Abstract
Objectives
To compare combined percutaneous radiofrequency ablation and ethanol injection (RFA-PEI) with hepatic resection (HR) in the treatment of resectable solitary hepatocellular carcinoma (HCC) with 2.1–5.0 cm diameter.
Methods
From June 2009 to December 2015, 271 patients whom underwent RFA-PEI (n = 141) or HR (n = 130) in three centres were enrolled. The overall survival (OS) and recurrence-free survival (RFS) between groups were compared with Kaplan–Meier method and log-rank tests. Complications, hospital stay and cost were assessed.
Results
The OS rates at 1, 3 and 5 years were 93.5%, 72.7%, 58.6% in RFA-PEI group and 82.3%, 57.5%, 51.8% in HR group (p = 0.021). The corresponding 1-, 3- and 5-year RFS rates were 65.8%, 41.3%, 34.3% in RFA-PEI group and 50.5%, 33.8%, 28.4% in HR group (p = 0.038). For patients with 2.1–3.0 cm tumours, the 1-, 3- and 5-year OS after RFA-PEI and HR were 98.0%, 82.3%, 74.2% and 89.4%, 65.1%, 61.9%, respectively (p = 0.024). The corresponding RFS were 79.6%, 54.7%, 45.1% in RFA-PEI group, and 57.6%, 43.9%, 31.7% in HR group, respectively (p = 0.020). RFA-PEI was superior to HR in major complication rates, length of hospital stay and cost (all p < 0.001).
Conclusion
RFA-PEI had a survival benefit over HR in the treatment of solitary HCCs, especially for those with 2.1–3.0 cm in diameter.
Key Points
• RFA-PEI provided superior survival to HR in solitary HCC with 2.1–5.0 cm in diameter.
• RFA-PEI is superior to HR in complications, length of hospital stay and cost.
• RFA-PEI might be an alternative treatment for solitary HCC within 5.0 cm in diameter.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) is the third most common cause of cancer-related mortality worldwide [1]. For small, solitary tumours without macrovascular invasion or extrahepatic metastasis, hepatic resection (HR), liver transplantation or percutaneous ablation is recommended as the initial curative treatment modality according to the international guidelines [2, 3]. The shortage of donors limits the widespread use of liver transplantation [4]. For solitary HCCs no larger than 2 cm in diameter, the latest Barcelona Clinic Liver Cancer (BCLC) system has considered ablation as a non-inferior and more cost-effective option to HR and liver transplantation [5]. For solitary tumours larger than 2.0 cm in diameter, HR has been considered the first-choice treatment, reserving liver transplantation for patients with recurrence of cancer after treatment, and those with microvascular invasion or satellites detected by pathological analysis [5]. Percutaneous ablation is usually considered to be a second-choice treatment for unresectable tumours owing to impaired liver function and associated diseases [5]. However, for solitary resectable tumours measuring 2.1–5.0 cm in diameter, there has been no randomized controlled trial (RCT) or large-scale cohort study comparing the efficacy of HR with ablation. Because HR inevitably accompanies invasiveness, micro-invasive treatment such as ablation might hold great promise as an alternative strategy with comparable or even superior efficacy to HR.
Several studies have shown that radiofrequency ablation (RFA) may provide similar survival rates but less invasiveness compared to HR for patients with solitary HCC no larger than 5.0 cm in diameter [6,7,8]. Since local tumour control of RFA was reported to be less efficient than HR, it has been suggested to combine other therapies with RFA to improve the local efficacy. Among them, the combination of RFA with percutaneous ethanol injection (PEI) (RFA-PEI) with a multipronged needle has been reported, through which a complete ablation rate of 94% could be achieved after one session of treatment in patients with tumours measuring 3.0–7.0 cm in diameter [9]. This strategy was compared with RFA alone for HCC larger than 3 cm in diameter in an RCT, and higher complete ablation rate, better survival outcomes and comparable safety were achieved [10, 11]. To the best of our knowledge, no study has directly compared RFA-PEI with HR for solitary HCCs up to 5.0 cm so far. Thus, we retrospectively compared the efficacy of RFA-PEI with HR in the treatment of solitary resectable HCC with diameter 2.1–5.0 cm.
