Abstract
Objectives
To propose a follow-up strategy for desmoid-type fibromatosis (DF) based on tumor growth behavior and the signal on T2-weighted MRI.
Methods
We retrospectively reviewed 296 MRI studies of 34 patients with histologically proven DF. In each study, tumor volume and T2 signal relatively normal striated muscle were assessed. Volume variation and monthly growth rates were analyzed to determine lesion growth behavior (progressing versus stable/regressing lesions). Growth behavior was correlated with T2 signal, tumor location, β-catenin status, treatment strategy, and follow-up duration. Interobserver variability of volume measurements and interobserver measurement variation ratio were assessed.
Results
There were 25 women and 9 men with a mean age of 39.9 ± 19 (4–73) years. Mean follow-up time in the patients included was 55 ± 41 (12–148) months. In progressing lesions, the mean average monthly growth ratio was 10.9 ± 9.2 (1.1–42.5) %. Interobserver variability of volume measurements was excellent (ICC = 0.96). Mean interobserver measurement variation ratio was 20.4 ± 23.6%. The only factor correlated with tumor growth behavior was T2 signal ratio (p < 0.0001). Seventeen out of 34 (50%) patients presented a signal change over the threshold of 1 during follow-up. There were five occurrences of secondary growth after a period of stability with a mean delay until growth of 38.2 ± 44.2 (17–116) months.
Conclusion
DF growth rate was quantitatively assessed. A threshold for volume variation detection was established. DF growth behavior was significantly related to T2 signal. An evidence-based follow-up strategy is proposed.
Key Points
• In progressing desmoid fibromatosis, the mean average monthly growth ratio was 10.9 ± 9.2%.
• Lesions with muscle/tumor T2 signal ratios lower than 1 tended to be stable or regress over time.
• Given the interobserver measurement variability and MRI in-plane spatial resolution, a variation higher than 42.6% in tumor volume is required to confirm punctual progression.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Desmoid-type fibromatosis (DF) is a rare (2–4 case per million per year) soft tissue tumor arising from the connective tissue of the muscle, aponeurosis, or overlying fascia [1]. DFs do not metastasize, but these tumors have unpredictable behavior and can be locally aggressive, with an average recurrence rate of 24 to 77% [2, 3]. There are considerable controversies about prognostic factors for DF and currently, a wait-and-see strategy is recommended as the first line of management in asymptomatic DF with progression-free survival rate up to 50% [4]. The different systemic medical treatments available for DF (anti-inflammatory drugs, hormonal therapy, tyrosine kinase inhibitors, isolated limb perfusion) [5, 6] are reported to have variable response rates ranging from 17 to 100% [7]. Percutaneous ablation treatments such as Cryoablation are reported to be an effective alternative treatment for local control of small and moderately sized extra-abdominal desmoid tumors [8]. Magnetic resonance imaging (MRI) plays a cardinal role in DF follow-up, and since tumor size remains the most important criterion for the evaluation of treatment efficacy, the imaging follow-up strategy may influence patient management. Several groups have issued formal guidelines for DF management, including the British Sarcoma Group, the National Comprehensive Cancer Network, and the European Society for Medical Oncology [9, 10]. These guidelines, although helpful, are diverse and not evidence-based, leading to variable management of DF patients [11]. Previous reports have also indicated that DF growth behavior can be associated with multiple factors such as signal intensity, contrast enhancement, and adjuvant therapy [12,13,14]. We hypothesize that these factors should be considered in a DF-specific imaging follow-up strategy.
The aim of this study is to propose a follow-up strategy for DF based on the analysis of tumor growth behavior and the signal on T2-weighted MRI. Other factors that might influence tumor growth (β-catenin status, location, treatment strategy, and follow-up duration) were also evaluated. This information may help to standardize DF image follow-up with a potentially positive impact on patient management.
