films deposited from baths A and B display the dispersed nanoparticle and preferentially grown faceted octahedral surface morphology, respectively. According to Scherrer’s formula, the mean crystallite sizes estimated for BiVO4 crystals deposited from baths A and B are 10 nm and 511 nm, respectively.
3.2.2 Chemical bonding analysis
The chemical bonding characteristics of the BiVO4 thin films deposited from baths A and B were probed with Raman spectroscopy, as shown in Fig. 3. The Raman peaks P1 (131 cm−1) and P2 (212 cm−1) at lower frequency regions are assigned to the external rotation or translation modes of BiVO4 [41]. All the BiVO4 samples display typical Raman bands P3 (325 cm−1) and P4 (367 cm−1), corresponding to the asymmetric (Bg symmetry) and symmetric (Ag symmetry) bending modes of the VO4 tetrahedron, respectively [42]. The peaks P5 (710 cm−1) and P6 (826 cm−1) are assigned to the asymmetric (V–O) stretching mode and symmetric (V–O) stretching modes, respectively [41,42,43]. The present Raman features confirm the pure monoclinic scheelite BiVO4 phase deposition from both baths. Furthermore, the functional groups present on the BiVO4 thin film surface were analyzed with FTIR spectroscopy, as shown in Fig. S2 (ESI). The BiVO4 thin films deposited from both baths display distinct IR bands corresponding to the monoclinic scheelite BiVO4 phase, indicating no organic impurities attached to the surface of deposited BiVO4 crystals.
3.2.3 XPS analysis
The XPS technique was used to probe the chemical constituents and oxidation states of elements in BiVO4 thin films. The survey XPS spectra commonly show the spectral features for the Bi, V and O elements in BiVO4 thin films (Fig. S3 ESI). Figure 4 represents the Bi 4f, V 2p and O 1 s core level XPS spectra of BiVO4 thin films deposited from baths A and B. Figure 4a shows the high-resolution Bi 4f XPS spectra of the BiVO4 thin films. Both BiVO4 thin films deposited from baths A and B show intense peak doublet at 164.2 eV and 158.9 eV assigned to the Bi 4f5/2 and Bi 4f7/2, respectively, confirming the Bi+3 state [44]. The V 2p XPS spectra of BiVO4 thin films in Fig. 4b show two broad peaks at binding energies (BEs) of 516.4 and 520.8 eV corresponding to the spin–orbit splitting of V 2p3/2 and V 2p1/2, respectively [45]. These spectral features are characteristic of pentavalent V in BiVO4 thin films. As shown in Fig. 4c, the high-resolution O 1 s XPS spectra of BiVO4 thin films exhibit a broad peak at 529.5 eV with a shoulder at 533.1 eV. The broad peak at 529.5 eV is assigned to the oxygen from the BiVO4 lattice, and the shoulder is attributed to the OH− groups (originating from structural or adsorbed water molecules and oxygen vacancies) on the surface of the sample [44]. The present XPS results clearly show the trivalent and pentavalent states of Bi and V in BiVO4, respectively, which confirms the formation of pure phase BiVO4 from both baths.
3.2.4 Morphological and compositional analysis
SEM analysis was used to investigate the surface morphology of BiVO4 thin films. The anionic precursor variation significantly affected the BiVO4 morphology, as shown in Fig. 5. The BiVO4 thin films deposited from bath A (OAlk) show dispersed particle morphology with an average size of 0.45 µm. In addition, ONut and OAcd samples have similar nanoparticulate morphology as that of OAlk, as shown in Fig. S4 (ESI). On the other hand, that deposited from bath B (MAlk) shows faceted microcrystal morphology with an average size of 5 µm. The present SEM analysis provides strong evidence for the change in morphology from the dispersed particles to facet-controlled BiVO4 thin films by changing the anionic precursor.