Materials and methods
Study design
This is a retrospective multicentre study on prospectively collected data in the First Affiliated Hospital of Sun Yat-sen University, Cancer Center of Sun Yat-sen University and the Affiliated Eastern Hepatobiliary Surgery Hospital of the Second Military Medical University. The study was approved by the ethics committee of each centre.
Patients presented with a first-time diagnosis of HCC in each centre from June 2009 to December 2015 were considered potential candidates for this study. HCC was diagnosed according to the most current clinical guidelines at the time of treatment [12, 13]; for example, two dynamic contrast-enhanced imaging modalities showing typical features of HCC, or one imaging study together with elevated serum alpha fetoprotein (AFP) level over 400 ng/dL, or a cytologic/histologic confirmation of HCC. The maximal diameter of the tumour was measured on cross-sectional imaging of computed tomography (CT) or magnetic resonance imaging (MRI). The histological diagnosis was confirmed by biopsy in 4 (2.8%) patients in the RFA-PEI group and by resected specimen for all patients in the HR group. Patients who met the following criteria were included in the study: (1) age between 18 and 75 years; (2) solitary tumour with 2.1–5.0 cm in diameter; (3) tumour defined resectable after evaluation of the feasibility of removing the lesion while leaving an adequate remaining liver remnant by our surgery team; (4) lesions visible on ultrasound with an acceptable and safe path for electrode insertion; (5) Child–Pugh A liver function; (6) no any other previous treatment; (7) an Eastern Cooperative Oncology Group (ECOG) performance status of 0.
The exclusion criteria were as follows: (1) underwent liver transplantation; (2) radiological evidence of vascular invasion or extrahepatic metastases; (2) severe coagulopathy (prothrombin activity < 40% or a platelet count < 40,000/mm3); (3) evidence of hepatic decompensation; (4) an American Society of Anesthesiologists score of 3 or more; (5) history of any other concurrent malignancies; (6) complicated with severe comorbidities; (7) allergic to ethanol; (8) fitted with a pacemaker.
Our multidisciplinary team including surgeons, interventional oncologists and radiologists recommended both the RFA-PEI and HR as a curative treatment choice for HCC measuring 2.1–5.0 cm in diameter, according to the previous experience [9, 14, 15]. The final choice of treatment was decided by patients, after being informed of the advantages and disadvantages of both treatments in detail by the attending physicians. Reasons for choosing RFA-PEI instead of HR included psychological resistance to invasive treatment and refusal of general anaesthesia. Informed consent was obtained from all patients before treatment.
RFA-PEI procedure
At each centre, RFA-PEI was performed percutaneously by the interventional radiologists with at least 10 years of experience in RFA according to the methods described in previous studies [9, 10]. The whole procedure was under the real-time guidance of ultrasound. For PEI, an 18-gauge needle (Hakko Co., Ltd, Japan) was used. RFA was performed by using a commercially available Cool-tip™ RFA system (Valleylab, Boulder, CO, USA) or an RF 2000 system (Radio-Therapeutics Mountain View, CA). First, the radiofrequency needle was inserted into the low-centre of the target tumour. Then, the 18-gauge needle was placed immediately adjacent to the radiofrequency needle in another access path. The ethanol was slowly injected in an injection–rotation–injection manner. The mean volume of ethanol used was 10.2 ± 2.0 mL (range, 5–30 mL). RFA was performed 3–5 min after PEI. Multiple overlapping techniques were conducted as appropriate [16]. The detailed procedures are described in the Supplementary Material.
Open hepatic resection
Open HR was performed under general anaesthesia by surgeons with 10–40 years of experience. Intraoperative US was routinely used to assist in operative evaluation including tumour burden, liver remnant and the possibility of a negative resection margin. The type of hepatectomy was defined according to the current guideline [17]. Anatomic resection was defined as the complete removal of at least one Couinaud segment containing the tumour and the corresponding hepatic territory. Other types of resection, such as wedge resection or tumour enucleation, were classified as non-anatomic resection. The surgical approach was chosen on the basis of hepatic functional reserve, tumour location and preference of the operator. Generally, anatomic resection was performed if the patient’s liver functional reserve permitted.
