Abstract
Objectives
It is challenging to know at the first which patients with desmoid fibromatosis (DF) are better suited to conservative or aggressive treatment. To investigate whether the low signal intensity bundles on T1- or T2-weighted images (WI), termed the “black fiber sign (BFS),” can predict non-progressive behavior in the conservative approach.
Methods
This retrospective study included 59 patients with primary DF managed with wait-and-see approach from 2005 to 2018 and serial MR images were analyzed. Three observers blinded to the patient information verified the presence or absence of BFS on baseline T1 or T2WI. The likelihood of progression-free survival (PFS) after ascertaining the presence or absence of the BFS was estimated using the Kaplan–Meier method and analyzed with the log-rank test.
Results
PFS was significantly higher in cases with BFS than without BFS on T1WI (p < 0.01), but there was no significant difference in PFS between cases with and without BFS on T2WI. Multivariable Cox proportional hazards analysis revealed that the absence of BFS on T1WI was a high-risk factor for progression (hazard ratio, 14.9; p < 0.01). Drastic tumor regression was apparent with significantly increased low-signal area in cases with BFS on T1WI. Intra- and interobserver reliabilities of BFS on T1WI were in almost-perfect agreement (κ > 0.8).
Conclusion
Our retrospective observational data support that presence of BFS in baseline MRI may be a predictor for progression-free survival of DF. BFS on T1WI is easily identifiable and can be utilized clinically in patients with DF.
Key Points
• We proposed a new imaging marker for prediction of desmoid fibromatosis progression.
• The absence of black fiber sign predicted a high risk of disease progression.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Extra-abdominal desmoid fibromatosis (DF) has a locally aggressive infiltrative character, with no metastasis; therefore, DF therapy aims to achieve local control. Historically, complete surgical resection with an aggressive wide margin has been the standard care, but resection of this type often results in significant functional impairment and the need for soft tissue reconstruction. Moreover, DF has a high risk of local recurrence after resection ranging from 42 to 86% [1, 2].
Given this unpredictable biological behavior, including the possibility of spontaneous regression, attention has increasingly been directed toward initial nonsurgical management. An observational approach, known as the wait-and-see policy, has now become the more standard initial approach for DF [3, 4]. Fiore et al reported that the 5-year progression-free survival (PFS) was 49.9% in the wait-and-see group [5].
Castellazzi et al suggested that DF has variable characteristics on MRI and that it is impossible to predict tumor behavior based on MRI [6]. Therefore, it is important to identify predictors of DF progression during aggressive observation in clinical decision-making.
Histologically, DF is monoclonal proliferation of myofibroblasts with variable collagen deposition. In general, DF has histologically active and inactive areas. The transcriptionally inactive region has sparse cells with narrow, darker-staining nuclei and few mitoses with more extensive collagen [7, 8]. The histologically active region is characterized by light-staining oval nuclei, greater cell density, and increased mitotic activity with less collagen deposition. Furthermore, Rhim et al showed that low-signal-intensity bands on T1-weighted images (WI) correlated well with hypocellular and dense collagenous stroma on pathologic specimens [9]. Loss of T1 and T2 signals, suggesting a response to chemotherapy or radiation therapy in soft tissue tumor including DF, was associated with increased collagen deposition and decreased cellularity [10,11,12].
We therefore hypothesized that the cases with low-signal-intensity bands, called the “black fiber sign” (BFS), which might be associated with inactive DF regions, have a high likelihood of spontaneous regression or stabilization while under wait-and-see care. The objective of this retrospective cohort study was to examine whether tumors with BFS on T1WI and/or T2WI had non-progressive behavior, based on serial MRI evaluations, and to identify whether the BFS could be a reliable prognostic predictor of progression of extra-abdominal DF managed with observation.
Materials and methods
Patients
In total, 107 patients with pathologically confirmed extra-abdominal DF were treated in three institutions between January 2005 and April 2018 (Fig. 1). To preserve homogeneity in the study, we included only primary DF managed by observation over 3 months, as the first-line approach, with or without administration of drugs, including non-steroidal anti-inflammatory drugs, tranilast, and tamoxifen. Patients with recurrent disease or were treated by immediate active intervention, including surgery, radiotherapy, and chemotherapy (methotrexate, vinblastine, and imatinib), as first-line management, and without consecutive MRI studies were excluded. To avoid the effect of responses to active intervention, patients who were switched to active treatment, including surgery, radiotherapy, and chemotherapy during the study period, were analyzed until intervention commencement.