Furthermore, the chemical composition of the BiVO4 thin films deposited from baths A and B was probed with EDS analysis, as shown in Figs. S5–S6 (ESI) and Fig. 6. The BiVO4 thin films deposited from baths A and B display uniform distribution of constituent elements [bismuth (Bi), vanadium (V) and oxygen (O)] in the nanometer scale mapping region with a Bi/V ratio of 1. Present results indicate the uniform growth of BiVO4 from both baths without any special phase separation.
As shown in Figs. 5 and 7, all the BiVO4 thin films deposited from bath B display shape-selective octahedral crystals, which agrees with the XRD results showing an intense (121) peak and quenched (040) XRD peaks in these films. The pH of bath B significantly affects the crystal shape and size. The shape of the BiVO4 crystals changes from faceted microcrystals to well-defined octahedra as the pH changes from alkaline to acidic media. The MAlk thin film show coarse octahedral-shaped microcrystals with significantly truncated vertices and blunt edges. On the other hand, the MAcd thin film show well-defined octahedral-shaped microcrystals with mildly truncated vertices and sharp edges. MNut sample has a similar type of octahedral morphology as compared to MAcd sample. In addition, from the XRD data, the R values of MNut and MAcd are 9.75 and 6.44, respectively. Though the MAcd sample displays slightly less R value, it showed compact and sharp BiVO4 microcrystallites. In addition, the size of octahedral microcrystals deposited at acidic pH of 5.5 is reduced compared to that synthesized at alkaline pH. The present result underscores the importance of pH variation for the shape-selective synthesis of BiVO4. Among the three samples, BiVO4 deposited from an acidic bath shows the well-defined facet-controlled octahedral morphology, which can be advantageous for visible light-induced PEC measurements.
3.2.5 Optical analysis
The band structure and optical properties of the BiVO4 thin films are studied with diffuse reflectance UV–Vis spectroscopy. As shown in Fig. 8, the dispersed nanoparticles and faceted BiVO4 thin films display significant absorption in the visible light region, indicating the visible-light harvesting ability of BiVO4 thin films. The electronic structure of monoclinic scheelite BiVO4 comprises valance and conduction bands composed of hybrid Bi 6 s/O 2p and V3d orbitals, respectively [46]. BiVO4 thin films deposited from baths A and B absorb visible light in the wavelength range of < 515 and < 530 nm, respectively, corresponding to the bandgap energies of 2.40 and 2.33 eV. The calculated bandgap energies of BiVO4 thin films deposited from baths A and B are displayed in Table S1 (ESI). The BiVO4 thin films deposited from ‘bath A’ display a blue shift in absorption edge compared to films prepared from ‘bath B’, which is attributed to the smaller size of BiVO4 dispersed nanoparticles deposited from ‘bath A’. The lower bandgap values of BiVO4 thin films deposited from ‘bath B’ are attributed to the growth of facet-controlled micrometer-size octahedral BiVO4 crystals. This type of faceted morphology can assist the easy transport of excitons to the surface of the semiconductor, leading to effective charge separation and improved PEC activity [47].
UV–Vis absorbance spectra of BiVO4 thin films deposited from a bath A [OAcd (i), ONut (ii), OAlk (iii)] and b bath B [MAcd (i), MNut (ii), MAlk (iii)]. Diffuse reflectance UV–Vis spectra (plotted as the Kubelka–Munk function of the reflectance, R) of BiVO4 thin films deposited from c bath A [OAcd (i), ONut (ii), OAlk (iii)] and d bath B [MAcd (i), MNut (ii), MAlk (iii)]
4 Photoelectrochemical measurements
The PEC characteristics of the BiVO4 photoanodes deposited on SS substrates were studied with LSV and chronoamperometry measurements. All the BiVO4 photoanodes exhibited notable photocurrent in potassium phosphate buffer electrolyte, highlighting their functional PEC performance (J–V curves in Fig. S7 in ESI). The photocurrent densities at 1.23 V (vs. RHE) for all BiVO4 photoanodes are summarized in Table S2 in ESI. As shown in Fig. 9a, the BiVO4 thin film photoanode deposited from bath B display higher current density than the nanoparticulate BiVO4 photoanode indicating its improved PEC performance. The variation in the PEC performances of BiVO4 thin films deposited from baths A and B can be ascribed to their distinct morphologies.
