Abstract
Background
Patients with single small hepatocellular carcinoma (HCC) can be managed by surgical resection or radio frequency ablation (RFA), with similar recurrence and survival rates. Recently, minimally invasive surgery (MIS) has been introduced in liver surgery, and the advantage/drawback balance between surgery and RFA needs reassessment.
Methods
Patients with Child-Pugh class A or B cirrhosis, and with single 1–3 cm HCC, undergoing MIS (laparoscopic or robot-assisted) or RFA from July 1998 to December 2012 were compared.
Results
Overall, 45 patients underwent MIS, and 60 underwent RFA. Groups were not statistically different regarding type of underlying liver disease, HCC size, and AFP. However, RFA patients showed worse liver synthetic function with lower albumin and higher bilirubin serum levels, and higher ASA scores. Patients with HCC in segments 2–6 were more often treated by MIS. The incidence of complications was similar between groups (RFA: 6/60, 10 % vs. MIS: 5/45, 11 %, p = 0.854), and there was no measurable difference in the rate of procedure-related blood transfusions (RFA: 1/60, 1.7 % vs. MIS: 3/45, 6.7 %, p = 0.185). Local recurrence was only detected after RFA (11.7 %, p = 0.056, log-rank). Overall survival was higher in the MIS group (p = 0.042), with median survivals of 100 ± 13.5 versus 68 ± 15.9 months.
Conclusion
The present data need further validation. Selected patients with single ≤3-cm HCCs can be safely treated by MIS, without increased risk of perioperative complication, and with a lower risk of local recurrence. MIS should be especially favoured in patients with peripheral HCCs in segments 2–6, and/or when a histological assessment is desirable.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
The management and expected outcome of patients with hepatocellular carcinoma (HCC) vary greatly according to the underlying liver disease and the stage of the cancer. According to the BCLC algorithm, therapeutic options include liver resection, radio frequency ablation (RFA), liver transplantation, chemo-embolization, sorafenib, and best supportive care [1].
Patients with single early (≤3 cm) HCCs demonstrate the best potential outcomes with expected five-year survival rates of 40–70 % [1]. In the presence of a poorly compensated liver disease and/or an increased hepatic venous pressure gradient, patients are best treated by RFA. Conversely, patients with well-compensated (mostly Child A) liver disease can undergo liver surgery, and even more when the location of the HCC only requires a minor resection. In addition, a number of patients can be equally managed by both RFA and liver resection procedures.
The published evidence has compared RFA with the classical open liver surgery. Taking all data together, both approaches appear similar in terms of survival and recurrence rates for the management of patients with early (≤3 cm) HCCs [2, 3]. However, RFA is associated with fewer complications and shorter length of stay, appears more cost-effective, and leads to a better post-treatment quality of life than open surgery [4–6]. Conversely, surgery offers a lower risk of local intra-hepatic HCC recurrence and provides a specimen for pathological analysis [3].
Recently, minimally invasive liver surgery (MIS) has emerged as a valuable treatment option for selected patients, and the number of published laparoscopic and robotic-assisted cases is increasing rapidly [7, 8]. Such an approach appears associated with less blood loss and fewer complications than open liver surgery [7, 9]. As a consequence, the advantage/drawback balance of both RFA and liver surgery must be reassessed. Specifically, one can wonder whether the increased rate of complication after liver surgery for HCC holds at the MIS era.
The aim of this case–control study was to compare RFA with MIS for the management of patients with single ≤3-cm HCCs.
Patients and methods
The study compared baseline characteristics and outcomes of patients undergoing percutaneous RFA versus MIS in a case–control design. It retrospectively assessed prospectively acquired databases at the General Hospital Henry Mondor, Creteil, France, and at the University Hospitals of Geneva, Geneva, Switzerland. The two centers were selected because they share common policies, with the aim to reach a meaningful sample size.
Patients undergoing HCC treatment by either MIS, including laparoscopic or robot-assisted surgery, or imaging-guided RFA from July 1998 to December 2012 were considered. According to the local policy of treating patients with compensated liver disease, only patients with Child-Pugh class A or B biopsy-proven cirrhosis were included, and to homogenize the population, patients with a single, 1–3 cm HCC were selected.
