Abstract
Background
Inoperable hepatocellular carcinoma (HCC) can be treated with laparoscopic radiofrequency ablation (LRFA), which is generally a more accurate and accessible procedure than percutaneous RFA (PRFA). However, few studies have compared survival outcomes between LRFA and PRFA in patients with HCC.
Aims
This study aimed to compare the efficacy of LRFA and PRFA for HCC treatment.
Methods
Patients who underwent PRFA or LRFA as an initial treatment modality between April 2005 and April 2016 were enrolled in this study. The overall and recurrence-free survival rates were examined for each patient. Additionally, propensity score matching was performed for both groups.
Results
The baseline characteristics of patients in the PRFA and LRFA groups showed several minor differences. Multivariate analysis showed that the RFA method was not a critical determinant of recurrence-free or overall survival (p = 0.069 and p = 0.406). Among patients who underwent RFA as the initial treatment modality, there was no significant effect between either RFA procedures on survival. After propensity score matching, univariate analysis showed a significant difference in overall survival between PRFA and LRFA (p = 0.031). Multivariate analysis showed that LRFA is a strong factor that contributed to an improved overall survival in HCC patients (hazard ratio 0.108, p = 0.040). Furthermore, our data showed that LRFA was able to limit multiple intrahepatic recurrences, as well as prevent marginal recurrence.
Conclusions
LRFA appears to be superior to PRFA in terms of survival. LRFA may help reduce mortality in HCC patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) is the most common primary malignant neoplasm of the liver and the third principal cause of cancer-related death [1]. Among the HCC treatment modalities, hepatic resection is the gold standard for complete cure and prevention of loco-regional recurrence in patients with hepatic malignancy [2]. However, surgical resection is limited by certain patient-related factors such as hepatic dysfunction, and by tumor-related factors such as tumor size, location, and multiplicity [3]. Therefore, non-surgical techniques such as percutaneous radiofrequency ablation (PRFA) [4, 5], percutaneous ethanol injection [6, 7], and percutaneous microwave coagulation therapy [8, 9] are commonly used for treating HCCs that are unsuitable for hepatic resection. Among these methods, both radiofrequency ablation and microwave ablation are commonly used as therapeutic procedures that provide tumor control and/or radical cure as a part of a multifaceted HCC treatment [10]. Despite several studies showing the clinical advantage of microwave ablation over RFA (microwave ablation materializes faster, has larger and more precise ablation zones), RFA is chosen over microwave ablation in our country, as restrictions from national insurance coverage makes microwave ablation cost-prohibitive [10,11,12]. However, PRFA also can damage or perforate adjacent visceral organs or cause diaphragmatic injury during, before, or after the ablation procedure [3, 13, 14]. To overcome the limitations of PRFA, new devices have been developed. One example is the Certus microwave ablation system with 17-gauge PR (short tip) or LK (long tip) antenna (NeuWave Medical, Madison, Wisconsin). This system was designed to treat deep-seated lesions, even tumors adjacent to vital organs, with hydroinfusion techniques [11, 15]. However, the application of these percutaneous methods is limited for relatively inaccessible hepatic tumor lesions or tumors difficult to detect on ultrasound imaging [16]. Moreover, after PRFA, HCC tumor nodules located near the peripheral hepatic capsule relapse more frequently than other nodules because the safety margin of ablation is relatively small [17, 18]. To overcome these limitations, laparoscopic RFA (LRFA) can be used as an alternative ablation method [4].
LRFA not only ablates tumors that are relatively inaccessible percutaneously but also prevents damage to organs during the procedure. Thus, it can be advantageous for tumors that are inaccessible by PRFA. Moreover, in subcapsular lesions, seeding of malignant tissue to the outside of the tumor during ablation procedure can occur, even if the tumor is not punctured directly for both percutaneous and laparoscopic RFA methods. In this situation at our institution, wet gauze is directly packed on the superficial area of the ablation site of subcapsular tumors during LRFA. It also allows placement of RFA electrodes in multiple sites with enhanced accuracy under real-time intraoperative ultrasound imaging, which can be reconstructed by computed tomography (CT) or magnetic resonance imaging (MRI) [16]. Therefore, LRFA is considered a more accurate and effective treatment modality for HCCs that arise in relatively inaccessible locations compared to PRFA [19, 20]. LRFA is not only a good alternative therapy for HCC that is difficult to access by PRFA, but is also a reliable modality for HCCs that are located on the surface, have multiplicity, or contain tiny HCC nodules that are undetectable by CT or MRI.