Evaluation
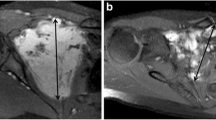
MRI was mainly performed using Magnetom series at 1.5 T (Siemens Healthcare), Signa series at 1.5 T (GE Healthcare), or Gyroscan and Ingenia series at 1.5 T (PHILIPS Healthcare) with a fast spin echo sequence. The contrast agent, gadopentetate dimeglumine (Magnevist; Bayer Healthcare), gadodiamide hydrate (Omniscan; Daiichisankyo), or gadoteridol (ProHance, Bracco) were mainly used. Consecutive MRI investigations were obtained over a period ranging from 3 to 12 months. An expert musculoskeletal oncologist (observer 1, Y.M.) with 12 years of experience, and blinded to the clinical information, analyzed the MR images. Tumor size at baseline and in serial images was calculated using the Response Evaluation Criteria in Solid Tumors guidelines, based on T2WI [13]. On the baseline MRI, the blinded observer determined the presence or absence of the BFS. A T1 or T2WI with very low signal intensity (the same as air) known as signal void more than 1 mm in diameter, and that had multiple, well-defined bundles inside the tumor, not at its periphery, was scored as positive for the BFS (Fig. 2a–d). Images with no significant low signal intensity and ill-defined low-signal area borders inside the tumor on T1 or T2WI were scored as negative (Fig. 2c).
Black fiber sign on T1- or T2-weighted MRI. a, b A 15-year-old female with desmoid fibromatosis in the posterior compartment of the left thigh. The black fiber sign is present in the tumor on (a) T1 and (b) T2-weighted imaging (arrow head). c, d A 58-year-old female with desmoid fibromatosis in the posterior compartment of the left thigh. The black fiber sign is absent on (c) T1-weighted imaging and presents on (d) T2-weighted imaging (arrow head). Scale bar, 2 cm
Inter- and intraobserver reliabilities for determining of BFS on T1WI were assessed using kappa values. These were interpreted as follows: 0.00–0.20, poor; 0.21–0.40, fair; 0.41–0.60, moderate; 0.61–0.80, substantial; and > 0.80, almost-perfect agreement [14]. To ensure interobserver concordance, two blinded observers (M.E., observer 2: musculoskeletal oncologist with 16 years of experience; J.S., observer 3: musculoskeletal oncologist with 8 years of experience) independently evaluated the presence or absence of BFS on T1WI. To assess intraobserver reliability, the observers made a double evaluation, 3 months apart.
PFS was calculated from the date of the first examination to the date of imaging progression, using the Response Evaluation Criteria in Solid Tumors. Time to intervention was defined as the time between the first examination and the date on which intervention (surgery, radiotherapy, or chemotherapy) was initiated. Furthermore, we examined whether tumor growth was related to fiber size or characterization of fiber borders (sharp or indistinct) on baseline T1WI. We analyzed the ratio of the very-low-signal area to the tumor (as the fiber size ratio) by drawing each region of interest at its greatest cross-sectional diameter using axial images.
We analyzed the ratio of the very-low-signal-intensity area (same as air) to the tumor with the presence of the BFS on T1WI. T1WI values were assessed by two methods. First, the oncologist drew the largest electronic region of interest within the boundaries of each tumor at its greatest cross-sectional diameter using the axial images and calculated the tumor size. Second, the size of the very-low-signal-intensity area was calculated. These measurements were repeated at the same location in the tumor on the first and the latest studies. Then, we analyzed the tumor size and the ratio of the very-low-signal area to the tumor at the first and the latest examination.
We finally assessed tumor T2 signal intensity using the modified Choi technique as previously described [15]. Briefly, the largest circular region of interest within the boundaries of each tumor at its greatest cross-sectional diameter and then, another circular region of interest at the adjacent, normally appearing muscle was drawn on the baseline examination. Finally, the ratio between tumor and muscle mean T2 signal intensities was calculated.