Data of patients having undergone MIS were compared with those of patients having undergone RFA. Matching criteria included number of HCC = 1, HCC diameter = 1–3 cm, presence of histology-proven cirrhosis, Child score = A or B, and absence of ascites.
Diagnosis of HCC was based on noninvasive findings or histopathology, according to the European Association for Study of Liver (EASL) consensus criteria [10].
The type of treatment was determined in dedicated multidisciplinary team discussions including surgeons, hepatologists, oncologists, interventional radiologists, and pathologists, according to HCC location, liver function, and the presence of comorbidities. Exclusion criteria for MIS or RFA were extra-hepatic metastasis, macroscopic evidence of tumor invasion in the portal vein, hepatic vein or inferior vena cava, portal hypertension (HVPG > 10 mmHg), and decompensated liver cirrhosis with ascites.
Collected variables
Patient characteristics included gender, age, type of underlying liver disease, Child-Pugh score, American Society of Anesthesiology (ASA) score, body mass index (BMI), bilirubin, prothrombin time, creatinin, albumin, and platelet count. HCC data included long-axis diameter, location, and alpha-fetoprotein (AFP). Patients underwent similar preoperative assessments, including abdominal ultrasonography (US), computed tomography (CT), and/or magnetic resonance (MR) imaging. Procedure-related variables included operative time, blood loss, blood transfusion, and length of hospital stay. Complications were classified according to the Dindo–Clavien grading system [11].
Survival and recurrence rates were assessed from the time of the initial MIS or RFA intervention until last follow-up visit. Patients undergoing subsequent liver transplantation were censored at the time of transplantation.
Recurrence-free survival was calculated from the time of the initial MIS or RFA until the follow-up visit with evidence of local tumor progression or new tumor. Local tumor progression was defined as the appearance of foci of HCC close to a tumor that had been previously considered as completely ablated or resected. New recurrence was considered as new tumor growing at a distance from the original site.
MIS procedure
The MIS procedure has been described previously [12–15]. Port placement and size were based on tumor location. Three to four trocars between 5 and 12 mm were used. Liver parenchymal transection was carried out using a combination of harmonic scalpel, Cavitron ultrasonic surgical aspirator, and vascular staplers. In a number of patients, the procedure was robot assisted, using the Da Vinci SI robot (Intuitive, Sunnyvale, CA). The liver specimen was placed into a plastic bag and extracted through the umbilical incision without fragmentation. A Pringle’s maneuver was performed if necessary. The hand-assisted technique was not used.
Tumor-free resection margin (R0) was defined as >1 mm.
RFA procedure
All RFA procedures were performed percutaneously under general anesthesia or short-acting intravenous sedation. Placement of RFA electrodes was performed under US, CT, or MRI guidance [16]. Of note, CT and rarely MRI were only considered when HCC was not visible on US. One bipolar electrode with an appropriate active tip length was placed centrally within small HCC nodules (10–15 mm). Two or three electrodes were placed in parallel in the outer third of larger HCC nodules (16–30 mm). Whenever a nodule was in the vicinity of a large vessel, at least one electrode was placed between the HCC and the vessel. Percutaneous RFA ablations were achieved with a 470-kHz RF generator (CelonLab Power), which can manage simultaneously up to three bipolar electrodes by automatic impedance feedback. The initial power output was chosen between 20 and 120 W (mean, 61 ± 32 W), depending on tumor size and number of electrodes. The endpoint of energy deposition was based on empirical parameters, taking into account tumor size and geometry, and an intended safety margin of at least 10 mm.
The outcome of RFA was based on recommendations by the International Working Group on Image-Guided Tumor Ablation [17]. Technical success and early complications were assessed by a contrast-enhanced CT performed 24 h after RFA. Ablative margins were evaluated by image fusion in the axial and coronal planes. One month after treatment, technical effectiveness was assessed by using contrast-enhanced CT or MR imaging.
Postoperative care
Post-procedural follow-up was similar between groups, and at both institutions, including AFP, and CT or MRI every 3 months for the first year after resection or RFA, every 4 months for the second year, and every 6 months thereafter. The primary and secondary technique efficacy rates were calculated during the imaging follow-up.