If a patient with HCC has a tumor that is treatable by either PRFA or LRFA, it is important for clinicians to select the best ablation method to prevent recurrence and enhance survival. On laparoscopic approach, ablation zone enlargement can be achieved by a 30–40% reduction in liver blood flow by pneumoperitoneum and multifaceted ablations. In addition, tumor ablation limitations, such as, lesions centrally located in the liver parenchyma, were overcome by LRFA [21, 22]. However, LRFA involves higher risks and is more invasive than PRFA, and also carries the inconvenience of requiring general anesthesia. Despite these differences, only a few studies thus far have compared the efficacy of PRFA and LRFA with respect to recurrence and survival in patients.
Therefore, this study aimed to compare LRFA and PRFA in terms of recurrence, mortality, and survival outcomes in patients with HCC.
Methods
Patients
We searched the electronic medical records of Chungnam National University Hospital and enrolled 328 patients who underwent RFA for HCC between April 2005 and April 2016. Of these, 84 patients were excluded owing to having received RFA for secondary hepatic neoplasms or intrahepatic cholangiocarcinoma, or having undergone initial RFA at another institution, transferred to another institution, or being lost to follow-up. Ultimately, the study cohort compromised 244 patients with HCC, 173 who underwent PRFA and 71 who underwent LRFA (Fig. 1). Written informed consent was obtained from all individual participants included in this study before treatment. The study was approved by the Institutional Review Board of Chungnam National University Hospital (IRB Number: 2016-06-009).
Laboratory Measurements
HCC was diagnosed based on imaging tools (such as 3-phase dynamic contrast-enhanced CT and MRI) and tumor markers. Elevation of tumor markers such as α-fetoprotein (AFP) or prothrombin induced by vitamin K absence II (PIVKA-II), history of chronic hepatitis B or C virus infection, and history of alcohol consumption were taken into account. Additionally, we used a model to derive the end-stage liver disease score for both patients with chronic liver disease and those with liver cirrhosis to assess the severity of hepatic dysfunction and mortality risk.
Before ablative treatment, all patients were subjected to basal laboratory tests to determine hemoglobin level, platelet count, total bilirubin level, albumin level, blood urea nitrogen level, creatinine level, and prothrombin time. Additionally, the number of tumors as well as the hepatic segmental locations of tumor nodules was determined. The tumor locations were categorized as present in segments V to VIII or else according to the Couinaud’s classification in the remaining segments. With respect to tumor location, we defined a subcapsular lesion as a tumor located within 1 cm of the Glisson’s capsule. Some of the enrolled patients had previously received therapeutic modalities such as transarterial chemoembolization (TACE) or hepatic resection for HCC before ablation therapy. After these treatments, we considered the tumor of these patients to be in a nearly controlled state based on radiologic evaluation, except in some patients who received TACE with incomplete lipiodol uptake. All complications were graded according to the Clavien–Dindo grading system for classification of surgical complications.
Procedure and Indication for PRFA
PRFA was considered for HCC patients who could not endure surgical removal or ablation of the tumor, or who were not eligible for liver transplantation; it was considered the most appropriate modality for HCC masses measuring <3 cm in diameter and located away from vital organs such as the bowel, bile duct, ureter, or diaphragm because of the risk of perforation or injury. Importantly, PRFA was only considered for tumors that were accessible percutaneously; for example, although not always the case, some tumors with poor visualization or demarcation of the tumor margins on real-time percutaneous ultrasound, such as lesions that were positioned in deep posterior surface segments VI, VII, or VIII of the liver, were not considered for PRFA.
To facilitate the detection and ablation of HCC using PRFA, we use matched images derived from 3-dimensional CT or MRI reconstructions as well as real-time ultrasonography guidance. Moreover, the patients received additional planning ultrasound examination for setting up of probe ablation map the day before procedure. After confirming the feasibility for ablation of a tumor nodule in the liver, patients were administered local anesthesia (20 mL lidocaine) onto the skin surface. During PRFA procedure, the patient was placed in multiple positions to gain the optimal access to the tumor, using either a lateral or posterior approach. If the space between the HCC mass and nearby tissues was <5 mm, normal saline solution was infused into the para-hepatic area using a 7-Fr, 18-gauge needle to create artificial ascites. After confirming the presence of sufficient artificial ascites, standard mono-polar RFA was administered using one of two possible RFA electrodes: a 15-cm-long, 15-gauge or 17-gauge RFA electrode equipped with a 3-cm-long, bare metallic tip (Cool-tip™, Covidien, Medtronic, MN, USA); or either a 15-cm or 20-cm-long, 15-gauge or 17-gauge RFA electrode equipped with a 3-cm-long, bare metallic tip (Proteus™, Starmed, Ilsan, Korea). Patients were treated with a 480-kHz radiofrequency generator (Model CC-1-220; Valleylab, TycoHealthcare, USA) and were administered ablation. A sphere-shaped coagulation area of 2.0–4.0 cm in diameter was produced in one session, which was 12 min in duration on average. The length of the tip, power (wattage), and deploying time of the electrodes were dependent on the size and shape of the tumor. Typically used delivered power was 80–100 W. If the electrode automatically shut down to protect the adjacent normal liver tissue from serious thermal injury, the procedure was stopped. When completing the procedure, additional ablation along the electrode track was performed to destroy viable cells that may have been seeded along the track during electrode withdrawal.