Treatment assessment and follow-up
In the RFA-PEI group, contrast-enhanced ultrasound (CEUS) was performed the following morning after treatment to evaluate the technical success. An additional RFA-PEI was given if residual viable tumour was found. If enhanced areas were still observed in CEUS after the additional RFA-PEI, the treatment was considered a failure and the patients were referred to other therapies [18]. In the HR group, resection margins and status (R0 or R1 resection) were evaluated according to the absence of microscopic tumour invasion at the resection margin [19].
In both groups, contrast-enhanced dynamic CT (CECT) and CEUS were conducted 4 weeks after the treatment for evaluation of technique efficacy [20]. Thereafter, the patients were followed up once every 3 months for the first 2 years, once every 6 months from 2 to 5 years and then once every 12 months after 5 years. At each follow-up visit, CEUS and blood tests including liver function tests and serum AFP level assay were performed. CECT was performed every 6 months. Chest radiography, CT of the chest, MRI and bone scan were performed when clinically indicated.
When local tumour progression (LTP) (defined as the appearance of tumour enhancement around the ablation or resection margins) [20], intrahepatic distant recurrence or extrahepatic recurrence was detected during the follow-up, corresponding treatments were given according to the tumour characteristics, patients’ liver function and requests, etc.
Complications were reported using the Dino–Clavien classifications [21]. Major complications were defined as clinical events leading to additional therapeutic interventions or prolonged hospitalisation [22]. Hospital costs were estimated with the frequency and unit cost of drugs, procedures, inpatient and outpatient visits, laboratory testing and imaging examination which included the direct costs for RFA-PEI or HR and also the costs for re-treatments after failure of these treatments. All the costs were converted to US dollars in 2016.
Overall survival (OS) was defined as the time interval from the date of diagnosis to the date of death from any cause or to the date of the last follow-up visit. Recurrence-free survival (RFS) was defined as the time interval between the date of diagnosis and the date of recurrence or last follow-up. This study was censored on 30 June 2016.
Statistical analysis
Continuous variables are presented as means ± SD and categorical variables as numbers and percentages. Differences between the two groups were compared with the t test for continuous variables and χ2 test for categorical variables. Survival curves were generated by the Kaplan–Meier method and compared by the log-rank test. Subgroup analyses were performed according to the tumour size. The prognostic relevance of potential survival predictors was analysed by univariate and multivariate Cox proportional hazard regression models. Statistical significance was considered as a two-sided p value of less than 0.05. The above statistical analysis was performed with SPSS 20.0 (SPSS Inc., Chicago, IL, USA).
Results
Patient characteristics
From June 2009 to December 2015, 3448 consecutive patients received a first-time diagnosis of HCC in our three centres. On the basis of the inclusion and exclusion criteria, 3177 patients were excluded because of tumour size greater than 2.1 cm or greater than 5.0 cm (n = 1122), tumour number greater than 1 (n = 1031), Child–Pugh B or C liver function (n = 300), ECOG score greater than 0 (n = 154), vascular invasion or extrahepatic tumour (n = 570). Finally, 271 patients (219 men, 52 women; mean age, 59.0 years; range, 20–75 years) with solitary HCC (2.1–5.0 cm) who had undergone percutaneous RFA-PEI (n = 141) or open HR (n = 130) as their initial therapies in these three centres (Fig. 1) were enrolled in this study. In the RFA-PEI group, there were 77 patients with tumours measuring 2.1–3.0 cm in diameter and 64 with tumours measuring 3.1–5.0 cm in diameter. In the HR group, there were 70 patients with tumours measuring 2.1–3.0 cm in diameter and 60 with tumours measuring 3.1–5.0 cm in diameter. Baseline characteristics of the study population are summarised in Table 1. No significant difference in demographic data was detected between the RFA-PEI and HR groups.