Statistical analysis
Patient characteristics were compared with the use of Fischer and Mann–Whitney tests. Survival and incidence of HCC recurrence were assessed using the Kaplan–Meier method and compared with log-rank test. Results were provided as median (minimum–maximum). Standard alpha level of 0.05 indicated statistical significance. Analyses were conducted using SPSS 18.0 (SPSS, Chicago, IL).
Results
Patient and HCC characteristics
During the study period, 156 and 235 patients were treated by MIS or percutaneous RFA for HCC, respectively. Among them, 105 with Child-Pugh class A or B cirrhosis and single 1–3 cm HCC were included in the study. Forty-five patients underwent MIS, including 40 laparoscopic resections and five robotic-assisted resections. Sixty patients underwent RFA under US (n = 49), CT (n = 7), or MR (n = 4) imaging guidance.
Baseline characteristics are shown in Table 1. Both groups were not statistically different regarding age, type of underlying liver disease, and Child-Pugh score. However, patients undergoing RFA demonstrated worse liver synthetic function with lower albumin serum concentrations and higher bilirubin levels. In addition, RFA patients also tended to have more comorbidities as reflected by more ASA score 3 patients (45 vs. 15.6 %, p = 0.002). Platelet counts were similar between groups.
HCC size and AFP were similar between groups (2.3 vs. 2.1 cm, p = 0.378; 10.2 vs. 9 ng/ml, p = 0.847, Table 2). However, fewer HCCs were located in segments 7 and 8 in the MIS group compared with the RFA group (3/45, 6.7 % vs. 23/60, 38 %, p < 0.001, Table 2). In the RFA group, 13 patients demonstrated an HCC in the vicinity (<5 mm) with a large vessel (>3 mm). Five patients did so in the MIS group.
Peri-procedural characteristics
The MIS procedures included 28 wedge resections, 9 segmentectomies, and 8 bisegmentectomies. Procedure duration was longer in the MIS group (180 vs. 28 min, p < 0.001, Table 3). The median intra-operative blood loss was 200 (20–2000) ml in the MIS group. One percutaneous RFA patient had a post-procedure subcapsular hematoma and required the transfusion of two red blood cell units. As a result, the number of patients requiring a procedure-related transfusion was not statistically different between groups (RFA: 1/60, 1.7 % vs. MIS: 3/45, 6.7 %, p = 0.185). All MIS patients demonstrated a R0 resection, and 12/45 demonstrated microvascular invasion on the specimen.
The rate of complications was similar between groups. Most were Dindo/Clavien classification stage 1 and 2. Stage 3 complications included a biloma requiring percutaneous drainage (after MIS), and a subcapsular hemorrhage requiring arterial embolization and a pneumothorax requiring chest tube insertion (after RFA). No procedure-related 30-day mortality was observed.
The median hospital stay was 6 (1–16) days in the MIS group versus 1 day (range 1–12 days) in the RFA group (p < 0.001).
HCC recurrence and survival
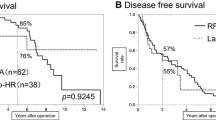
After a median follow-up of 26 (2–129) months, a local tumor progression was detected in 11.7 % (7/60) of patients in the RFA group, while no MIS patient had a local recurrence (p = 0.056, log-rank, Fig. 1A). These RFA-related local tumor progressions were identified during follow-up imaging in five patients, and on the explant pathology following transplantation in two patients. The risk of local HCC progression was not different between patients with HCC in the vicinity of a large vessel versus others (2/13 vs. 5/47, p = 0.64). Patients with local HCC progression after RFA were treated by RFA (n = 3), trans-arterial chemo-embolization (n = 2), and transplantation (n = 2). The incidence of all-site recurrence was not statistically different between groups (21/45, 46.7 % vs. 34/60, 56.7 %, p = 0.906 on log-rank, Fig. 1B). During follow-up, the primary and secondary technique efficacy rates of percutaneous RFA were 88 and 93 %, respectively. Overall survival was significantly higher in the MIS group (p = 0.042, Fig. 2A), with median survivals of 100 ± 13.5 and 68 ± 15.9 months for the MIS and RFA groups, respectively. In the MIS group, 6 deaths were related to HCC, 3 to non-liver-related causes, and 1 to a decompensate cirrhosis. In the RFA group, 10 deaths were related to HCC, 7 to non-liver-related causes, and 2 to a decompensate cirrhosis. In an effort to understand whether this observed difference was linked to the severity of the original liver disease, an overall survival analysis was also conducted only looking at Child-Pugh A patients. The trend remained with longer median survivals in the MI group (100 ± 10.8 and 75 ± 26.4 months, p = 0.147, Fig. 2B).