Statistical analysis
Data were expressed as the means ± standard deviation using EZR, a graphical user interface for R (The R Foundation for Statistical Computing) [16]. Statistical significance was evaluated using Welch’s t test for comparison. The difference in the characterization of tumor borders (sharp or indistinct) between partial response (PR) and stable disease (SD) was analyzed using Fisher’s exact test. The likelihood of PFS and intervention-free survival after ascertaining the presence or absence of the BFS on the first examination was estimated using the Kaplan–Meier method. Differences between cases with and without the BFS were analyzed with the log-rank test. The Cox proportional hazard model was performed to determine independent predictors of PFS. Univariable and multivariable analyses were used to identify potential predictors of PFS. Variables with a p value < 0.5 on univariable analysis were included in the multivariable analysis [17]. For all statistical analyses, p values < 0.05 were considered significant.
Ethics and registration
This study was carried out in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice guidelines. The study was approved by the Institutional Review Board for Clinical Research at our hospital (reference number 312-2; June 5, 2019). Written informed consent was waived for the patients with DF because of the retrospective nature of this study.
Results
Patient and tumor characteristics
Table 1 shows the demographic characteristics and treatment factors in enrolled patients with DF. The ratio of male to female patients was approximately 1:2.5. The mean age was 46.4 years (range, 15–76 years). The most common location of the tumor was the trunk (66.1%); the mean tumor size was 57 mm. Only one patient had a confirmed diagnosis of familial adenomatous polyposis, and 58 patients had the sporadic disease.
We analyzed the baseline and serial MR images using the Response Evaluation Criteria in Solid Tumors; the rates of complete response, partial response (PR), stable disease (SD), and progressive disease (PD) were 0, 11.9%, 50.8%, and 37.3%, respectively. Overall, 26 patients (44.1%) dropped out from the wait-and-see policy and switched to active treatment for pain or contracture due to tumor growth (Fig. 1). Eleven patients underwent resection, six patients were treated by radiotherapy, and nine patients received chemotherapy.
Association of the BFS with tumor progression
The BFS was present in 19 cases (32.2%) on T1WI and 43 cases (72.9%) on T2WI. Among the 19 patients with the presence of the BFS on T1WI, five (26.3%) had PR and 13 (68.4%) had SD. A 20% increase in the tumor size, in the long-axis (PD), was observed in only one patient (5.3%) at 5 months after the first examination. Among the 40 patients without the BFS on T1WI, two (5%) demonstrated a PR and 17 (42.5%) had SD. PD was observed in 21 patients (52.5%) at a median of 4 months (range, 2–45) after baseline examination. Among the 43 patients with the presence of the BFS on T2WI, five (11.6%) had PR, 22 (51.2%) had SD, and 16 (37.2%) had PD. Among the 16 patients without the BFS on T2WI, two (12.5%) demonstrated a PR, eight (50%) had SD, and six (37.5%) had PD.
The PFS rate was significantly higher in the presence than in the absence of the BFS on T1WI (p < 0.001; Fig. 3a). The intervention-free survival rate was also significantly higher in the presence than in the absence of the BFS on T1WI (p = 0.008; Fig. 3b). Four out of 26 patients who switched to active intervention had the BFS on T1WI. There was no statistically significant difference in PFS and intervention-free survival rates between the presence and absence of the BFS on T2WI (Fig. 3c, d). Twenty out of 26 patients who switched to active intervention had the BFS on T2WI. We further examined whether tumor behavior was related to the fiber size or characterization of fiber borders on T1WI. There was no significant difference in the fiber size ratio or characterization of fiber borders between PR and SD in patients with BFS on T1WI (Table 2).
The results of Cox proportional hazards analysis of progressive disease are summarized in Table 3. In univariable analyses, the BFS on T1WI showed a statistical significance (p = 0.008). We used a composite of five factors with a p value < 0.5 (age, size, BFS on T1WI, and drug administration including non-steroidal anti-inflammatory drugs and tamoxifen) in multivariable analysis. The absence of BFS on T1WI predicted a high risk of PD (p = 0.009) in multivariable analysis, with a hazard ratio of 14.89.
To clarify the change in the size of the low-signal-intensity area in the tumor, we calculated this size on T1WI obtained at the first and the latest examinations. Representative T1-weighted MR images demonstrate tumor regression with an increasing low-signal area after 72 months (Fig. 4a, b). In quantitative outcome data, the size of the tumor was decreased significantly, and the ratio of the low-signal area to the tumor was statistically significantly increased at the latest examination (Fig. 4c).