Technical success of RFA-PEI and open hepatic resection
In the RFA-PEI group, technical success was achieved in all the patients, including 137 after a single treatment session, and 4 after two sessions of RFA-PEI. In the HR group, 79 patients had anatomic resection and 51 patients had non-anatomic resection. Among the patients with anatomic resection, 59 patients had resection of one liver segment and 20 patients had resection of more than one segment. R0 resection was achieved in all the patients in the HR group. Table S1 summarises the operative data and perioperative outcomes for patients in the HR group.
Treatment outcomes and survival analysis
The median follow-up duration was 63.9 months (range, 11–84 months) in the RFA-PEI group and 61.8 months (range, 12–84 months) in the HR group, respectively.
During follow-up, recurrence occurred in 87 patients after RFA-PEI (8 LTP and 79 of distant recurrence) and 96 patients after HR (1 LTP, 95 distant recurrence and 1 extrahepatic recurrence with distant recurrence) (Table 2). For the whole population, LTP rate was higher in the RFA-PEI group than in the HR group (9 vs 1, p = 0.046). However, LTP rate was comparable between RFA-PEI and HR in both the subgroups of 2.1–3.0 cm (p = 0.686) and 3.1–5.0 cm (p = 0.225) tumours. The intrahepatic distant recurrence rate was significantly higher in the HR group than in the RFA-PEI group in the subgroup of 2.1–3.0 cm tumours (p = 0.023) but was comparable between RFA and HR in the subgroup of 3.1–5.0 cm tumours (p = 0.999). The modalities used to treat recurrent HCC are summarised in Table 3. At the time of censoring, a total of 54 patients in the RFA-PEI group and 67 patients in the HR group died, and causes of death are reported in Table S2.
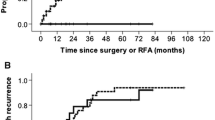
The cumulative OS rates at 1, 3 and 5 years were 93.5%, 72.7% and 58.6% in the RFA-PEI group and 82.3%, 57.5% and 51.8% in the HR group, respectively (p = 0.021, Fig. 2a). Correspondingly, the 1-, 3- and 5-year RFS rates were 65.8%, 41.3% and 34.3% in the RFA-PEI group and 50.5%, 33.8% and 28.4% in the HR group, respectively (p = 0.038, Fig. 2b).
On subgroup analysis, for patients with tumours measuring 2.1–3.0 cm in diameter, the 1-, 3- and 5-year OS rates after RFA-PEI (n = 77) and HR (n = 70) were 98.0%, 82.3%, 74.2% and 89.4%, 65.1%, 61.9%, respectively (p = 0.024, Fig. 3a). The corresponding RFS rates were 79.6%, 54.7% and 45.1% in the RFA-PEI group and 57.6%, 43.9% and 31.7% in the HR group, respectively (p = 0.020, Fig. 3b). For patients with tumours measuring 3.1–5.0 cm in diameter, the 1-, 3- and 5-year OS rates after RFA-PEI (n = 64) and HR (n = 60) were 86.4%, 65.1%, 55.4% and 88.9%, 64.5%, 49.6%, respectively (p = 0.125, Fig. S1a). The corresponding RFS rates were 53.5%, 29.4% and 24.0% in the RFA-PEI group and 42.2%, 26.6% and 21.9% in the HR group, respectively (p = 0.715, Fig. S1b).
Univariate analysis showed that AFP (p = 0.045), tumour size (p = 0.033) and treatment type (p = 0.021) were significantly associated with OS, while tumour size (p = 0.030) and treatment type (p = 0.025) were associated with RFS. Multivariate analysis demonstrated that treatment type was the only independent predictor of OS (hazard ratio (HR) = 1.523, 95% CI, 1.063–2.182; p = 0.022) and RFS (HR = 1.357, 95% CI, 1.014–1.815; p = 0.040) (Table S3).