Discussion
This study suggests that MIS offers at least similar overall and disease-free survivals as percutaneous RFA in the management of selected patients with single ≤3-cm HCCs. Complication rates are similar between both strategies.
Patients with well-compensated liver disease (Child A and early Child B) and low (≤10 mmHg) hepatic venous pressure gradient have the potential of being treated by both RFA and MIS. The present data confirm that both strategies are valuable and allow for similar post-treatment survivals.
Of interest, the increased risk of complication previously seen in HCC patients treated by open liver surgery is not observed anymore with the use of liver MIS and modern perioperative patient management [5]. Both MIS and percutaneous RFA were associated with low complication rates (≤11 %), with most being minor and easily treatable (Dindo–Clavien stages 1 and 2, Table 3). Overall, the surgical stress is less intense after MIS compared with open liver surgery with fewer complications, shorter length of stay, and less bleeding, and the gap between MIS and RFA is closing [9]. In addition, MIS helps preserving the integrity of the abdominal wall and round ligament porto-systemic shunts and could help maintaining the portal pressure low.
In practice, the choice between MIS and RFA should be guided by a number of points. HCC location remains a key element as the MIS approach should still be favored in patients with HCCs located in the left and inferior segments [18]. To illustrate, most studied patients with HCCs in segments 2–6 were treated by MIS. Conversely, central lesions would require a larger surgical parenchymal sacrifice and are better treated by RFA. In addition, a radical surgical resection should be favored when an anatomo-pathological expertise of the HCC is desirable. This is of special interest when guiding a potential subsequent liver transplantation according to the HCC stage and microvascular invasion. Finally, the surgical approach helps minimizing the risk of local intra-hepatic recurrence (none observed in the present study). Such recurrences can appear because of preexisting microscopic tumor foci that are undetected by imaging, or malignant cells that have been disseminated during RFA. A local recurrence was found 7/60 patients treated by percutaneous RFA.
The present study includes a reasonable number of patients for the MIS field (n = 45). However, it is limited by its retrospective nature, and all studied patients were likely not eligible for both procedures. To illustrate, MIS patients tended to demonstrate better liver function, with higher albumin and lower bilirubin serum levels. Ideally, a randomized clinical trial would be needed to solve these limitations. Alternatively, further validation would require the inclusion of more patients, which could allow for a better adjustment for the stage of the underlying liver disease and for the potential presence of portal hypertension. Such data are especially desirable to better capture the impact of MIS on survival.
Overall, the main message of the present assessment is that patients with single ≤3-cm HCCs can be safely treated by MIS, without increased risk of perioperative complication, and with a lower risk of local recurrence. This strategy should be especially favored in patients with peripheral HCCs in segments 2–6, and/or when a histological assessment is desirable.