Procedure and Indication for LRFA
LRFA was indicated for tumors that were ineligible for PRFA and for patients who could not undergo tumor resection due to advanced liver cirrhosis. Moreover, LRFA was applied for hepatic tumors near visceral organs or the diaphragm because CO2 is injected into the abdominal cavity of the patient to secure the space between the liver and other organs during laparoscopy. Furthermore, as LRFA can be used to manage emergency surgical problems, it is ideal for treating subcapsular lesions that have a strong tendency to pop-up (seed to the outside of the tumor of malignant tissue) during RFA.
The LRFA procedure was performed as follows. The patient was placed under general anesthesia. A 20-cm-long, 17-gauge RFA electrode equipped with a 2-cm-long, bare metallic tip (Cool-tip™, Covidien, Medtronic, MN, USA), or a 25-cm-long, 17-gauge electrode equipped with a 3-cm-long, bare metallic tip (Cool-tip™, Covidien, Medtronic, MN, USA) was used for ablation. Patients were treated with a 480-kHz radiofrequency generator (Model CC-1-220; Valleylab, TycoHealthcare, USA) and administered standard mono-polar RFA. The length of the tip, wattage, and deploying time of the electrodes depended on the size and shape of the tumor. Usually, used delivered power was 80–100 W on average. If the tumor size was >2 cm, additional ablation was performed to secure a larger safety margin zone of at least 1 cm. For all ablations, a single RF current was transmitted for 12 min.
If the tumor had poor border demarcation on ultrasound or CT imaging, or the tumor did not show typical HCC features, a dynamic liver MRI was performed to check the tumor characteristics, confirm locations, numbers, and sizes of the tumors, and to clarify the tumor boundary. In addition, liver MRI was conducted to examine the existence of tiny tumor nodule besides of the tumor planned to treatment before RFA. To avoid normal liver tissue injury and ensure accurate tumor site targeting, the whole liver was scanned by using intraoperative ultrasonography. The patient’s position varied according to the tumor location; for example, the supine position was chosen if the tumor was seated anteriorly, while 45° right-side-up recumbent position was chosen if the tumor was located on the posterior surface of segment VI or VII to stabilize the tumor during the ablation process. The electrode size was selected such that it was larger than the tumor diameter. The first trocar for the laparoscope was interpolated to the intraperitoneal cavity after the injection of CO2. Other remaining trocars were inserted under the guidance of the first inserted laparoscope. Finally, an RF electrode was carefully inserted at the tumor site for ablation. If the tumor was located superficially, the RFA electrode was inserted through the normal liver tissue to prevent tumor pop-up during ablation.
Tumor Characteristics and Staging
A subcapsular HCC tumor was defined as a tumor located within 1 cm of the hepatic Glisson’s capsule. The primary HCC tumor stages were determined according to the American Joint Committee on Cancer (AJCC) 7th staging system.
Follow-Up
Three-phase dynamic contrast-enhanced CT was performed immediately after LRFA or PRFA and every 3–4 months in the first 2 years for every patient. At each follow-up visit, laboratory tests to measure liver function or α-fetoprotein levels were performed. If HCC recurred during the follow-up period, patients received appropriate management depending on their hepatic function and tumor status.
Propensity Score Matching
The following variables were applied to the propensity model: 3 patient factors that included serum total bilirubin level, background liver disease status, and Child–Pugh classification score; and 4 tumor factors that encompassed subcapsular tumor presence, tumor number, TNM stage, and previous history of TACE after RFA for HCC.
Statistical Analysis
The 2 groups were compared using the Student’s t test for continuous data and the Chi-square test for categorical data. Variables with relative significance for recurrence rate and overall survival rate were analyzed using univariate logistic regression analysis and multivariate Cox proportional hazards regression analysis. The disease-free and overall survival rates were calculated using the Kaplan–Meier method.
The logistic regression model for propensity score matching for both groups and the listed covariates were based on the assessment of the Hosmer–Lemeshow goodness-of-fit statistical test. Subsequently, we used the R package program (MatchIt), and nearest neighbor matching method to adjust for covariance between the 2 groups. All statistical analyses were performed using SPSS version 19.0 (SPSS Inc., Chicago, IL). Differences between 2 groups were analyzed by a 2-tailed Student’s t test. A p value <0.05 was considered statistically significant.