Complications and hospital costs
There was no treatment-related mortality in our study population. Major complications were observed in 3 of the 141 (2.1%) in the RFA-PEI group and 33 of the 130 patients (25.4%) in the HR group (p < 0.001) (Table 4). Pain and fever were the two most common minor complications. All the minor complications were also more common in the HR group than in the RFA-PEI group (all p < 0.05). The hospital stay was significantly shorter in the RFA-PEI group compared with that in the HR group (5 vs 11 days, p < 0.001). The hospital cost was $3456.70 ± 940.98 for the RFA-PEI group and $6516.50 ± 965.70 for the HR group (p < 0.001).
Discussion
Our multicentre study showed that RFA-PEI achieved better long-term survival outcomes, fewer major complications, shorter hospital stay and lower cost than HR for patients with solitary HCCs measuring 2.1–5.0 cm in diameter.
For local tumour control, no significant difference in LTP rate was observed between the RFA-PEI and HR groups in either the subgroup of 2.1–3.0 cm tumours or 3.1–5.0 cm tumours. Considering the inferior local efficacy of RFA to HR, the local efficacy of RFA-PEI relative to HR was improved, which might be explained by the increased ablation zone resulting from the following factors. First, ethanol could destruct the vessels within or around the tumours to reduce the heat-sink effect of RFA. Second, ethanol could diffuse into the tumour area not reached by radiofrequency power. Third, thermal conduction could be improved by the lower extent of carbonization of tissue around electrode with ethanol [23, 24].
RFA-PEI presented a lower distant recurrence rate than HR in the subgroup of 2.1–3.0 cm tumours but a comparable rate to HR in the subgroup of larger (3.1–5.0 cm) tumours. It is reasonable to assume that in the subgroup of smaller (2.1–3.0 cm) tumours, RFA-PEI and HR could both achieve sufficient safety margin to ensure complete ablation and resection. It is also conceivable that the activating effect of RFA on the immune system might to some extent inhibit the intrahepatic distant metastasis (true recurrence) [25, 26]. Conversely, HR may suppress the immune system without the potential to prevent distant recurrence. Moreover, blood loss and blood transfusion, which are more likely to be required in HR than in RFA-PEI, can promote tumour recurrence [27, 28]. We believe that these are potential reasons to explain why more distant recurrence occurred after HR when compared to RFA-PEI for 2.1–3.0 cm HCCs. However, in the subgroup of 3.1–5.0 cm tumours, RFA-PEI may have not consistently produced sufficient safety margin because of the larger tumour size. Hazard from potentially inadequate safety margin in the RFA-PEI group may have outweighed the positive immunological effect of RFA-PEI on suppressing tumour recurrence. Thus, for 3.1–5.0 cm HCCs, no significant difference in the distant recurrence was observed between these two groups.
In our study, RFA-PEI achieved better OS and RFS compared to HR in patients with HCCs measuring 2.1–5.0 cm in diameter. More specifically, subgroup analyses revealed that the improved OS and RFS for RFA-PEI compared to HR was for 2.1–3.0 cm tumours, but not for 3.1–5.0 cm tumours. There might be several underlying reasons. First, a safety margin was reported to be one of the most important factors affecting recurrence after RFA and HR [29,30,31]. The enlarged ablation zone with wider safety margin by RFA-PEI can lead to a good local tumour control with full ablation of target tumour and effective clearance of micrometastases and microvascular invasion around the tumour. Thus, in our study, RFA-PEI achieved comparable LTP rates to HR, which suggested that RFA-PEI can obtain enough ablative margin at most cases, at least not inferior to that of HR. Second, in the case of complete ablation and resection, the intrahepatic distant recurrence may have contributed to the difference of RFS between the groups. The higher intrahepatic distant recurrence rate in the HR group in the subgroup of 2.1–3.0 cm tumours might contribute to a poorer RFS of patients after HR. However, in the subgroup of 3.1–5.0 cm tumours, the RFS rate was not significantly different with comparable intrahepatic distant recurrence between the groups. Third, prior studies including meta-analyses have shown poorer survival outcomes for non-anatomic resections, compared to anatomic resections for HCC patients after hepatectomy [32,33,34]. In our study, a relatively high proportion of non-anatomical resection (51/130, 39.2%) might be one of the reasons for better survival outcomes of the RFA-PEI group than the HR group. Fourth, RFA-PEI has the advantage over HR in causing less damage to liver function, which enables patients to endure repeated curative treatments in the case of relapse after RFA-PEI. And the interventions for recurrence might have a significant influence in the prognosis. Fifth, RFA-PEI has the advantage over HR in terms of micro-invasiveness with much fewer major complications and shorter hospital stay, which boosted the patients’ recovery and might therefore have improved their prognosis.
