Abstract
In this work, the surface of BiVO4 nanoporous films was modified by ultrathin CoOx nanoparticles through a facile electrochemical deposition route. By adjusting the deposition time, the effect of CoOx loading amount (or time) was investigated with respect to the photoelectrochemical (PEC) performance of the BiVO4 photoanode. No significant morphological changes were observed after depositing CoOx cocatalysts onto the BiVO4 surface, except for a rougher surface and the appearance of nanosheets at 40 s. After optimizing the deposition time, the highest photocurrent density of 3.36 mA/cm2 was achieved at 1.23 VRHE for the 20-CB sample under solar irradiation, exhibiting a nearly threefold increase in the photocurrent as compared to that of pristine BiVO4 (1.24 mA/cm2). It was found that the CoOx loading reduced the onset potential and interfacial charge transfer resistance, leading to the significant enhancement of the PEC activity. However, when the deposition time was extended or the loading amount increased, the PEC activity actually decreased, which might be related to the increase in carrier recombination loss. This research will help us understand the mechanism of surface CoOx modification for improving the PEC activity of semiconductor photoanodes like BiVO4.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Currently, the increasing energy shortage has caused people to turn their attention towards clean energy like solar and hydrogen energies. Photoelectrochemical (PEC) water splitting provides a feasible method for utilizing solar energy to produce H2, which is usually carried out in a two-electrode or three-electrode system.1,2 The H2 production rate is usually faster than powder-based photocatalytic systems and cheaper than electrocatalysis.3 At present, PEC water splitting is faced with challenges in developing a suitable photoelectrode candidate that has broad light-harvesting ability, suitable bandgap, and high catalytic activity, and which can quickly split water into H2 or O2 under illumination. Currently, the solar-to-hydrogen (STH) efficiency of PEC cells is still limited by the photoanode, which involves a four-electron transfer process rather than a two-electron transfer process that occurs on the cathode.4 As is commonly known, it is difficult to achieve ideal PEC activity in photoanodes with a single component like ZnO, TiO2, or BiVO4, because they have certain limitations, including narrow spectral response, slow carrier separation, and possible photo-corrosion behavior. In most cases, surface engineering techniques such as metal deposition, doping, and the construction of a heterojunction or nanocomposites are commonly employed for enhancing both the PEC activity and stability of a semiconductor electrode.5,6,7
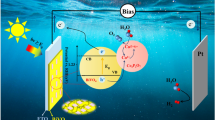
BiVO4 is a hot candidate for fabricating solar water-splitting photoanodes due to its suitable bandgap of 2.4 eV, good stability, environmentally friendly nature, and low cost. In theory, the conversion efficiency of the BiVO4 photoanode from sunlight can reach 9.2%, corresponding to a photocurrent of 7.5 mA/cm2 under solar irradiation.8 However, the PEC performance of BiVO4 is limited by the slow water oxidation kinetic on its surface, which could be enhanced significantly by modifying its surface with cocatalysts.9 To date, much effort has been devoted to optimizing BiVO4 photoanodes and exploring the underlying mechanism. He et al. developed a novel, high-efficiency, solar-driven BiVO4-related photoanode via sandwiching a photothermal CoOx layer between a BiVO4 photoanode film and an FeOOH/NiOOH sheet electrocatalyst, leading to a superior photocurrent density of 6.34 mA/cm2 at 1.23 V versus a reversible reference electrode (VRHE).10 In their study, CoOx plays at least two roles in enhancing the PEC activity of BiVO4 photoanodes. First, CoOx can be