Results
Patient Characteristics
All patient and tumor characteristics are summarized in Table 1. There were no significant differences between the 2 groups in terms of age, sex, and blood chemistry results. Moreover, the α-fetoprotein levels, PIVKA-II levels, and model of end-stage liver disease scores were not significantly different between 2 groups. However, the LRFA group comprised more patients with liver cirrhosis (84.5 vs. 74.6, p = 0.018), multiple tumors (≥2), higher TNM stages, and higher Child–Pugh scores than the PRFA group. Additionally, we did not observe any remarkable disparity in the segmental distribution of HCC tumors or any significant difference in the subcapsular tumor location or site of RFA between the PRFA and LRFA groups. A major proportion of the RFA targets was new lesions in both groups. The PRFA group showed a lower incidence rate of complications than the LRFA group, although this difference was not significant (8.7 vs. 2.8%, respectively, p = 0.104).
Prior Treatments
Some patients in both groups underwent TACE or hepatic resection prior to RFA, with no significant difference in the proportions of these patients between the groups. Eighty-one patients underwent additional TACE for recurring HCC after RFA: 68 in the PRFA group and 13 in the LRFA group (p = 0.002). All prior treatments and patients proportions are presented in Table 1. All complications were grade I pleural effusions.
Recurrence and Overall Survival
The numbers of patients with incomplete ablation (1.7 vs. 0.0%), marginal recurrence (11.0 vs. 2.8%), and new HCC lesions (37.0 vs. 33.8%) were higher in the PRFA group than in the LRFA group, respectively, but without statistical significance on intrahepatic recurrence pattern (p = 0.166). The extrahepatic recurrence rate (11.6 vs. 2.8%, respectively, p = 0.030), but not the intrahepatic recurrence rate (49.7 vs. 36.6%, respectively, p = 0.062), showed a significant difference. Moreover, most of the patients with extrahepatic recurrence showed pulmonary metastases, bone metastases, or multiple, extensive lymph node metastases in their abdominal cavity. Among these patients, one patient was treated with sorafenib, and one patient received only conservative management in the LRFA group. Among patients who received PRFA, four patients underwent best supportive care only, and four patients were treated with palliative radiation therapy for bone metastases, and two of them received sorafenib. In addition, five patients were treated with sorafenib alone, and five with palliative TACE, and four of these patients received sorafenib. One patient underwent a palliative concurrent chemoradiation therapy. Univariate analyses showed that none of the analyzed factors influenced recurrence-free survival. Multivariable analysis showed that PRFA was not a significant predictor of recurrence compared to LRFA [hazard ratio 0.507, 95% confidence interval (CI) 0.243–1.055, p = 0.069] (Table 2). However, serum albumin level (hazard ratio 0.352, 95% CI 0.203–0.613) and extrahepatic recurrence (hazard ratio 3.849, 95% CI 1.800–8.230) were significant predictors of overall survival (Table 3).
Although both the Child–Pugh ‘C’ classification and ≥4 tumors were significant predictors of overall survival on multivariate analysis, we could not determine whether the proportion of patients in these categories were statistically significant because they occurred in no more than 1 patient in each group (Table 3). Moreover, the RFA method was not an important predictor of recurrence-free survival or overall survival (p = 0.607 and 0.085, respectively) (Fig. 2). Multivariate analysis showed that LRFA had a positive but statistically insignificant effect on recurrence-free and overall survivals (Fig. 3).
Effects of the RFA Procedure
To investigate the influence of the type of RFA procedure on recurrence and overall survival in patients with HCC, we selected patients who underwent RFA as their initial treatment. A total of 104 PRFA patients and 33 LRFA patients were selected from their respective groups. The variables related to the clinicopathological features of the tumor were similar between the 2 groups, except for the TNM stage (p = 0.001) and number of subcapsular tumors (p = 0.041) (Table 4). Univariate analysis for recurrence-free survival showed that serum albumin level (p = 0.028), PIVKA-II (p = 0.009), number of tumors (p = 0.039), and TNM stages (p = 0.006) significantly affected survival (Table 5). However, the RFA method did not have a significant effect on recurrence-free survival (p = 0.848). Among the above-mentioned factors, multivariate analysis showed only PIVKA-II to be a significant predictor of recurrence-free survival (p = 0.025).
With respect to overall survival, univariate analysis revealed serum albumin level (p < 0.001), prothrombin time (p = 0.001), pop-up during RFA (p = 0.010), days of hospital stay during initial RFA (p = 0.013), and extrahepatic recurrence (p = 0.004) to be significant predictors. Among these factors, serum albumin (hazard ratio 0.299, 95% CI 0.103–0.868) and extrahepatic recurrence (hazard ratio 6.823, 95% CI 2.025–22.995) were significant predictors of overall survival on multivariate analysis (Table 6). However, the RFA method did not have a remarkable effect on overall survival (hazard ratio 0.319, 95% CI 0.041–2.498) or recurrence-free survival in patients who underwent RFA as the initial treatment modality (Fig. 4). Furthermore, multivariate analysis showed that LRFA may help enhance the overall survival (p = 0.277) (Fig. 5).