Our study revealed that RFA-PEI was safe with a low incidence of major complications (2.1%), which was similar to those reported previously (0–4.6%) [9, 10]. Moreover, RFA-PEI resulted in much fewer major complications and shorter hospital stay than those of HR. The average cost in the HR group was approximately 1.9 times higher than that in the RFA-PEI group, and RFA-PEI might be more emotionally acceptable for some patients because RFA-PEI was performed with local anaesthesia while HR with general anaesthesia. Therefore, RFA-PEI was superior to HR in terms of being less invasive, more cost-effective and in facilitating patients’ fast recovery, which make RFA-PEI clinically preferable in the era of micro-invasive treatment.
Recently, combined RFA with traditional or drug-eluting beads TACE (RFA-TACE) has shown favourable efficacy for HCC [35,36,37,38]. Compared to RFA-TACE, RFA-PEI can be performed in the same session with lower cost, less effort and fewer adverse effects. Besides, microwave ablation (MWA), an increasingly popular technique, is able to create larger ablation zones and to overcome the heat-sink effect more effectively than RFA [39, 40]. Compared to MWA, RFA-PEI might be more suitable to treat tumours adjacent to critical structures such as the subcapsular region and areas near large vessels or the gallbladder [9, 10]. However, since no study has been performed to compare RFA-PEI with RFA-TACE or MWA so far, this warrants future studies.
This multicentre retrospective study is the first investigation comparing RFA-PEI with HR in the treatment of solitary HCCs with diameter of 2.1–5.0 cm in the largest study population ever reported; however, there are limitations to our study. First, it is a retrospective study with all its inherent defects. Non-randomization regarding choices of treatment inevitably introduced selection bias. Second, our study was based on a cohort of mono-ethnic Chinese patients with hepatitis B viral infection as the predominant aetiology of HCC, and the results must be validated in other areas with different demographics and underlying causes of liver disease.
In conclusion, RFA-PEI is superior to HR in terms of long-term survival for patients with solitary HCCs with 2.1–3.0 cm in diameter whereas it provides comparable survival outcomes to HR for patients with HCCs with 3.1–5.0 cm in diameter. However, the results need to be validated in RCTs with a large sample size.
Abbreviations
- BCLC:
-
Barcelona clinic liver cancer
- CECT:
-
Contrast-enhanced computed tomography
- CEUS:
-
Contrast-enhanced ultrasound
- ECOG:
-
Eastern cooperative oncology group
- HCC:
-
Hepatocellular carcinoma
- HR:
-
Hepatic resection
- LTP:
-
Local tumour progression
- MWA:
-
Microwave ablation
- OS:
-
Overall survival
- PEI:
-
Percutaneous ethanol injection
- RCT:
-
Randomized controlled trial
- RFA:
-
Radiofrequency ablation
- RFS:
-
Recurrence-free survival
- TACE:
-
Transarterial chemoembolization
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2011) Global cancer statistics. CA Cancer J Clin 61:69–90
European Association for the Study of the Liver; European Organisation for Research and Treatment of Cancer (2012) EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 56:908–943
Bruix J, Sherman M (2011) Management of hepatocellular carcinoma: an update. Hepatology 53:1020–1022
O’Leary JG, Lepe R, Davis GL (2008) Indications for liver transplantation. Gastroenterology 134:1764–1776
Bruix J, Reig M, Sherman M (2016) Evidence-based diagnosis, staging, and treatment of patients with hepatocellular carcinoma. Gastroenterology 150:835–853
Chen MS, Li JQ, Zheng Y et al (2006) A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg 243:321–328