Increase in low-signal-intensity area in T1-weighted MRI. T1-weighted MRI of the back, depicting desmoid fibromatosis, with a low signal intensity at baseline (first examination); (a) and after 72 months (b) under the wait-and-see policy. Scale bar, 2 cm. c Changes in the total tumor size and the ratio of the low-signal-intensity area to the tumor. Data are expressed as mean ± standard deviation. *p < 0.001 between baseline and the latest examination by Welch’s t test
A previous study has shown that the T2 signal ratio was associated with DF growth behavior [15]; thus, we evaluated the association of the T2 signal ratio with tumor growth in the current study. The group with a T2 ratio < 1 tended to have higher PFS and intervention-free survival rates than the group with a T2 ratio ≥ 1, but there was no statistically significant difference (Fig. 5a, b). Furthermore, we examined the value of the T2 ratio in the cases with or without BFS on T1WI. BFS-positive cases had a significantly lower T2 ratio than BFS-negative cases (Fig. 5c). Low-signal-intensity area (signal void) on T1WI was very low (signal void) in most cases even on the T2WI, and showed no enhancement on T1WI after administration of gadolinium-based contrast material. In contrast, low-signal-intensity area (signal void) on T2WI had a heterogeneous, low- to isointense to muscle on T1WI.
Inter- and intraobserver variability in the identification of the BFS
Observers 2 and 3 independently analyzed the T1WI of all eligible tumors and found 16 (27.1%) and 17 cases (28.8%) with the BFS, respectively. The interobserver variability between the three readers was excellent (Table 4). Intraobserver reproducibility for MRI detection of the BFS was in almost-perfect agreement (κ = 0.920; 95% CI, 0.811–1.029).
Discussion
We proposed a new marker for prediction of desmoid fibromatosis progression based on the low-signal intensity bundle, known as the black fiber sign, on T1-weighted images. The black fiber sign on T1-weighted images was significantly associated with the risk of progression and a switch from the wait-and-see approach to active intervention. Multivariable Cox proportional hazards analysis revealed that the absence of the black fiber sign on T1-weighted images was a high-risk factor for progression in patients treated with the wait-and-see policy. Furthermore, intra- and interobserver reliabilities in this study were almost perfect.
Most studies only reported the prognostic factors for local recurrence and few assessed tumor behaviors in the conservative approach [18,19,20,21,22]. In previous studies, the age, location, size, and surgical margin were independent prognostic factors for local recurrence [19, 20]. In some studies on the natural history of the disease with the wait-and-see policy, multivariable analysis identified no clinical variables or MRI characteristics as independent predictors of PFS [5, 6]. In the current study, there was also no statistically significant association between progression and age, sex, and tumor location, or size, according to univariable and multivariable Cox proportional hazards analysis. In contrast, Salas et al demonstrated that age, tumor localization, tumor size, and surgical margin influenced PFS in 426 cases of DF treated with different therapeutic strategies, including surgery, radiotherapy, medical treatment, and the conservative approach [21].
MRI may reveal changes associated with increased collagen deposition and decreased cellularity, such as a loss of T2 signal, which suggests either a response to treatment or a spontaneous regression in disease activity [9, 10, 23,24,25,26]. Rhim et al showed that the low-signal-intensity bands in T1WI of DF in the head and neck correlated with dense collagenous stroma within the mass [9]. Histologically, more collagen deposition is evident in regions in which cells have narrow, darker-staining nuclei and few mitoses, and appear transcriptionally inactive [7, 27]. Typically, the areas with more “transcriptionally inactive” cells are often separated by extensive collagen. Consecutive MR images typically show tumor shrinkage and reduction in T1- and T2-signal intensity in response to active intervention including chemotherapy and radiotherapy [10, 11]. If the collagen deposition represents the very-low-signal-intensity bands on T1WI, the BFS may have a histologically inactive area. Reduction in the contrast effect following systemic therapy is related to clinical response and tumor volume, and contrast enhancement of the tumor may be related to tumor activity [12, 25, 28]. In this study, the BFS on T1WI had almost no contrast enhancement, which suggests that the BFS on T1WI may be the area of low activity. In DF with the BFS on T1WI, tumor size decreased with an increase in the ratio of the low-signal area to the tumor. The BFS on initial T1WI may reflect the area that will later change to the collagen deposition area, appearing transcriptionally inactive. Meanwhile, the relationship between fiber characteristics (size and border) and tumor behavior has not been clarified. There was no significant difference in PFS and intervention-free survival between the cases with and without BFS on T2WI; thus, the BFS on T2WI did not serve as a prognostic indicator. The BFS on T2WI had a heterogeneous, low- to isointense to muscle on T1WI, and might contain some area without collagen deposition which appear transcriptionally inactive.