As represented in Fig. 9b, the shape-selective BiVO4 thin films display significant variation in PEC performance with bath pH. Among the shape-selective BiVO4 thin films, MAcd thin film exhibited the highest photocurrent density of 2.75 mA cm−2 at 1.23 V (vs. RHE), indicating improved PEC performance. The improved performance of MAcd thin film is attributed to the well-defined compact octahedral BiVO4 morphology with exposed {121} facets, which provides more surface-active facets with abundant active sites for effective PEC reactions. The water oxidation kinetics as well as charge-separation and transfer properties of BiVO4 photoanodes are highly influenced by its crystal facets. From experimental findings it is concluded that, the water oxidation kinetics of BiVO4 photoanode is based on the adsorption and dissociation energy of water molecules on its crystal facets. Theory calculations suggested that, the adsorption of water molecules on the {121} facets of BiVO4 is energetically favorable for successive dissociation and oxidation compared to the {010} and {110} low-index facets [48]. Hence, the present octahedral BiVO4 photoanodes with {121} facets are highly efficient for improved PEC performance. However, the shape-selective MNut and MAlk thin films display lower PEC performance than MAcd, which is attributed to the decreased compactness and sharpness of BiVO4 microcrystallites.
The PEC performance of BiVO4 thin film photoanodes is highly dependent on their thickness. It is observed that; as the pH of deposition bath decreases, thickness of BiVO4 thin film increases. The PEC performance of BiVO4 thin film increases with increase in BiVO4 film thickness. The plot of thickness of BiVO4 thin film with pH variation is shown in Fig. S8 (ESI).
Furthermore, the photocurrent density vs. time (J–t) measurements were used to probe the long-term PEC stability of BiVO4 thin film photoanodes under a constant electrical bias of 1.23 V vs. RHE and illumination. 0.1 M potassium phosphate buffer solution (pH 7) mixed with 0.2 M Na2SO3 hole scavenger was used as an electrolyte [49]. As shown in Fig. 10a, b, all the BiVO4 thin film photoanodes deposited from baths A and B display stable photocurrent density over 60 min with a slight decrease at the initial 5 min. The BiVO4 thin film photoanode deposited with alkaline bath A (OAlk) exhibited stabilized photocurrent density of 0.39 mA cm−2. On the other hand, BiVO4 thin film photoanodes deposited from acidic bath B (MAcd) display the highest stabilized photocurrent density of 2.73 mA cm−2. Present results highlighting the high photostability of BiVO4 thin film photoanodes against applied bias and light illumination.
In addition, the ABPE was calculated for all BiVO4 thin film photoanodes from respective J–V curves obtained and plotted in Fig. 10c, d. The shape-selective BiVO4 thin film photoanodes display a higher ABPE of 0.084% compared to nanoparticulate BiVO4. The improved ABPE of shape-selective BiVO4 thin film photoanodes is attributed to the fast charge transfer kinetics on the {121} crystal facet and quick transport of excitons in well-defined compact octahedral BiVO4 crystals. Furthermore, the charge separation efficiency of BiVO4 photoanodes was evaluated and displayed in Fig. 11. The shape-selective BiVO4 thin film photoanodes display a maximum charge separation efficiency of 45.5% for the MAcd sample.
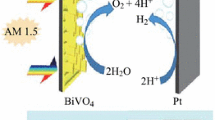
5 Photoelectrochemical reaction mechanism
The band structure of BiVO4 thin film photoanodes was mapped using CV measurements and UV–Vis spectroscopy. The scheme of the PEC reaction mechanism with band structure for BiVO4 thin film photoanodes is displayed in Fig. 12. The lowest edge of the conduction band (CB) can be determined from the onset potential of the reduction peak in the CV curve (Fig. S9 in ESI) [50]. The onset potential of the reduction peak is observed at the − 0.26 V vs. Ag/AgCl corresponds to the 4.39 eV (vs. vacuum). Thus, the lowest edge of the CB is located at 4.39 eV (vs. vacuum). Judging from the bandgap energy (2.33 eV) of BiVO4 thin film photoanodes, the valence band (VB) position is located at 6.72 eV (vs. vacuum) [51]. The mapped band structure of BiVO4 thin film photoanodes indicates that these band positions favor the oxidation of water molecules [52].