Feng K, Yan J, Li XW et al (2012) A randomized controlled trial of radiofrequency ablation and surgical resection in the treatment of small hepatocellular carcinoma. J Hepatol 57:794–802
Liang HH, Chen MS, Peng ZW et al (2008) Percutaneous radiofrequency ablation versus repeat hepatectomy for recurrent hepatocellular carcinoma: a retrospective study. Ann Surg Oncol 15:3484–3493
Huang G, Lin M, Xie X et al (2014) Combined radiofrequency ablation and ethanol injection with a multipronged needle for the treatment of medium and large hepatocellular carcinoma. Eur Radiol 24:1565–1571
Zhang YJ, Liang HH, Chen MS et al (2007) Hepatocellular carcinoma treated with radiofrequency ablation with or without ethanol injection: a prospective randomized trial. Radiology 244:599–607
Vallone P, Catalano O, Izzo F, Siani A (2006) Combined ethanol injection therapy and radiofrequency ablation therapy in percutaneous treatment of hepatocellular carcinoma larger than 4 cm. Cardiovasc Intervent Radiol 29:544–551
Bruix J, Sherman M, Llovet JM et al (2001) Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol 35:421–430
Bruix J, Sherman M, Practice Guidelines Committee, American Association for the Study of Liver Diseases (2005) Management of hepatocellular carcinoma. Hepatology 42:1208–1236
Kuang M, Lu MD, Xie XY et al (2009) Ethanol ablation of hepatocellular carcinoma up to 5.0 cm by using a multipronged injection needle with high-dose strategy. Radiology 253:552–561
Yin XY, Xie XY, Lu MD et al (2009) Percutaneous thermal ablation of medium and large hepatocellular carcinoma: long-term outcome and prognostic factors. Cancer 115:1914–1923
Chen MH, Yang W, Yan K et al (2004) Large liver tumors: protocol for radiofrequency ablation and its clinical application in 110 patients–mathematic model, overlapping mode, and electrode placement process. Radiology 232:260–271
Strasberg SM (2005) Nomenclature of hepatic anatomy and resections: a review of the Brisbane 2000 system. J Hepato-Biliary-Pancreat Surg 12:351–355
Peng ZW, Zhang YJ, Chen MS et al (2013) Radiofrequency ablation with or without transcatheter arterial chemoembolization in the treatment of hepatocellular carcinoma: a prospective randomized trial. J Clin Oncol 31:426–432
Couinaud C (1956) Contribution of anatomical research to liver surgery. Fr Med 19:5–12
Ahmed M, Solbiati L, Brace CL et al (2014) Image-guided tumor ablation: standardization of terminology and reporting criteria—a 10-year update. Radiology 273:241–260
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240:205–213
Omary RA, Bettmann MA, Cardella JF et al (2003) Quality improvement guidelines for the reporting and archiving of interventional radiology procedures. J Vasc Interv Radiol 14:S293–S295
Goldberg SN, Kruskal JB, Oliver BS, Clouse ME, Gazelle GS (2000) Percutaneous tumor ablation: increased coagulation by combining radio-frequency ablation and ethanol instillation in a rat breast tumor model. Radiology 217:827–831
Wong SN, Lin CJ, Lin CC, Chen WT, Cua IH, Lin SM (2008) Combined percutaneous radiofrequency ablation and ethanol injection for hepatocellular carcinoma in high-risk locations. AJR Am J Roentgenol 190:W187–W195
Mizukoshi E, Yamashita T, Arai K et al (2013) Enhancement of tumor-associated antigen-specific T cell responses by radiofrequency ablation of hepatocellular carcinoma. Hepatology 57:1448–1457
Zerbini A, Pilli M, Fagnoni F et al (2008) Increased immunostimulatory activity conferred to antigen-presenting cells by exposure to antigen extract from hepatocellular carcinoma after radiofrequency thermal ablation. J Immunother 31:271–282
Katz SC, Shia J, Liau KH et al (2009) Operative blood loss independently predicts recurrence and survival after resection of hepatocellular carcinoma. Ann Surg 249:617–623