Various reports underscore the importance of T2 signal assessment in DF growth behavior [15, 25, 26, 29]. Gondim Teixeira et al showed that DF growth behavior was significantly related to hyperintense signal on T2WI and established a threshold of T2 value which predicts tumor growth [15]. In the current study, DF with T2 ratio < 1 tended to have higher PFS and intervention-free survival than that with ratio ≥ 1, but there was no statistically significant difference. This may be due to the small sample size in our study and differences in methods of DF behavior assessment between both studies. The tumors with BFS on T1WI had significantly lower signal on T2WI than those without BFS; thus, the BFS on T1WI may be related to T2 values of the whole tumor. However, some cases with BFS on T1WI had high T2 signal values, and some cases with BFS on T1WI had low T2 signal values. In the current study, the BFS appears to be more involved in the DF growth behavior than the T2 signal.
However, the study had some limitations, including the small sample size and relatively short follow-up period. To validate our results, further prospective studies are warranted. To preserve homogeneity, patients with recurrence after resection were excluded in the current study. In the future, it should be investigated whether the BFS is associated with tumor behavior in recurrent DF. Furthermore, MRIs were performed only with a fast spin echo sequence, and results of the BFS with other sequences such as the gradient echo sequence are unclear.
In conclusion, we demonstrated that the absence of the black fiber sign on T1-weighted images is a significant risk factor for the progression of desmoid fibromatosis managed with the wait-and-see policy. The black fiber sign could be easily ascertained in clinical settings, and, therefore, may be a factor in choosing the treatment.
Abbreviations
- BFS:
-
Black fiber sign
- CR:
-
Complete response
- DF:
-
Desmoid fibromatosis
- FAP:
-
Familial adenomatous polyposis
- HR:
-
Hazard ratio
- MRI:
-
Magnetic resonance images
- NSAIDs:
-
Non-steroidal anti-inflammatory drugs
- PD:
-
Progressive disease
- PFS:
-
Progression-free survival
- PR:
-
Partial response
- RECIST:
-
Response Evaluation Criteria in Solid Tumors
- ROI:
-
Region of interest
- SD:
-
Stable disease
- WI:
-
Weighted imaging
References
Smith K, Desai J, Lazarakis S, Gyorki D (2018) Systematic review of clinical outcomes following various treatment options for patients with extraabdominal desmoid tumors. Ann Surg Oncol 25:1544–1554
Kriz J, Eich HT, Haverkamp U et al (2014) Radiotherapy is effective for desmoid tumors (aggressive fibromatosis) - long-term results of a German multicenter study. Oncol Res Treat 37:255–260
Bonvalot S, Desai A, Coppola S et al (2012) The treatment of desmoid tumors: a stepwise clinical approach. Ann Oncol 23:x158–x166
Gronchi A, Colombo C, Le Péchoux C et al (2014) Sporadic desmoid-type fibromatosis: a stepwise approach to a non-metastasising neoplasm--a position paper from the Italian and the French Sarcoma Group. Ann Oncol 25:578–583
Fiore M, Rimareix F, Mariani L et al (2009) Desmoid-type fibromatosis: a front-line conservative approach to select patients for surgical treatment. Ann Surg Oncol 16:2587–2593
Castellazzi G, Vanel D, Le Cesne A et al (2009) Can the MRI signal of aggressive fibromatosis be used to predict its behavior? Eur J Radiol 69:222–229
Francastel C, Schübeler D, Martin DI, Groudine M (2000) Nuclear compartmentalization and gene activity. Nat Rev Mol Cell Biol 1:137–143
Skubitz KM (2017) Biology and treatment of aggressive fibromatosis or desmoid tumor. Mayo Clin Proc 92:947–964
Rhim JH, Kim JH, Moon KC et al (2013) Desmoid-type fibromatosis in the head and neck: CT and MR imaging characteristics. Neuroradiology 55:351–359
Gounder MM, Lefkowitz RA, Keohan ML et al (2011) Activity of Sorafenib against desmoid tumor/deep fibromatosis. Clin Cancer Res 17:4082–4090