used as a photothermal material, utilizing the heat in the solar spectrum, which corresponds to the energy over the infrared band. Second, CoOx also has a strong hole-extraction capability, making it a suitable hole cocatalyst with suitable valence band edges (VBE). In another report, Song et al. demonstrated a high photocurrent density of 5.85 mA/cm2 at 1.23 VRHE under solar irradiation in a BiVO4 nanoporous photoanode through incorporating MoOx/MXene quantum dots (MQD) into the hole transfer layer.11 In addition, Seo et al. found that the colloidal CeO2 quantum dot (CeQD) layer simultaneously enhanced the charge-separation efficiency and transfer kinetics, resulting in the highest photocurrent density (4.0 mA/cm2) at 1.23 VRHE under visible light irradiation.12 In their study, the CeQDs with average sizes of 1.8–3.0 nm served as the hole extraction layer. However, there are currently few reports on surface modification of BiVO4 photoanodes with CoOx or Co3O4 cocatalysts, and the mechanism requires a deeper investigation. More attention has been directed towards other surface modifiers (cocatalysts) like CoOx, Co3O4, CoPi, and CoOOH.13,14,15
In this work, BiVO4 nanoporous films were modified with thin CoOx nanoparticles (NPs) via a facile electrochemical deposition route. The existence of CoOx was firstly demonstrated by x-ray diffraction (XRD) patterns. After loading of a suitable amount of CoOx cocatalyst, the CoOx-modified BiVO4 photoanode displayed the highest photocurrent density of ~3.36 mA/cm2 at 1.23 VRHE under simulated solar irradiation. The enhanced PEC performance was attributed to the reduced interface charge transfer resistance, as indicated by the electrochemical impedance spectroscopy (EIS) results.
Experimental Details
Preparation of BiVO4 Nanoporous Photoanodes
The nanoporous BiVO4 photoanodes used in this work were prepared according to our previous work.16 Briefly, the BiOI intermediate was obtained through electrodeposition and then converted into a BiVO4 porous film by a solution drop-casting process. Specifically, a 0.04 mol/L Bi(NO3)3 solution was prepared by slowly dissolving Bi(NO3)3·5H2O in 50 mL of 0.4 mol/L KI aqueous solution with pH of 1.75. Then, 20 mL of absolute ethanol containing 0.23 mol/L p-benzoquinone was mixed into the solution. The BiOI electrodeposition onto a fluorine-doped tin oxide (FTO) glass substrate was carried out in a three-electrode electrochemical cell consisting of a working electrode (WE), counter electrode (CE), and reference electrode (RE) at −0.143 V versus a saturated calomel electrode (SCE) for 10 min. Then, 0.15 mL of VO(acac)2/DMSO solution (0.2 mol/L) was dripped onto the BiOI films (2 × 2 cm2), followed by drying in an oven (60°C, 3 h) and annealing in air (450°C, 2 h). Finally, the pristine BiVO4 electrodes were obtained by soaking the samples into a 1.0 mol/L NaOH aqueous solution for 30 min in order to remove the excess of V2O5.
Deposition of CoOx on BiVO4 Photoanode
The deposition of the CoOx nanosheet on the BiVO4 photoanode comprised two steps.11 First, the Co(OH)2 nanosheet was produced by electrochemical deposition. The as-prepared BiVO4 photoanode was used as the working electrode, the SCE as a reference electrode, and Pt foils as counter electrode. The electrolyte solution was prepared by dissolving Co(NO3)2·6H2O (15 mM) in deionized (DI) water. The working electrode was applied at a constant voltage (−1.0 V versus SCE) and the amount of Co(OH)2 deposited was controlled by varying the deposition time from 5 s to 40 s. Second, the obtained Co(OH)2/BiVO4 film was rinsed with DI water and dried in air, followed by an annealing process in a muffle furnace at 350°C with a ramping rate of 2°C/min for 2 h. Finally, the CoOx/BiVO4 photoanode samples were designated as BVO, 5-CB, 10-CB, 20-CB, 30-CB, and 40-CB, respectively, according to the CoOx deposition time.