Materials and methods
Population
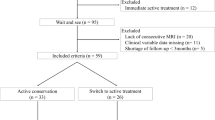
From January 2000 to January 2018, the MRI studies of 48 patients with histologically proven DF were retrospectively evaluated. These patients had been identified by performing a search in our institution’s hospital information system (Xplore, EDL® version 6.2.933) using the keyword “desmoid fibromatosis” and derived terms. Fourteen patients were excluded: two for which there were less than three follow-up studies available, follow-up being shorter than 12 months for seven patients, and five with complete surgical resection without recurrence.
In our institution, retrospective studies with fully anonymized patient data did not require ethics committee approval (IRB waived).
Imaging protocol
MR imaging was performed at 1.5-T (Signa Advantage, Signa H23, Signa HDxt) or 3.0-T MR750 (GE Healthcare) using dedicated coils. The acquisition protocol included at least two fast spin-echo T2 fat-saturated sequences in two different orthogonal planes. Acquisition protocols were adapted to the patient anatomy and tumor location: repetition time (TR) 3500−10,000 ms; echo time (TE) 48–77 ms; number of excitations (NEX) 1–4; bandwidth 13–42 kHz; echo train length (ETL) 10–23; field-of-view (FOV) 200–440 mm; slice thickness 3.5–5 mm; gap 0.5–3 mm; and matrix 224 × 256–416 × 352. In-plane voxel size varied from 0.27 to 3.36 mm3. T1-weighted with and without contrast enhancement was also part of the imaging protocol.
In our institution, in accordance with the National Comprehensive Cancer Network guidelines for soft tissues sarcoma, the follow-up strategy for DF was MRI every 6 months for the patients with stable or regressing DF up to 3 years and then, if lesion size remained stable, yearly follow-up. Patients with progressing DF were imaged every 3 months until progression stopped or surgical treatment was implemented [9].
Image analysis
One radiologist in training with 1 year of clinical experience in musculoskeletal MRI reviewed the images from all studies performed in the patients included. A second reader with 4 years of clinical experience with musculoskeletal MRI independently reviewed the first and the last MRI studies of each patient included (68 studies) allowing interobserver reproducibility assessment. Images were evaluated on a picture archiving and communication system workstation (Synapse 4.1, FUJIFILM Medical systems). T2-weighted fat-saturated images in two orthogonal planes were browsed to select the images showing the tumor’s greatest diameters. The readers then measured in millimeters the greatest tumor diameters in three orthogonal planes. Peritumoral edema was not included in the measurements. Tumor volume was calculated using the following equation (volume of an ellipse):
where D1, D2, and D3 are the maximal orthogonal diameters of a given lesion. This calculation method has been shown to correlate closely with true volumetric calculations based on slice-by-slice tumor segmentation [15]. Time versus tumor volume graphs were constructed. The average monthly growth rate (AMGR) of each previously defined tumor behavior period was calculated as follows:
where n is the number of months between the initial and final volumes. Based on these parameters, a third radiologist with 12 years of experience with musculoskeletal imaging determined the tumor growth behavior.
Tumor T2 signal intensity was objectively assessed using the modified Choi technique similar to that described by Stacchioti et al [16]: First, the image showing the largest tumor diameter on T2-weighted were selected. Then, the largest circular region-of-interest possible was drawn within the tumor. A second region-of-interest was drawn on the adjacent, normally appearing striated muscle. Finally, the ratio between tumor and muscle mean signal intensity was calculated and used for inter-patient comparisons.