Propensity Score Matching
The following variables were applied to the propensity score matching model: serum total bilirubin level, background liver disease, Child–Pugh classification, subcapsular tumor location, number of tumors, TNM stage of the tumor, and TACE treatment after RFA. After propensity score matching, 33 patients each from the PRFA and LRFA groups were selected for additional investigation.
Univariate and Multivariate Analyses
Patients’ clinicopathological factors and tumor factors were comparable between the 2 matched groups, except for a minor difference in the serum albumin levels (Table 7). Univariate and multivariate analyses showed serum albumin level (hazard ratio 0.319, 95% CI 0.118–0.861, p = 0.024), number of tumors (especially ≥2 tumors on multivariate analyses) (hazard ratio 5.569, 95% CI 1.452–21.361, p = 0.012), and number of days of hospital stay during initial RFA procedure (hazard ratio 1.104, 95% CI 1.007–1.210, p = 0.035) were significant factors influencing recurrence-free survival on univariate and multivariate analyses (Table 8).
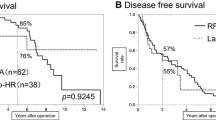
Surprisingly, univariate analysis showed that the RFA method, serum albumin level, and the number of days of hospital stay during initial RFA procedure were important predictors of overall survival. Multivariate analyses showed that the LRFA method, serum albumin level, and number of days of hospital stay were significant factors influencing overall survival (Table 9). Although the RFA method did not have a significant effect on the cumulative recurrence-free survival rate (hazard ratio 1.069, 95% CI 0.336–3.400, p = 0.910), it showed a significant effect on the cumulative overall survival rate (hazard ratio 0.108, 95% CI 0.013–0.906, p = 0.040) (Fig. 6). Furthermore, neither PRFA nor LRFA showed a significant difference in recurrence-free survival rate (p = 0.196); however, LRFA was a significant predictor of overall survival (p = 0.031) (Fig. 7).
Discussion
RFA has been used as a tool for radical cure of HCC that is inoperable owing to patient- and/or tumor-related factors. PRFA is generally used for ablation of superficially accessible tumors. However, it is difficult to properly position the ablation electrode at the site of subcapsular HCCs from the outside and ablate the tumor percutaneously, as it may damage the superficial hepatic area near the tumor and cause pop-up [23, 24]. Moreover, PRFA may lead to insufficient ablation of primary tumors located on the hepatic surface area because the tumor site needs to be approached percutaneously, especially for superficial tumors situated adjacent to the internal organs or diaphragm [3, 13, 14, 25]. Recently, other institutions have infused extremely diluted contrast solution into the para-hepatic area, not only to secure space between liver and adjacent organs, but also to adequately demarcate liver tissue. In addition, to ablate such subcapsular nodules, LRFA or laparoscopic hepatic resection modalities have been used instead [25]; moreover, PRFA itself has recently undergone improvements including the use of multiple imaging techniques and improved ablation tools [26]. Therefore, the appropriate RFA modality ultimately depends on the patients’ condition and tumor status [4]; clinicians should decide whether to use PRFA or LRFA for HCC treatment after considering the risk/benefit ratio in terms of safety margins and surgical invasiveness, as well as the patients wishes [4].
According to several previous studies, LRFA is a useful RFA strategy for ablating HCC tumors; since the tumor location is not a concern, subcapsular neoplasms in the liver or neoplasms close to internal organs can be easily approached, and the local tumor progression rate is low, at only 16.7% [27]. Moreover, LRFA enables direct contact between the intraoperative laparoscopic ultrasonography probe and the surface of a patient’s liver, which has helped discover 25% of small-sized malignant neoplasms that could not be identified by other imaging modalities [24]. Moreover, LRFA allows for performing the Pringle maneuver to reduce hepatic vascular inflow, expand the area of the injured site by thermal coagulation, and ablate tumors located centrally near major blood vessels with a low risk of hemorrhage [4, 20]. As the intraabdominal pressure is elevated by CO2 infusion during the laparoscopic procedure, intrahepatic vascular flow decreases significantly due to vascular collapse, which helps improve the ablation outcome [28]. Moreover, LRFA can be used during emergencies such as tumor pop-ups or hemorrhaging during ablation of surface HCCs or tumors near a major vessel. LRFA also allows repetitive overlapping ablation for complete coagulation of HCC tumors, when necessary, by enabling the reinsertion of the ablating needle through multiple parallel approaches [20]. Thus, LRFA can be an effective treatment for surface HCCs, those that are difficult to access, or those that are small satellite lesions, with large safety margins. Consequently, LRFA may produce lower recurrence and mortality rates than PRFA among patients with HCC.