Material Characterization
The structural analysis of samples was carried out by X-ray diffraction (XRD, Haoyuan DX-2700) with a Cu Kɑ source. The surface morphology of the as-prepared photoanodes was determined by field-emission scanning electron microscopy (FESEM, Hitachi SU8220 next-generation, Japan) equipped with X-ray energy-dispersive spectroscopy (EDS) system. The light absorption properties of as-prepared samples were measured by ultraviolet–visible (UV–Vis) diffuse reflectance spectroscopy (DRS, Cary 300 UV–Vis spectrophotometer, Varian Co.), in which a BaSO4 whiteboard was used as the internal reflectance standard.
PEC Measurements
The PEC measurements of photoanodes were performed using a CHI 660E electrochemical workstation in a standard three-electrode system. The obtained photoanode working electrode, SCE reference electrode, and a Pt foil counter electrode were used. An aqueous solution of 0.1 M Na2SO4 (pH = 7) was used as the electrolyte after N2 bubbling for 0.5 h. The intensity of simulated sunlight was controlled at 100 mW/cm2 using a 300 W Xe arc lamp with an AM 1.5G filter (CHF-XM-500 W, Beijing Changtuo Technology Co., Ltd.). All photoanodes were illuminated by simulated sunlight through the FTO side (i.e., a backside illumination). The tested area of the photoanode controlled by epoxy resin was about 6.0 cm2. The photocurrent–potential curves were measured by scanning with a continuous voltage change at a scan rate of 20 mV/s. The measured potentials versus SCE electrode were converted to the reversible hydrogen electrode (RHE) using the Nernst equation:
where VRHE is the converted potential versus RHE, and VSCE is the experimentally measured potential against the SCE reference electrode. In addition, the electrochemical impedance spectra were recorded under irradiation over a frequency range of 0.1–5 × 104 Hz with an amplitude of 20 mV and an applied potential of 0.0 V versus SCE.
Results and Discussion
Structure and Surface Morphology
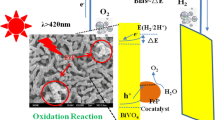
The BiVO4 photoanode was prepared via the electrodeposition solution drop-casting process. Figure 1 presents the FESEM images of BiVO4 and CoOx/BiVO4 heterostructure photoanodes. Pure BiVO4 possesses a network structure composed of interconnected rod-like particles (200–300 nm in diameter) with a smooth surface and no impurities present on its surface. The nanoporous structure and vertically oriented worm-like morphology of the BiVO4 photoanode was retained even after loading of a large amount of CoOx cocatalysts. Such a surface morphology is beneficial for obtaining a large surface area and electrolyte infiltration. The above results also indicate that the CoOx cocatalyst is so small that it does not block the pores of BiVO4 and could not be distinguished by SEM. It was also found that the CoOx loading would roughen the BiVO4 particle surface, but there were no CoOx nanosheets until the electrodeposition time reached 40 s (Fig. 1f).
Figure 2 compares the XRD patterns of BiVO4 and the BiVO4/CoOx heterostructure with different deposition times (from 5 s to 40 s). For the pure BiVO4 sample, all the diffraction peaks are assigned to the monoclinic scheelite crystal structure (JCPDS no. 14-0688) of BiVO4 and the rutile crystal structure (JCPDS no. 41-1445) of SnO2 derived from the FTO. No diffraction peaks of other phases or impurities were observed, indicating the high crystal quality and purity of the samples. There are no obvious peaks of CoOx species in the XRD pattern, which might be ascribed to a low deposition amount of Co(OH)2 as well as overlapping of the CoOx-related peaks with those of BiVO4. It is worth noting that the dominant peak of CoOx located at 18.9° was overlapped with the (011) crystal plane of BiVO4.
Figure 3 displays the cross-sectional SEM image of a typical CoOx/BiVO4 sample (40-CB). As can be seen, the BiVO4 film, the FTO conductive layer, and glass substrate are identified with clear interfaces. The BiVO4 nanoporous film displays a thickness of ~700 nm and worm-like porous morphology, where a small amount of CoOx nanosheets appear on the BiVO4 microparticles.