Statistical analysis
Statistical analysis was performed with the R Development Core Team software (version 3.0.12013). AMGR and monthly volume variation were calculated based on the first and the last imaging study of each behavior period evaluated. Quantitative data are presented as mean ± standard deviation (range). Confidence intervals (CIs) are also presented in parentheses. Intraclass correlation coefficients (ICC) were calculated to assess the interobserver variability of volume measurements and T2 ratios. ICC values of 0–0.20 were considered to represent slight; 0.21–0.40, fair; 0.41–0.60, moderate; 0.61–0.80, substantial; and 0.81–1, excellent agreement. A generalized linear mixed model fit by maximum likelihood (Laplace approximation to the deviance) was used to evaluate the influence of multiple variables (T2 ratios, β-catenin mutation, treatment, follow-up duration, and lesion location) on tumor behavior (progression versus stability/regression) and on AMGR. During the process for generalized linear mixed model estimation, the (marginal) likelihood is maximized through Laplace approximation, which corresponds to an adaptive Gaussian quadrature with only one quadrature point. The threshold for statistical significance was set to p < 0.05.
Results
Study population
Among the 34 subjects included, there were 25 females and nine males with a mean age of 39.9 (4–73) years. The mean follow-up time in the patients included was 55 ± 41 (12–148) months yielding a total of 296 MRI studies (mean per patient 8.7 ± 5.3 (3–22) studies). Twenty lesions were located in the extremities (12 in lower the extremity and eight in the upper extremity), and 14 lesions were located in the body (seven in the head and neck, four in the abdomen, and three in the chest wall). Among the 34 patients included, 22 were positive for β-catenin mutation, and 9 were negative. For one patient, despite an immunohistochemical analysis consistent with DF, β-catenin mutation assessment was not performed. Two patients had a history of familial adenomatous polyposis and Gardner syndrome. Nineteen patients received adjuvant therapy (tamoxifen, methotrexate, or radiotherapy), and a wait-and-see strategy was adopted for 15 patients (Table 1). The mean initial lesion volume was 157.1 ± 291.6 (0.20–1570).
The mean lesion T2 signal ratio in all follow-up studies evaluated was 1.5 ± 1.1 (0.127–7.5). The interobserver variability for T2 ratios was considered excellent (ICC = 0.84 [95% CI = 0.76; 0.90]).
The mean AMGR was 10.1 ± 9.2 (1.1–42.5) % in progressing lesions, 0.0 ± 0.5 (− 0.7–0.8) %, in stable lesions, and − 6.1 ± 4.5 (− 15.7 to − 1.1) % in regressing lesions. DF behavior (progression, stability, and regression) was significantly related to the lesion T2 ratio (p = 0.000001). When AMGR versus T2 ratio graph was considered, higher AMGR values were found in lesions with T2 ratios greater than 1 (Fig. 1); thus, this was considered to be the threshold for T2 signal variations. The duration of patient follow-up, β-catenin mutation status, treatment status, and tumor topography (body and upper extremity versus lower extremity) did not significantly influence DF growth behavior (p > 0.05). None of these factors directly influenced AMGR. Information on tumor growth rate and volume with respect to lesion T2 signal, location, mutation status, and treatment strategy in the study population is presented in Table 2.
Criteria for DF behavior assessment
The interobserver variability of volume measurements was considered excellent (ICC = 0.96 [95% CI = 0.93; 0.97]. The mean lesion volume variation ratio between the two readers was 20.4 ± 23.6% (95% CI = 15.9%; 24.9%). The upper limit of the 95% CI (24.9%) was considered the interobserver variation threshold. The lowest in-plane spatial resolution used (3.36 mm2 pixel size) would only allow identification of volume variations higher than 17.7%. As both interobserver measurement variability and spatial resolution affect the detectability of volume variation, in light of these results, the minimal identifiable volume variation was considered to be 42.6%. Punctual volume variations inferior to this threshold were not considered significant.
In accordance with these results, DF growth behavior was classified as follows:
Tumor progression: defined by a volume increase in at least two consecutive control studies or a volume increase higher than 42.6% compared with that in the previous study. Additionally, the AMGR of the corresponding period had to be higher than 1%.
Tumor regression: defined by a volume decrease in at least two consecutive control studies or a volume decrease higher than 42.6% compared with that in the previous study. Additionally, the AMGR of the corresponding period had to be lower than − 1%.
Tumor stability: defined by follow-up periods with an AMGR between − 1 and 1%.