Randomized controlled trials are required to compare PRFA and LRFA and determine their effect on recurrence and mortality. However, such comparative studies may not be feasible because some HCCs are clinically eligible for only one of the RFA procedures. Furthermore, many patients with HCC cannot endure general anesthesia because of their medical condition and have to undergo PRFA by default [20]. Indeed, patients in our study who underwent LRFA had poorer hepatic functional statuses than those who underwent PRFA. Moreover, there were several differences in the baseline characteristics of patients in the PRFA versus LRFA groups.
Local recurrence is one of the main setbacks experienced by patients undergoing PRFA, as it is a major indicator of a poor prognosis [29]. Because LRFA has a broad ablation margin, it may lead to lower recurrence and mortality rates. Several studies found that LRFA produces better tumor loco-regional recurrence control as compared to PRFA; a previous meta-analysis indicated that LRFA showed more prominent local control compared to PRFA, independent of the size of the HCC [30]. Masashi et al. concluded that the safety ablation margin was much broader when using LRFA than PRFA; as such, LRFA is more efficacious for curative treatment of HCCs measuring >2 cm in diameter [19].
In our study, the 1- and 2-year loco-regional recurrence rates of patients who underwent LRFA were 0% each, compared to 0 and 17.5% on other study, respectively, for PRFA [19]. Previous studies revealed HCC loco-regional recurrence rates of 1.3–38.6% after PRFA, most reported recurrence rates of approximately 20% [25, 26, 31,32,33,34,35]. On the other hand, the loco-regional recurrence rates of HCC after LRFA were 3.3–47.4% (mean, 10%) [25, 27, 36, 37]. In our study, the mean local intrahepatic recurrence rate of HCC following LRFA was 36.6% and that following PRFA was 49.7% (p = 0.062), likely because our LRFA patient population had a higher proportion of patients with high tumor stages of HCC, as well as multiple tumors, than the PRFA patient population.
Patients in the PRFA group (26.6%) had significantly higher mortality than those in the LRFA group (7.0%) (p = 0.001), although the recurrence and overall survival rates did not differ significantly between these groups. Furthermore, PRFA did not significantly influence recurrence and mortality. A previous study showed no significant differences in tumor recurrence or survival rates between PRFA and LRFA, whether short term or long term [20]. Such historical data are consistent with our results, even though our patients underwent RFA as the initial treatment modality for HCC and excluded those with secondary hepatic metastasis.
Patients in the PRFA and LRFA groups showed difference in mortalities, but not in recurrence rates. Since the baseline characteristics for these patients were significantly different, we accounted for several factors relating to recurrence and survival by using propensity score matching to adjust for patient background status and tumor conditions between the 2 groups. Strikingly, LRFA was found to be important for overall survival among HCC patients; those underwent LRFA had a higher rate of survival, but not of recurrence, than those who underwent PRFA. Hence, one of our most important findings was that survival rates in patients who underwent LRFA were superior to those who underwent PRFA after propensity score matching.
To understand this key outcome, we assessed both intrahepatic and extrahepatic recurrence rates but found no statistical difference between these parameters. On analyzing the recurrence patterns in patients individually, however, we found that PRFA patients had a higher proportion of marginal tumor recurrences (28.6%) and intrahepatic multiple tumor recurrences (57.1%); on the other hand, LRFA patients experienced a higher proportion of intrahepatic solitary tumor recurrences (60.0%) and no marginal recurrences. These results suggested that, under similar patient and tumor conditions, LRFA did not curtail HCC recurrence when used as the initial treatment modality. However, LRFA may have helped reduce the disease-critical recurrences of HCC and therefore lower patient mortality rates. Moreover, the short-term advantages of the low invasiveness of the PRFA procedure did not outweigh its long-term risk of mortality. Therefore, PRFA should only be performed in patients who cannot endure LRFA with general anesthesia.
We found that LRFA could be a potentially contributing factor in terms of improving overall survival and it has profound implications for selecting the appropriate RFA modality for HCC patients. At the same time, our study had several limitations. First, the number of patients was relatively small, especially in the LRFA group. Second, our study was retrospective, observational, and non-randomized; therefore, selection bias was unavoidable. Additional randomized, large-scale studies are needed to compare LRFA and PRFA with propensity score matching. Although our study did not definitively determine the superiority of LRFA over PRFA for recurrence and overall survival, our findings suggest that LRFA may contribute to an improved overall survival of the HCC patients. Recently, new ablative devices were developed to overcome the limitations of the conventional PRFA method. However, ablation using microwave ablation (MWA) device was not covered by national insurance and was not commonly available on our country during the study enroll periods. Therefore, if the current generation microwave devices were actively used in our country, it would be novel to compare percutaneous ablation with the current generation of microwave devices, such as the Neuwave MWA device or Medtronic MWA Emprint devices, to either laparoscopic ablation with conventional LRFA devices or the previous generation of microwave devices.