Solar energy can be converted into chemical energy in PEC cells via a water-splitting reaction. The overall PEC water splitting can occur via the following half-cell reactions:
When photons having energy equal to or greater than the bandgap energy of BiVO4 \((h\upnu \ge {E}_{gBiV{O}_{4}})\) are incident on it, then electron–hole (e−–h+) pairs are generated. The photogenerated electrons are transferred to the CB, whereas holes are transferred to the VB of BiVO4. After the charge separation, photogenerated electrons are transferred to the surface of the cathode (Pt plate), where a reduction reaction (H2 evolution) occurs, while photogenerated holes are transferred to the surface of the anode (BiVO4), where an oxidation reaction (O2 evolution) takes place [4].
6 Conclusions
The highly photoactive facet-controlled BiVO4 thin film photoanodes are synthesized via a cost-effective CBD method in the present work. The anionic precursor variation leads to the tailoring of the crystal shape of BiVO4 from dispersed nanoparticles to faceted microcrystals. The shape-selective faceted BiVO4 photoanodes display enhanced photoactivity compared to the nanoparticulate type, which clearly underscores the effectiveness of crystal facets control in improving the PEC performance of BiVO4 thin films. The BiVO4 thin film photoanode deposited from an acidic bath exhibited the maximum photocurrent density (2.75 mA cm−2 at 1.23 V vs. RHE), good photostability and charge separation efficiency (45.5%) attributed to its well-defined compact octahedral morphology. Thus, the present results highlight the effectiveness of faceted microstructure for improved photoactivity in PEC water splitting.
Author contributions
SPK: investigation, methodology, writing—original manuscript, YMC: validation, VVM: validation, PDS: formal analysis, SVT: software, SAP: software, UMP: data curation, KVG: writing—review and editing, DBM: resources, AUP: resources, JLG: conceptualization, supervision, funding acquisition, writing—review and editing.
Data availability
The authors declare that the data supporting the findings of this study are available within this paper and its Supplementary Information files.
References
R.M.N. Yerga, M.C.A. Galvan, F.D. Valle, J.A.V. Mano, J.L.G. Fierro, Chem. Sus. Chem. 2, 471–485 (2009)
A.A. Ismail, D.W. Bahnemann, Sol. Energy Mater. Sol. Cells 128, 85–101 (2014)
I. Paramasivam, H. Jha, N. Liu, P. Schmuki, Small 8, 3073–3103 (2012)
W. Yang, R.R. Prabhakar, J. Tan, S.D. Tilley, J. Moon, Chem. Soc. Rev. 48, 4979–5015 (2019)
J. Li, N. Wu, Catal. Sci. Technol. 5, 1360–1384 (2015)
M. Zayed, A.M. Ahmed, M. Shaban, Int. J. Hydrog. Energy 44, 17630–17648 (2019)