In one patient, punctual changes in tumor volume over 42.6% were not considered to represent progression because the tumor was small in size (< 10 cm3) and with very irregular contours explaining the high variation in volume measurements. After image review and the analysis of time versus volume graphs by reader 3, this lesion was considered stable.
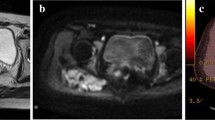
Changes in T2 signal relative to muscle were frequent in the patients studied and 17 out of 34 (50%) patients presented a signal change over the threshold of 1(e.g., T2 signal went from less than that of the muscle or greater than that of muscle or vice-versa) during follow-up (Fig. 2). Moreover, in 13 out of 17 patients (76.5%), this signal change was associated with a behavioral change (progression versus regression/stability).
There were 29 follow-up periods in which more than two follow-up studies performed in stable or regressing DF. The mean follow-up time in these periods was 40 ± 33.1 (7–116) months. There were five occurrences (17.2%) of tumor progression after two or more controls indicating tumor stability/regression with a mean secondary progression delay of 38.2 ± 44.2 (7–116) months. In four out of five occurrences, T2 ratios were higher than 1 at the time of tumor growth. In two of the patients with a secondary progression, a change in treatment preceded the behavior change. The other three patients resumed progression without any change in therapy (one was on tamoxifen and the other two on a wait-and-see strategy). The other 24 (85.7%) remained stable or regressed until the end of the available follow-up.
Follow-up interval determination
Among the DF evaluated, there were 27 periods of tumor progression, eight periods of tumor stability, and 26 periods of tumor regression adding up to 61 follow-up periods. When lesions presented a T2 ratio < 1 (17 follow-up periods), the upper limit of the 95% CI of the AMGR was − 0.2%, indicating that these lesions tend to be stable over time. In lesions with T2 ratios ≥ 1 (44 follow-up periods), the upper limit of the 95% CI of AMGR was 6.7%. Thus, given the threshold for volume variation identification, a follow-up interval of 6.3 months (42.6%/6.7%) would be needed to detect this growth.
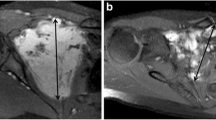
Figure 3 shows an example of the relationship between the signal and DF growth behavior.
a, b An 18-year-old female with a DF to the posterior compartment of the left knee (white stars). a Axial T2-weighted fat-saturated MR image showing a DF with a T2 ratio < 1. b T2-weighted fat-saturated axial MR image of the same patient, 80 months later, showing stable lesion volume and signal. c, d A 62-year-old male with a DF to the medial part of the left knee (white arrows). c Coronal T2-weighted fat-saturated MR image showing a DF with a T2 ratio > 1. d Coronal T2-weighted fat-saturated MR image of the same patient 17 months later showing an increase in lesion volume and signal
Based on these findings, a follow-up algorithm can be proposed (Fig. 4). This algorithm considers the fact that T2 signal ratios were significantly associated with DF growth behavior and the AMGR of DF with lower and higher T2 ratios. Moreover, follow-up intervals were determined taking into account from one side the DF AMGR and on the other the influence both of spatial resolution and interobserver measurement variability on volume estimates (e.g., the least amount of time necessary for growth identification considering lesion AMGR and the volume variation detection capabilities of MRI).