Martin-Liberal J, Benson C, McCarty H, Thway K, Messiou C, Judson I (2013) Pazopanib is an active treatment in desmoid tumour/aggressive fibromatosis. Clin Sarcoma Res 3:13
Stacchiotti S, Collini P, Messina A et al (2009) High-grade soft-tissue sarcomas: tumor response assessment--pilot study to assess the correlation between radiologic and pathologic response by using RECIST and Choi criteria. Radiology 251:447–456
Eisenhauer EA, Therasse P, Bogaerts J et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247
Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics 33:159–174
Gondim Teixeira PA, Biouichi H, Abou Arab W et al (2020) Evidence-based MR imaging follow-up strategy for desmoid-type fibromatosis. Eur Radiol 30:895–902
Kanda Y (2013) Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant 48:452–458
Steyerberg EW (2009) Clinical prediction models: a practical approach to development, validation, and updating. Springer, Berlin Heidelberg, New York
van Broekhoven DL, Verhoef C, Elias SG et al (2013) Local recurrence after surgery for primary extra-abdominal desmoid-type fibromatosis. Br J Surg 100:1214–1219
Crago AM, Denton B, Salas S et al (2013) A prognostic nomogram for prediction of recurrence in desmoid fibromatosis. Ann Surg 258:347–353
Cates JM, Stricker TP (2014) Surgical resection margins in desmoid-type fibromatosis: a critical reassessment. Am J Surg Pathol 38:1707–1714
Salas S, Dufresne A, Bui B et al (2011) Prognostic factors influencing progression-free survival determined from a series of sporadic desmoid tumors: a wait-and-see policy according to tumor presentation. J Clin Oncol 29:3553–3558
Prodinger PM, Rechl H, Keller M et al (2013) Surgical resection and radiation therapy of desmoid tumours of the extremities: results of a supra-regional tumour centre. Int Orthop 37:1987–1993
Gondim Teixeira PA, Chanson A, Verhaeghe JL et al (2019) Correlation between tumor growth and hormonal therapy with MR signal characteristics of desmoid-type fibromatosis: a preliminary study. Diagn Interv Imaging 100:47–55
Vandevenne JE, De Schepper AM, De Beuckeleer L et al (1997) New concepts in understanding evolution of desmoid tumors: MR imaging of 30 lesions. Eur Radiol 7:1013–1019
Braschi-Amirfarzan M, Keraliya AR, Krajewski KM et al (2016) Role of imaging in management of desmoid-type fibromatosis: a primer for radiologists. Radiographics 36:767–782
Ben Haj Amor M, Ploton L, Ceugnart L, Taïeb S (2020) Magnetic resonance imaging of desmoid-type fibromatosis: current evaluation criteria. Bull Cancer. https://doi.org/10.1016/j.bulcan.2019.11.009
Misemer BS, Skubitz AP, Carlos Manivel J et al (2014) Expression of FAP, ADAM12, WISP1, and SOX11 is heterogeneous in aggressive fibromatosis and spatially relates to the histologic features of tumor activity. Cancer Med 3:81–90
Sheth PJ, Del Moral S, Wilky BA et al (2016) Desmoid fibromatosis: MRI features of response to systemic therapy. Skeletal Radiol 45:1365–1373
Cassidy MR, Lefkowitz RA, Long N et al (2018) Association of MRI T2 signal intensity with desmoid tumor progression during active observation: a retrospective cohort study. Ann Surg. https://doi.org/10.1097/SLA.0000000000003073
Acknowledgments
We thank M Naka for technical assistance.
Funding
This study has received funding by JSPS KAKENHI Grant Number 17K10974.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Toshihiko Yamashita.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
Hirofumi Ohnishi kindly provided statistical advice for this manuscript.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• retrospective
• prognostic study
• multicenter study
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Murahashi, Y., Emori, M., Shimizu, J. et al. The value of the black fiber sign on T1-weighted images for predicting stability of desmoid fibromatosis managed conservatively. Eur Radiol 30, 5768–5776 (2020). https://doi.org/10.1007/s00330-020-06953-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-020-06953-z