In conclusion, LRFA has various advantages over PRFA and can accurately approach tumors that are difficult to access and ablate by the latter, including those located in the subcapsular area or those in nearby hollow viscous or vessels. Even relatively huge HCC tumors were pierced several times until entire HCC mass was ablated by LRFA. Furthermore, by controlling the patient’s breathing by using general anesthesia, LRFA allows for more accurate and safe ablation than PRFA. Although LRFA does not reduce the recurrence rate compared to LRFA, it can curtail recurrences that directly influence patients’ mortality and can therefore be considered a radical curative modality for HCC. Our study confirmed that LRFA not only has technical merits but also exhibits superiority in terms of survival. Thus, LRFA is a more effective treatment modality than PRFA for patients with HCC.
References
Thomas MB, Jaffe D, Choti MM, et al. Hepatocellular carcinoma: consensus recommendations of the National Cancer Institute Clinical Trials Planning Meeting. J Clin Oncol. 2010;28:3994–4005.
Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022.
Lau WY, Leung TWT, Yu SCH, Ho SKW. Percutaneous local ablative therapy for hepatocellular carcinoma: a review and look into the future. Ann Surg. 2003;237:171–179.
Tanabe KK, Curley SA, Dodd GD, Siperstein AE, Goldberg SN. Radiofrequency ablation: the experts weigh in. Cancer. 2004;100:641–650.
Cha J, Rhim H, Lee JY, et al. Percutaneous radiofrequency ablation of hepatocellular carcinoma: assessment of safety in patients with ascites. Am J Roentgenol. 2009;193:424–429.
Shiina S, Tateishi R, Imamura M, et al. Percutaneous ethanol injection for hepatocellular carcinoma: 20-year outcome and prognostic factors. Liver Int. 2012;32:1434–1442.
Mahnken AH, Bruners P, Gunther RW. Local ablative therapies in HCC: percutaneous ethanol injection and radiofrequency ablation. Dig Dis. 2009;27:148–156.
Inokuchi R, Seki T, Ikeda K, et al. Percutaneous microwave coagulation therapy for hepatocellular carcinoma: increased coagulation diameter using a new electrode and microwave generator. Oncol Rep. 2010;24:621–627.
Ohmoto K, Mimura N, Iguchi Y, et al. Percutaneous microwave coagulation therapy for superficial hepatocellular carcinoma on the surface of the liver. Hepatogastroenterology. 2003;50:1547–1551.
Poulou LS, Botsa E, Thanou I, Ziakas PD, Thanos L. Percutaneous microwave ablation vs radiofrequency ablation in the treatment of hepatocellular carcinoma. World J Hepatol. 2015;7:1054–1063.
Thamtorawat S, Hicks RM, Yu J, et al. Preliminary outcome of microwave ablation of hepatocellular carcinoma: breaking the 3-cm barrier? J Vasc Interv Radiol. 2016;27:623–630.
Facciorusso A, Di Maso M, Muscatiello N. Microwave ablation versus radiofrequency ablation for the treatment of hepatocellular carcinoma: a systematic review and meta-analysis. Int J Hyperthermia. 2016;32:339–344.
Curley SA, Marra P, Beaty K, et al. Early and late complications after radiofrequency ablation of malignant liver tumors in 608 patients. Ann Surg. 2004;239:450–458.
Kong W-T, Zhang W-W, Qiu Y-D, et al. Major complications after radiofrequency ablation for liver tumors: analysis of 255 patients. World J Gastroenterol. 2009;15:2651–2656.
Mcwilliams JP, Plotnik AN, Sako EY, et al. Safety of hydroinfusion in percutaneous thermal ablation of hepatic malignancies. J Vasc Interv Radiol. 2014;25:1118–1124.
Santambrogio R, Opocher E, Montorsi M. Laparoscopic radiofrequency ablation of hepatocellular carcinoma: a critical review from the surgeon’s perspective. J Ultrasound. 2008;11:1–7.
Inoue T, Minami Y, Chung H, et al. Radiofrequency ablation for hepatocellular carcinoma: assistant techniques for difficult cases. Oncology. 2010;78:94–101.
Thamtorawat S, Limsuwarn P, Tongdee T, Chaiyasoot W, Siriapisith T. Incidence of complication and tumor recurrence after radiofrequency ablation in high-risk location of hepatocellular carcinoma patients. J Med Assoc Thai. 2014;97:95–100.