Discussion
The distinction between indolent and aggressive forms of DF may have important consequences for patient management [4]. The presented results, in accordance with prior reports, show a correlation between T2 signal and DF growth behavior (p < 0.0001) and the quantitative analysis of T2 ratios allowed identification of two groups of DF. When T2 ratio was inferior to that of adjacent muscle (ratio < 1), there was a general tendency for stability or regression (mean AMGR, 1.8 ± 3.3%), whereas a clear progression tendency was seen with T2 signal higher than that of adjacent muscle (ratio ≥ 1) (mean AMGR, 3.3 ± 11.5%). T2 signal changes overtime were frequently seen (48.6%) and were often associated with growth behavior changes (76.5%). The relationship between T2W signal on MRI and DF behavior is controversial, and Castellazi et al did not find any correlation between T2 signal and DF growth behavior in 27 patients [13]. In a later study, Gondim Teixeira et al demonstrated that in stable or regressing DF, T2 signal was lower compared with progressing DF in which low T2 signal intensity was rare [12]. These findings were corroborated by Cassidy et al that analyzed the percentage of T2 hyperintense tumor volume in 37 patients and its impact on progression-free survival. These authors demonstrated that when more than 90% of tumor volume was hyperintense, tumor progression was frequent [17]. In light of these results, the T2 signal should be considered in the follow-up strategy of DF and may influence patient management.
Interobserver measurement variability (an important factor in DF, which frequently presents irregular and infiltrative contours) and MRI in-plane spatial resolution could explain volume variations up to 42.6% [18, 19]. This variation may seem high, but since lesion volume was considered, this threshold is actually more sensitive for tumor progression than RECIST criteria, as a greater diameter increase of 20% would lead to a volume variation of 72.8% (if growth was assumed to be similar in all planes of space) [20, 21]. These findings are in accordance with previous studies that support the use of volume assessment (3D orthogonal measurement or direct volume assessment) for the follow-up of tumors of different histological types such as lung cancer, rhabdomyosarcoma, and angiomyolipomas [18, 22, 23].
The presented follow-up algorithm uses an optimized follow-up delay based on tumor growth rate for lesions with high T2 ratios, which represent 72% of the follow-up periods studied. The available data were insufficient to determine the optimal follow-up delay for lesions with low T2 ratios, which tend to remain stable. Tumor secondary progression was relatively infrequent (17%) and occurred with a widely variable delay (from 7 to 116 months). Thus, in accordance with previous guidelines, an empirical 12-month delay was proposed for patients with low T2 ratios or high T2 ratios stable for two or more controls [10]. Compared with the current guidelines of the European consensus for DF and low-grade soft tissue sarcoma, this follow-up algorithm requires relatively fewer and more sparse control studies, which could increase cost-effectiveness [9, 10, 24]. Additionally, an additional factor affecting tumor behavior was included (T2 signal), which could potentially allow earlier detection of tumor growth.
Several limitations of this study need to be acknowledged. The number of patients included was relatively small, which precluded the evaluation of tumor growth in smaller subgroups (particularly the secondary progression subgroup) and probably limited the statistical significance volume variations. Clinical findings such as pain and the proximity to noble anatomical structures were not evaluated in this study. The evaluated variables (e.g., T2 signal, β-catenin, follow-up time, therapy, and location) did not directly influence tumor AMGR. This could be related to the small number of follow-up periods available for analysis and a large number of possible variables affecting tumor growth. In order to facilitate the applicability of this technique in clinical practice, signal intensity was evaluated only in the slice depicting the larger tumor diameter. As DF can have a heterogenous signal distribution, this method may be less representative of global tumor signal intensity than a volumetric approach. Larger, prospective multicentric studies are necessary to overcome these difficulties and to confirm the effectiveness of the proposed algorithm. The formula used for volume assessment was that of an ellipsoid, which in some cases, is ill-suited for DF, which can present with irregular contours and a multinodular appearance. The use of deep learning and artificial intelligence could assist tumor segmentation and improve the interobserver measurement variation ratio [25]. Tumor T2 signal characteristics such as distribution and heterogeneity were not evaluated in this study. Further studies, potentially using a texture analysis approach, are required to assess this matter.
In conclusion, the volume AMGR of progressing, stable, and regressing DF on MRI was presented. T2 signal ratio frequently varied over time and was significantly associated with DF growth behavior. Secondary tumor progression was relatively infrequent with a wide variation in the delay to secondary progression. Given the interobserver measurement variability and MRI in-plane spatial resolution, a variation higher than 42.6% in tumor volume was required to confirm punctual progression. Based on these findings, an evidence-based follow-up strategy for DF is proposed.