Figure 4 presents the surface morphology of a typical BiVO4/CoOx sample (40-CB) and its EDS elemental mapping results. As shown in Fig. 4a, the CoOx nanosheets are identified on the surface of BiVO4 nanoporous film. Four elements (including O, Bi, V, and Co) are distributed uniformly over the whole sample (Fig. 4b, c, d, and e), suggesting the successful loading of CoOx cocatalysts. Quantitative EDS analysis reveals that the atomic Co/Bi ratio is 1.46:23.61, corresponding to an overall CoOx loading of ~5.8% (relative to BiVO4). Although EDS is not a precise technique for determining the film composition, it confirms the existence of CoOx cocatalyst on the BiVO4 surface.
Optical Absorption Properties
Figure 5a compares the UV–Vis absorption spectra and their corresponding Tauc plots for the abovementioned CoOx/BiVO4 electrode samples. Apparently, BiVO4 displays an excellent visible-light response with a sharp absorption edge around 470 nm due to its wide bandgap of ∼ 2.45 eV. Both the UV and visible-light absorption of BiVO4 were enhanced significantly after surface modification with the CoOx cocatalysts due to the narrower bandgap of CoOx (Eg = ~ 1.6 eV). With increasing the deposition time, the optical absorbance is enhanced over the entire band, indicating the effect of CoOx as a narrow-bandgap material. The 40-CB sample has the highest visible light absorption. Figure 5b shows the Tauc plot of nanoporous BiVO4 and CoOx/BiVO4 films, which displays a bandgap of 2.45 eV for pure BiVO4 through a linear fitting of the curve.
PEC Performance
Figure 6 compares the PEC performance of BiVO4 nanoporous photoanodes before and after modifying with CoOx cocatalysts. It is found that the pristine BiVO4 photoanode exhibits a photocurrent of 1.24 mA/cm2 at 1.23 VRHE under simulated solar irradiation. After modifying with CoOx, the photocurrent density of BiVO4 is enhanced significantly along with a reduction in the onset potential. The highest photocurrent density (3.36 mA/cm2) at 1.23 VRHE appears in the 20-CB sample, which is nearly three times that of the pure BiVO4 photoanode. Apparently, the value is comparable with those reported in other published articles, as listed in Table I. The enhanced PEC performance might be ascribed to a more efficient visible light harvesting and a lowering of the interfacial charge transfer resistance. It is speculated that these CoOx cocatalysts act as the hole-extraction layer, leading to a significant enhancement of hole-injection efficiency (from photoanode to electrolyte). Nevertheless, it is strange that as the deposition time is further prolonged to 30 s or 40 s, the photocurrent (1.23 VRHE) of the obtained sample decreases. Namely, 30-CB exhibits lower photocurrent than 20-CB, while that of 40-CB is even lower than that of 30-CB. Overall, 20-CB exhibits the highest PEC activity among all samples. Although the photocurrent of 40-CB is comparable to that of the unmodified BiVO4 (1.23 VRHE), the former has a significantly lower onset potential than the latter. In addition, the applied bias photon-to-current conversion efficiency (ABPE) plots of the pristine BiVO4 and BiVO4/CoOx photoanodes are presented in Fig. 6b. The ABPE values were calculated using the following equation19:
where J refers to the photocurrent density (mA/cm), VRHE refers to the applied bias, and Pin is the input light power intensity (100 mW/cm2). As shown in Fig. 6b, 20-CB displays the maximum ABPE value of 0.72% at ~ 0.8 VRHE, which is about four times that of pristine BiVO4 (only 0.14% at 1.0 VRHE). It is also noted that 20-CB exhibits the highest ABPE peak value of all, which is consistent with the aforementioned linear sweep voltammetry (LSV) results. Figure 6c presents a comparison of the Nyquist plots of pristine BiVO4 and BiVO4/CoOx photoanodes under simulated solar irradiation, and the fitted results are shown in Table II. The Nyquist plots of all the samples consist of an arc and a semicircle, where the smaller arc shows the kinetics of charge transfer in the photoelectrode, while the larger semicircle represents the charge transfer resistance (RCT) from the