H. Magnan, D. Stanescu, M. Rioult, E. Fonda, A. Barbier, J. Phys. Chem. C 123, 5240–5248 (2019)
S. Shen, J. Chen, L. Cai, F. Ren, L. Guo, J. Materiomics 1, 134–145 (2015)
V.M. Aroutiounian, V.M. Arakelyan, G.E. Shahnazaryan, Sol. Energy 78, 581–592 (2005)
S. Chandrasekaran, L. Yao, L. Deng, C. Bowen, Y. Zhang, S. Chen, Z. Lin, F. Peng, P. Zhang, Chem. Soc. Rev. 48, 4178–4280 (2019)
W. Xu, W. Tian, L. Li, RRL Solar 5, 2000412 (2021)
M. Suryawanshi, S.W. Shin, U. Ghorpade, D. Song, C.W. Hong, S.-S. Han, J. Heo, S.H. Kang, J.H. Kim, J. Mater. Chem. A 5, 4695–4709 (2017)
M.G. Tecedor, D.C. Morcoso, R.F. Climent, S. Gimenez, Adv. Mater. Interfaces 6, 1900299 (2019)
Z.F. Huang, L. Pan, J.J. Zou, X. Zhang, L. Wang, Nanoscale 6, 14044–14063 (2014)
G. Xi, J. Ye, Chem. Commun. 46, 1893–1895 (2010)
J. Zhu, F. Fan, R. Chen, H. An, Z. Feng, C. Li, Angew. Chem. 127, 9239–9242 (2015)
G. Liu, Y. Zhu, Q. Yan, H. Wang, P. Wu, Y. Shen, Y. Doekhi-Bennani, Sci. Total. Environ. 762, 143086 (2021)
S.S. Han, J.Y. Park, H.S. Hwang, H.R. Choe, K.M. Nam, E.J. Cho, Chem. Sus. Chem. 12, 3018–3022 (2019)
X. Zhai, Z. Li, Z. Lu, G. Wang, P. Li, Y. Gao, X. Huang, W. Huang, H. Uji-i, G. Lu, J. Colloid, Interface Sci. 542, 207–212 (2019)
Y. Zhang, H. Gong, Y. Zhang, K. Liu, H. Cao, H. Yan, J. Zhu, Eur. J. Inorg. Chem. 2017, 2990–2997 (2017)
H. Li, G. Liu, X. Duan, Mater. Chem. Phys. 115, 9–13 (2009)
G.S. Kamble, Y.-C. Ling, Sci. Rep. 10, 12993 (2020)
I. Khan, A.Z. Khan, A. Sufyan, M.Y. Khan, S.I. Basha, A. Khan, Ultrason. Sonochem. 68, 105233 (2020)
G. Zhang, Y. Meng, B. Xie, Z. Ni, H. Lu, S. Xia, Appl. Catal. B Environ. 296, 120379 (2021)
D. Wang, H. Jiang, X. Zong, Q. Xu, Y. Ma, G. Li, C. Li, Chem. Eur. J. 17, 1275–1282 (2011)
R.S. Mane, C.D. Lokhande, Mater. Chem. Phys. 65, 1–31 (2000)
M.C. Neves, T. Trindade, Thin Solid Films 406, 93–97 (2002)
L. Xia, J. Li, J. Bai, L. Li, S. Chen, B. Zhou, Nano-Micro Lett. 10, 11–21 (2018)
G. Rahman, A. Akhtar, N.A. Khan, S.Y. Chae, A.U.H.A. Shah, O.-S. Joo, Optik 224, 165516 (2020)
M. Han, X. Chen, T. Sun, O.K. Tan, M.S. Tse, Cryst. Eng. Comm. 13, 6674–6679 (2011)
W. Luo, Z. Wang, L. Wan, Z. Li, T. Yu, Z. Zou, J. Phys. D Appl. Phys. 43, 405402–405409 (2010)
T.W. Kim, Y. Ping, G.A. Galli, K.S. Choi, Nat. Commun. 6, 1–10 (2015)
X. Zhong, H. He, M. Yang, G. Ke, Z. Zhao, F. Dong, B. Wang, Y. Chen, X. Shi, Y. Zhou, J. Mater. Chem. A 6, 10456–10465 (2018)
S. Bera, S.A. Lee, W.-J. Lee, J.-H. Kim, C. Kim, H.G. Kim, H. Khan, S. Jana, H.W. Jang, S.-H. Kwon, A.C.S. Appl, Mater. Interfaces 13, 14291–14301 (2021)