Shiba H, Ishida Y, Wakiyama S et al (2009) Negative impact of blood transfusion on recurrence and prognosis of hepatocellular carcinoma after hepatic resection. J Gastrointest Surg 13:1636–1642
Nakazawa T, Kokubu S, Shibuya A et al (2007) Radiofrequency ablation of hepatocellular carcinoma: correlation between local tumor progression after ablation and ablative margin. AJR Am J Roentgenol 188:480–488
Poon RT, Fan ST, Tsang FH, Wong J (2002) Locoregional therapies for hepatocellular carcinoma: a critical review from the surgeon's perspective. Ann Surg 235:466–486
Shi M, Guo RP, Lin XJ et al (2007) Partial hepatectomy with wide versus narrow resection margin for solitary hepatocellular carcinoma: a prospective randomized trial. Ann Surg 245:36–43
Ye JZ, Miao ZG, Wu FX, Zhao YN, Ye HH, Li LQ (2012) Recurrence after anatomic resection versus nonanatomic resection for hepatocellular carcinoma: a meta analysis. Asian Pac J Cancer Prev 13:1771–1777
Feng X, Su Y, Zheng S et al (2017) A double blinded prospective randomized trial comparing the effect of anatomic versus non-anatomic resection on hepatocellular carcinoma recurrence. HPB (Oxford) 19:667–674
Kaibori M, Kon M, Kitawaki T et al (2017) Comparison of anatomic and non-anatomic hepatic resection for hepatocellular carcinoma. J Hepatobiliary Pancreat Sci 24:616–626
Morimoto M, Numata K, KondouM NA, Morita S, Tanaka K (2010) Midterm outcomes in patients with intermediate-sized hepatocellular carcinoma: a randomized controlled trial for determining the efficacy of radiofrequency ablation combined with transcatheterarterial chemoembolization. Cancer 116:5452–5460
Iezzi R, Pompili M, La Torre MF et al (2015) Radiofrequency ablation plus drug-eluting beads transcatheter arterial chemoembolization for the treatment of single large hepatocellualr carcinoma. Dig Liver Dis 47:242–248
Maluccio M, Covey AM, Gandhi R et al (2005) Comparison of survival rates after bland arterial embolization and ablation versus surgical resection for treating solitary hepatocellular carcinoma up to 7 cm. J Vasc Interv Radiol 16:955–961
Elnekave E, Erinjeri JP, Brown KT et al (2013) Long-term outcomes comparing surgery to embolization-ablation for treatment of solitary HCC<7 cm. Ann Surg Oncol 20:2881–2886
Xu Y, Shen Q, Liu P et al (2017) Microwave ablation for the treatment of hepatocellular carcinoma that met up-to-seven criteria: feasibility, local efficacy and long-term outcomes. Eur Radiol 27:3877–3887
Ma S, Ding M, Li J et al (2017) Ultrasound-guided percutaneous microwave ablation for hepatocellular carcinoma: clinical outcomes and prognostic factors. J Cancer Res Clin Oncol 143:131–142
Funding
This study has received funding by the National Natural Science Foundation of China (No. 81272312; No. 81301842), Pearl River S&T Nova Program of Guangzhou, China (No. 2014J2200087) and Guangdong Medical Science and Technology Foundation (No. 20161192364982).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Ming Kuang.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors (Bin Li) has significant statistical expertise.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional review board approval was obtained.
Methodology
• retrospective
• observational
• multicentre study
Electronic supplementary material
ESM 1
(DOCX 92 kb)
Rights and permissions
About this article
Cite this article
Chen, S., Peng, Z., Lin, M. et al. Combined percutaneous radiofrequency ablation and ethanol injection versus hepatic resection for 2.1–5.0 cm solitary hepatocellular carcinoma: a retrospective comparative multicentre study. Eur Radiol 28, 3651–3660 (2018). https://doi.org/10.1007/s00330-018-5371-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-018-5371-9