Abbreviations
- AMGR:
-
Average monthly growth rate
- CI:
-
Confidence intervals
- DF:
-
Desmoid-type fibromatosis
- ETL:
-
Echo train length
- FOV:
-
Field-of-view
- ICC:
-
Intraclass correlation coefficients
- MRI:
-
Magnetic resonance imaging
- NEX:
-
Number of excitations
- TE:
-
Echo time
- TR:
-
Repetition time
References
Fletcher CDM, Unni KK, Mertens F (2002) Pathology and genetics of tumours of soft tissue and bone. IARC Press, Lyon, France, April 24 - 28, 2002. IARC Press, Lyon
Salas S, Dufresne A, Bui B et al (2011) Prognostic factors influencing progression-free survival determined from a series of sporadic desmoid tumors: a wait-and-see policy according to tumor presentation. J Clin Oncol 29:3553–3558 https://doi.org/10.1200/JCO.2010.33.5489
Shields CJ, Winter DC, Kirwan WO, Redmond HP (2001) Desmoid tumours. Eur J Surg Oncol 27:701–706 https://doi.org/10.1053/ejso.2001.1169
Gronchi A, Colombo C, Le Péchoux C et al (2014) Sporadic desmoid-type fibromatosis: a stepwise approach to a non-metastasising neoplasm—a position paper from the Italian and the French Sarcoma Group. Ann Oncol 25:578–583 https://doi.org/10.1093/annonc/mdt485
Bonvalot S, Desai A, Coppola S et al (2012) The treatment of desmoid tumors: a stepwise clinical approach. Ann Oncol 23:x158–x166 https://doi.org/10.1093/annonc/mds298
Bonvalot S, Rimareix F, Causeret S et al (2009) Hyperthermic isolated limb perfusion in locally advanced soft tissue sarcoma and progressive desmoid-type fibromatosis with TNF 1 mg and melphalan (T1-M HILP) is safe and efficient. Ann Surg Oncol 16:3350–3357 https://doi.org/10.1245/s10434-009-0733-9
Shinagare AB, Ramaiya NH, Jagannathan JP et al (2011) A to Z of desmoid tumors. AJR Am J Roentgenol 197:W1008–W1014 https://doi.org/10.2214/AJR.11.6657
Kujak JL, Liu PT, Johnson GB, Callstrom MR (2010) Early experience with percutaneous cryoablation of extra-abdominal desmoid tumors. Skeletal Radiol 39:175–182 https://doi.org/10.1007/s00256-009-0801-z
Grimer R, Judson I, Peake D, Seddon B (2010) Guidelines for the management of soft tissue sarcomas. Sarcoma 2010:1–15 https://doi.org/10.1155/2010/506182
Kasper B, Baumgarten C, Bonvalot S et al (2015) Management of sporadic desmoid-type fibromatosis: a European consensus approach based on patients’ and professionals’ expertise – a Sarcoma Patients EuroNet and European Organisation for Research and Treatment of Cancer/Soft Tissue and Bone Sarcoma Group initiative. Eur J Cancer 51:127–136 https://doi.org/10.1016/j.ejca.2014.11.005
Eastley N, Hennig IM, Esler CP, Ashford RU (2014) 99. Nationwide trends in the current management of desmoid (aggressive) fibromatosis. Eur J Surg Oncol 40:S47 https://doi.org/10.1016/j.ejso.2014.08.095
Gondim Teixeira PA, Chanson A, Verhaeghe JL et al (2019) Correlation between tumor growth and hormonal therapy with MR signal characteristics of desmoid-type fibromatosis: a preliminary study. Diagn Interv Imaging 100:47–55 https://doi.org/10.1016/j.diii.2018.06.007
Castellazzi G, Vanel D, Le Cesne A et al (2009) Can the MRI signal of aggressive fibromatosis be used to predict its behavior? Eur J Radiol 69:222–229 https://doi.org/10.1016/j.ejrad.2008.10.012
de Camargo VP, Keohan ML, D’Adamo DR, et al (2010) Clinical outcomes of systemic therapy for patients with deep fibromatosis (desmoid tumor). Cancer 116:2258–2265 https://doi.org/10.1002/cncr.25089