J.L. Gunjakar, A. Inamdar, B. Hou, S. Cha, S.M. Pawar, A.A.A. Talha, H.S. Chavan, J. Kim, S. Cho, S. Lee, Y. Jo, H. Kim, H. Im, Nanoscale 10, 8953–8961 (2018)
J. Livage, Coord. Chem. Rev. 178–180, 999–1018 (1998)
Y. Hayashi, Coord. Chem. Rev. 255, 2270–2280 (2011)
W. Sun, M. Xie, L. Jing, Y. Luan, H. Fu, J. Solid State Chem. 184, 3050–3054 (2011)
A.K. Adepu, V. Katta, N. Venkatathri, New J. Chem. 41, 2498–2504 (2017)
T.D. Nguyen, V.H. Nguyen, S. Nanda, D.N. Vo, V.H. Nguyen, T.V. Tran, L.X. Nong, T.T. Nguyen, L.G. Bach, B. Abdullah, S.S. Hong, T.V. Nguyen, Environ. Chem. Lett. 18, 1779–1801 (2020)
T.D. Nguyen, Q.T.P. Bui, T.B. Le, T.M. Altahtamouni, K.B. Vu, D.V.N. Vo, N.T.H. Le, T.D. Luu, S.S. Hong, K.T. Lim, RSC Adv. 9, 23526–23534 (2019)
T.S. Dabodiya, P. Selvarasu, A.V. Murugan, Inorg. Chem. 58, 5096–5110 (2019)
A. Helal, S.M. El-Sheikh, J. Yu, A.I. Eid, S.A. El-Haka, S.E. Samra, J. Nanopart. Res. 22, 132 (2020)
Z. Jiang, Y. Liu, T. Jing, B. Huang, X. Zhang, X. Qin, Y. Dai, M.-H. Whangbo, J. Phys. Chem. C 120, 2058–2063 (2016)
Y. Deng, L. Tang, G. Zeng, C. Feng, H. Dong, J. Wang, H. Feng, Y. Liu, Y. Zhou, Y. Pang, Environ. Sci. Nano 4, 1494–1511 (2017)
K.K. Dey, S. Gahlawat, P.P. Ingole, J. Mater. Chem. A 7, 21207–21221 (2019)
R.A. Rather, A. Mehta, Y. Lu, M. Valant, M. Fang, W. Liu, Int. J. Hydro. Eng. 46, 21866–21888 (2021)
M. Yang, H. He, A. Liao, J. Huang, Y. Tang, J. Wang, G. Ke, F. Dong, L. Yang, L. Bian, Y. Zhou, Inorg. Chem. 57, 15280–15288 (2018)
X. Zhang, B. Zhang, K. Cao, J. Brillet, J. Chen, M. Wang, Y. Shen, J. Mater. Chem. A 3, 21630–21636 (2015)
J.L. Gunjakar, T.W. Kim, H.N. Kim, I.Y. Kim, S.J. Hwang, J. Am. Chem. Soc. 133, 14998–15007 (2011)
K. Saruwatari, H. Sato, T. Idei, J. Kameda, A. Yamagishi, A. Takagaki, K. Domen, J. Phys. Chem. B 109, 12410–12416 (2005)
H. Shi, H. Guo, S. Wang, G. Zhang, Y. Hu, W. Jiang, G. Liu, Energy Fuels 36, 11404–11427 (2022)
Acknowledgements
The authors would like to thank the Science and Engineering Research Board (SERB), a statutory body of the Department of Science and Technology (DST), Government of India, for awarding the Ramanujan Fellowship (SB/S2/RJN-090/2017) and core research grant (CRG/2019/006059). The authors thank D. Y. Patil Education Society, Kasaba Bawada, Kolhapur, for financial support through research project sanction No. DYPES/DU/R&D/2022/2352.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kulkarni, S.P., Chitare, Y.M., Magdum, V.V. et al. Chemically synthesized facet-controlled visible light active BiVO4 thin films for photoelectrochemical water splitting. Appl. Phys. A 129, 876 (2023). https://doi.org/10.1007/s00339-023-07164-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-023-07164-1