impedance at the solid/liquid interface. It was found that BiVO4 had the largest semicircle diameter, indicating a high charge transfer resistance occurring at the solid/liquid interface. The CoOx loading can effectively reduce the interfacial transfer resistance, leading to the enhancement of the charge injection efficiency (towards the electrolyte). As a result, 20-CB has the smallest semicircle diameter and interface charge transfer resistance (39.0 Ω). However, a further increase in the deposition time would lead to a significant increase in the semicircle diameter (or RCT). The 40-CB sample displays a significantly higher RCT value than the other BiVO4/CoOx samples. This may be related to the thick coating layer, which increases the interfacial carrier recombination loss. This is also why an ultrathin cocatalyst layer is used in most reports. In other words, a larger number of holes are lost during the transport process before reaching the electrolyte. Figure 6d compares the Mott–Schottky (M–S) plots of BiVO4, 20-CB, and 40-CB measured in the dark. It is known that both the carrier density (ND) and flat-band potential (VFB) of a semiconductor can be calculated from the M–S plots, since there is a direct relationship between ND and VFB as follows20:
where C is the depletion layer capacitance, e = 1.6 × 10−19 C, ɛ is the dielectric constant (68 for BiVO4), ɛ0 is the permittivity in vacuum (8.85 × 10−12 F/m), A represents the active irradiation area, V is the applied potential, and kT/e is a temperature-dependent correction term. As shown in Fig. 6d, the BiVO4 photoanode displays an ND value of 3.76 × 1019 cm−3 with a VFB value of 0.22 V. The modification of CoOx cocatalyst reduces VFB of BiVO4 significantly while increasing the carrier concentration. The 20-CB sample shows a low VFB value (0.05 VRHE), while 40-CB shows a lower VFB value (just 0 VRHE). That is to say, the VFB decreases with increasing the CoOx deposition time, of which the variation trend is consistent with that of the onset potential (Fig. 6a). Obviously, the negative shift of VFB is beneficial for achieving better PEC performance, which depends not only on VFB, but also on several factors including visible-light absorption and interfacial transfer resistance.
Figure 7 presents a schematic model to reveal the mechanism for the enhanced PEC activity of a BiVO4 photoanode. As can be seen, CoOx acts as an oxygen evolution reaction (OER) cocatalyst. Under solar illumination, the electrons inside BiVO4 are excited, leaving the holes in the valence band (VB). Due to the loading of CoOx cocatalysts, the holes migrate to the VB of CoOx for water oxidation (into ·OH or O2). Apparently, the CoOx loading not only increases the carrier concentration in photoanode, but also enhances the band bending at the solid/liquid interface, leading to a more negative VFB. Although the VFB value becomes more negative, 40-CB still shows much lower PEC activity than 20-CB, which may be associated with its larger interfacial transfer resistance, as mentioned before (Fig. 6c). It is well known that increasing the deposition time is beneficial for achieving a thicker CoOx layer, leading to an increase in the interfacial transfer resistance value.
Conclusion
In summary, BiVO4 nanoporous photoanodes were modified with CoOx cocatalysts through a facile electrodeposition route. The successful loading of CoOx cocatalysts was verified by EDS mapping, XRD, and PEC measurements. It was found that the PEC activity of BiVO4 was greatly influenced by the electrodeposition time. Loading CoOx can enhance visible-light absorption and reduce the onset potential, resulting in higher photocurrent (1.23 VRHE). Nevertheless, extending the electrodeposition would result in a significant reduction of the PEC activity. The optimal PEC activity was obtained with the 20-CB sample (with a deposition time of 20 s). Although 40-CB has a similar photocurrent at 1.23 VRHE with the BiVO4 sample, the onset potential of the former is much lower than the latter. We hope that this study will contribute to a deeper understanding of the semiconductor PEC process.