Hirooka M, Kisaka Y, Uehara T, et al. Efficacy of laparoscopic radiofrequency ablation for hepatocellular carcinoma compared to percutaneous radiofrequency ablation with artificial ascites. Dig Endosc. 2009;21:82–86.
Wong J, Lee KF, Yu SCH, et al. Percutaneous radiofrequency ablation versus surgical radiofrequency ablation for malignant liver tumours: the long term results. HPB. 15:595–601.
Jakimowicz J, Stultiens G, Smulders F. Laparoscopic insufflation of the abdomen reduces portal venous flow. Surg Endosc. 1998;12:129–132.
Herbold T, Wahba R, Bangard C, et al. The laparoscopic approach for radiofrequency ablation of hepatocellular carcinoma-indication, technique and results. Langenbecks Arch Surg. 2013;398:47–53.
Okabayashi T, Kobayashi M, Akimori T, et al. Usefulness of laparoscopic radiofrequency ablation of hepatocellular carcinoma. Surg Technol Int. 2005;14:177–181.
Santambrogio R, Podda M, Zuin M, et al. Safety and efficacy of laparoscopic radiofrequency of hepatocellular carcinoma in patients with liver cirrhosis. Surg Endosc. 2003;17:1826–1832.
Ito T, Tanaka S, Iwai S, et al. Outcomes of laparoscopic hepatic resection versus percutaneous radiofrequency ablation for hepatocellular carcinoma located at the liver surface: a case–control study with propensity score matching. Hepatol Res. 2016;46:565–574.
Nishigaki Y, Hayashi H, Tomita E, et al. Usefulness of contrast-enhanced ultrasonography using Sonazoid for the assessment of therapeutic response to percutaneous radiofrequency ablation for hepatocellular carcinoma. Hepatol Res. 2015;45:432–440.
Lee SD, Han H-S, Cho JY, et al. Safety and efficacy of laparoscopic radiofrequency ablation for hepatic malignancies. J Korean Surg Soc. 2012;83:36–42.
Smith MK, Mutter D, Forbes LE, Mulier S, Marescaux J. The physiologic effect of the pneumoperitoneum on radiofrequency ablation. Surg Endosc. 2004;18:35–38.
Arimura E, Kotoh K, Nakamuta M, et al. Local recurrence is an important prognostic factor of hepatocellular carcinoma. World J Gastroenterol. 2005;11:5601–5606.
Mulier S, Ni Y, Jamart J, et al. Local recurrence after hepatic radiofrequency coagulation: multivariate meta-analysis and review of contributing factors. Ann Surg. 2005;242:158–171.
Tateishi R, Shiina S, Teratani T, et al. Percutaneous radiofrequency ablation for hepatocellular carcinoma. Cancer. 2005;103:1201–1209.
Curley SA, Izzo F, Ellis LM, Nicolas Vauthey J, Vallone P. Radiofrequency ablation of hepatocellular cancer in 110 patients with cirrhosis. Ann Surg. 2000;232:381–391.
Takahashi S, Kudo M, Chung H, et al. Initial treatment response is essential to improve survival in patients with hepatocellular carcinoma who underwent curative radiofrequency ablation therapy. Oncology. 2007;72:98–103.
Hori T, Nagata K, Hasuike S, et al. Risk factors for the local recurrence of hepatocellular carcinoma after a single session of percutaneous radiofrequency ablation. J Gastroenterol. 2003;38:977–981.
Abdelaziz A, Elbaz T, Shousha HI, et al. Efficacy and survival analysis of percutaneous radiofrequency versus microwave ablation for hepatocellular carcinoma: an Egyptian multidisciplinary clinic experience. Surg Endosc. 2014;28:3429–3434.
Doi K, Beppu T, Ishiko T, et al. Endoscopic radiofrequency ablation in elderly patients with hepatocellular carcinoma. Anticancer Res. 2015;35:3033–3040.
Sakaguchi H, Seki S, Tsuji K, et al. Endoscopic thermal ablation therapies for hepatocellular carcinoma: a multi-center study. Hepatol Res. 2009;39:47–52.
Acknowledgments
This study was approved by the Institutional Review Board of Chungnam National University Hospital (IRB Number: 2016-06-009).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human rights
For this type of retrospective study, formal consent is not required.
Informed consent
Informed consent was obtained from all individual participants included in this study.
Rights and permissions
About this article
Cite this article
Eun, H.S., Lee, B.S., Kwon, I.S. et al. Advantages of Laparoscopic Radiofrequency Ablation Over Percutaneous Radiofrequency Ablation in Hepatocellular Carcinoma. Dig Dis Sci 62, 2586–2600 (2017). https://doi.org/10.1007/s10620-017-4688-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-017-4688-6