Aghighi M, Boe J, Rosenberg J et al (2016) Three-dimensional radiologic assessment of chemotherapy response in Ewing sarcoma can be used to predict clinical outcome. Radiology 280:905–915 https://doi.org/10.1148/radiol.2016151301
Stacchiotti S, Collini P, Messina A et al (2009) High-grade soft-tissue sarcomas: tumor response assessment—pilot study to assess the correlation between radiologic and pathologic response by using RECIST and Choi criteria. Radiology 251:447–456 https://doi.org/10.1148/radiol.2512081403
Cassidy MR, Lefkowitz RA, Long N et al (2018) Association of MRI T2 signal intensity with desmoid tumor progression during active observation: a retrospective cohort study. Ann Surg:1 https://doi.org/10.1097/SLA.0000000000003073
Mozley PD, Bendtsen C, Zhao B et al (2012) Measurement of tumor volumes improves RECIST-based response assessments in advanced lung cancer. Transl Oncol 5:19–25 https://doi.org/10.1593/tlo.11232
Krajewski KM, Nishino M, Franchetti Y, Ramaiya NH, Van den Abbeele AD, Choueiri TK (2014) Intraobserver and interobserver variability in computed tomography size and attenuation measurements in patients with renal cell carcinoma receiving antiangiogenic therapy. Cancer 120:711–721 https://doi.org/10.1002/cncr.28493
Schwartz LH, Litière S, de Vries E, et al (2016) RECIST 1.1 – update and clarification: from the RECIST committee. Eur J Cancer 62:132–137. https://doi.org/10.1016/j.ejca.2016.03.081
Sheth PJ, del Moral S, Wilky BA et al (2016) Desmoid fibromatosis: MRI features of response to systemic therapy. Skeletal Radiol 45:1365–1373 https://doi.org/10.1007/s00256-016-2439-y
Burke M, Anderson JR, Kao SC et al (2007) Assessment of response to induction therapy and its influence on 5-year failure-free survival in group III rhabdomyosarcoma: the Intergroup Rhabdomyosarcoma Study-IV experience—a report from the Soft Tissue Sarcoma Committee of the Children’s Oncology Group. J Clin Oncol 25:4909–4913 https://doi.org/10.1200/JCO.2006.10.4257
Bissler JJ, McCormack FX, Young LR et al (2008) Sirolimus for angiomyolipoma in tuberous sclerosis complex or lymphangioleiomyomatosis. N Engl J Med 358:140–151
Dangoor A, Seddon B, Gerrand C, Grimer R, Whelan J, Judson I (2016) UK guidelines for the management of soft tissue sarcomas. Clin Sarcoma Res 6. https://doi.org/10.1186/s13569-016-0060-4
Havaei M, Davy A, Warde-Farley D et al (2017) Brain tumor segmentation with deep neural networks. Med Image Anal 35:18–31 https://doi.org/10.1016/j.media.2016.05.004
Acknowledgements
We are indebted to Dr. Jean-Luc Verhaeghe for the support in the preparation of this work.
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Professor Alain Blum.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors has significant statistical expertise.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was not required because retrospective studies with fully anonymized patient data do not require ethics committee approval.
Methodology
• retrospective
• observational
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gondim Teixeira, P.A., Biouichi, H., Abou Arab, W. et al. Evidence-based MR imaging follow-up strategy for desmoid-type fibromatosis. Eur Radiol 30, 895–902 (2020). https://doi.org/10.1007/s00330-019-06404-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-019-06404-4