References
T. Hisatomi, J. Kubota, and K. Domen, Chem. Soc. Rev. 43, 7520 (2014).
R. Tang, S. Zhou, Z. Zhang, R. Zheng, and J. Huang, Adv. Mater. 33, 2005389 (2021).
J.H. Kim, D. Hansora, P. Sharma, J.W. Jang, and J.S. Lee, Chem. Soc. Rev. 48, 1908 (2019).
T. Li, J. He, B. Peña, and C.P. Berlinguette, Angew. Chem. Int. Ed. 55, 1769 (2016).
Q. Wang, J. He, Y. Shi, S. Zhang, T. Niu, H. She, Y. Bi, and Z. Lei, Appl. Catal. B Environ. 214, 158 (2017).
X. Li, J.Q. Wan, Y.W. Ma, Y. Wang, and X.T. Li, Chem. Eng. J. 404, 127054 (2021).
B. Zhang, H.P. Zhang, Z.Y. Wang, X.Y. Zhang, X.Y. Qin, Y. Dai, Y.Y. Liu, P. Wang, Y.J. Li, and B.B. Huang, Appl. Catal. B Environ. 211, 258 (2017).
P.M. Rao, L. Cai, C. Liu, I.S. Cho, C.H. Lee, J.M. Weisse, P. Yang, and X. Zheng, Nano Lett. 14, 1099 (2014).
C. Zachaus, F.F. Abdi, L.M. Peter, and R. van de Krol, Chem. Sci. 8, 3712 (2017).
B. He, S.R. Jia, M.Y. Zhao, Y. Wang, T. Chen, S.Q. Zhao, Z. Li, Z.Q. Lin, Y.L. Zhao, and X.Q. Liu, Adv. Mater. 33, 2004406 (2021).
Y.R. Song, X.M. Zhang, Y.X. Zhang, P.L. Zhai, Z.W. Li, D.F. Jin, J.Q. Cao, C. Wang, B. Zhang, J.F. Gao, L.C. Sun, and J.G. Hou, Angew. Chem. Int. Ed. 61, e202200946 (2022).
D.H. Seo, S.Y. Hong, T.H. You, A. Sivanantham, and I.S. Cho, Chem. Eng. J. 450, 137917 (2022).
Z.Y. Liu, X.F. Wu, B.N. Zheng, Y. Sun, C.M. Hou, J. Wu, K.K. Huang, and S.H. Feng, Chem. Commun. 58, 9890 (2022).
T.T. Pan, Y.M. Tang, Y.X. Liao, J.C. Chen, Y.P. Li, J. Wang, L.S. Li, and X. Li, Mol. Catal. 549, 113527 (2023).
R. Yalavarthi, R. Zbořil, P. Schmuki, A. Naldoni, and Š Kment, J. Power. Sources 483, 229080 (2021).
H.M. Geng, P.Z. Ying, Y.L. Zhao, and X.Q. Gu, Int. J. Hydrog. Energy 46, 35380 (2021).
X.J. Zhao, Y.F. Rui, Y. Bai, J.W. Huang, H.D. She, J.H. Peng, and Q.Z. Wang, CrystEngComm 25, 6677 (2023).
J.K. Wang, J.D. Sun, Y.L. Liu, X. Zhang, K. Cheng, Y.P. Chen, F.Z. Zhou, J.J. Luo, T.B. Li, J.J. Guo, and B.S. Xu, J. Colloid Interface Sci. 666, 57 (2024).
Z. Li, W. Luo, M. Zhang, J. Feng, and Z. Zou, Energy Environ. Sci. 6, 347 (2013).
H.P. Maruska and A.K. Ghosh, Sol. Energy 20, 443 (1978).
Acknowledgments
This work is financially supported by Fundamental Research Funds for the Central Universities (2019ZDPY04).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sui, M., Gu, X. Fabrication of CoOx/BiVO4 Photoanodes with Enhanced Photoelectrochemical Water Splitting. J. Electron. Mater. 53, 5326–5333 (2024). https://doi.org/10.1007/s11664-024-11287-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11664-024-11287-3