References
Llovet JM, Ducreux M, Lencioni R, Di Bisceglie AM, Galle PR, Dufour JF, Greten TF, Raymond E, Roskams T, De Baere T, Ducreux M, Mazzaferro V, Bernardi M, Bruix J, Colombo M, Zhu A (2012) EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 56(4):908–943
Wang Y, Luo Q, Li Y, Deng S, Wei S, Li X (2014) Radiofrequency ablation versus hepatic resection for small hepatocellular carcinomas: a meta-analysis of randomized and nonrandomized controlled trials. PLoS ONE 9(1):e84484. doi:10.1371/journal.pone.0084484
Feng K, Yan J, Li X, Xia F, Ma K, Wang S, Bie P, Dong J (2012) A randomized controlled trial of radiofrequency ablation and surgical resection in the treatment of small hepatocellular carcinoma. J Hepatol 57(4):794–802. doi:10.1016/j.jhep.2012.05.007
Cucchetti A, Piscaglia F, Cescon M, Colecchia A, Ercolani G, Bolondi L, Pinna AD (2013) Cost-effectiveness of hepatic resection versus percutaneous radiofrequency ablation for early hepatocellular carcinoma. J Hepatol 59(2):300–307. doi:10.1016/j.jhep.2013.04.009
Weis S, Franke A, Mössner J, Jakobsen JC, Schoppmeyer K (2013) Radiofrequency (thermal) ablation versus no intervention or other interventions for hepatocellular carcinoma. Cochrane Database Syst Rev 12:CD003046. doi:10.1002/14651858
Huang G, Chen X, Lau WY, Shen F, Wang RY, Yuan SX, Geng WX, Zhou WP (2014) Quality of life after surgical resection compared with radiofrequency ablation for small hepatocellular carcinomas. Br J Surg 101(8):1006–1015. doi:10.1002/bjs.9539
Yin Z, Fan X, Ye H, Yin D, Wang J (2013) Short- and long-term outcomes after laparoscopic and open hepatectomy for hepatocellular carcinoma: a global systematic review and meta-analysis. Ann Surg Oncol 20(4):1203–1215. doi:10.1245/s10434-012-2705-8
Buchs NC, Oldani G, Orci LA, Majno PE, Mentha G, Morel P, Toso C (2013) Current status of robotic liver resection: a systematic review. Expert Rev Anticancer Ther 14(2):237–246. doi:10.1586/14737140.2014.863155
Afaneh C, Kluger MD (2013) Laparoscopic liver resection: lessons at the end of the second decade. Semin Liver Dis 33(3):226–235. doi:10.1055/s-0033-1351780
Llovet JM, Ducreux M, Lencioni R, Di Bisceglie AM, Galle PR, Dufour JF, Greten TF, Raymond E, Roskams T, De Baere T, Ducreux M, Mazzaferro V, Bernardi M, Bruix J, Colombo M, Zhu A (2012) EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 56(4):908–943
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240(2):205–213
Cherqui D, Laurent A, Mocellin N, Tayar C, Luciani A, Van Nhieu JT, Decaens T, Hurtova M, Memeo R, Mallat A, Duvoux C (2009) Liver resection for transplantable hepatocellular carcinoma, long-term survival and role of secondary liver transplantation. Ann Surg 250:738–746
Bryant R, Laurent A, Tayar C, Cherqui D (2009) Laparoscopic liver resection—understanding its role in current practice :the Henri Mondor Hospital experience. Ann Surg 250:103–111
Salloum C, Subar D, Memeo R, Tayar C, Laurent A, Malek A, Azoulay D (2014) Laparoscopic robotic liver surgery: the Henri Mondor initial experience of 20 cases. J Robot Surg 8:119–124
N’Kontchou G, Aout M, Laurent A, Nahon P, Ganne-Carrié N, Grando V, Baghad I, Roulot D, Trinchet JC, Sellier N, Cherqui D, Vicaut E, Beaugrand M, Seror O (2012) Survival after radiofrequency ablation and salvage transplantation in patients with hepatocellular carcinoma and Child-Pugh A cirrhosis. J Hepatol 56(1):160–166
Terraz S, Constantin C, Majno PE, Spahr L, Mentha G, Becker CD (2007) Image-guided multipolar radiofrequency ablation of liver tumours: initial clinical results. Eur Radiol 17(9):2253–2261
Ahmed M, Solbiati L, Brace CL, Breen DJ, Callstrom MR, Charboneau JW, Chen MH, Choi BI, de Baère T, Dodd GD 3rd, Dupuy DE, Gervais DA, Gianfelice D, Gillams AR, Lee FT Jr, Leen E, Lencioni R, Littrup PJ, Livraghi T, Lu DS, McGahan JP, Meloni MF, Nikolic B, Pereira PL, Liang P, Rhim H, Rose SC, Salem R, Sofocleous CT, Solomon SB, Soulen MC, Tanaka M, Vogl TJ, Wood BJ, Goldberg SN, International Working Group on Image-Guided Tumor Ablation, Interventional Oncology Sans Frontières Expert Panel, Technology Assessment Committee of the Society of Interventional Radiology, Standard of Practice Committee of the Cardiovascular and Interventional Radiological Society of Europe (2014) Image-guided tumor ablation: standardization of terminology and reporting criteria-a 10-year update. J Vasc Interv Radiol 25(11):1691–1705
Buell JF, Cherqui D, Geller DA, O’Rourke N, Iannitti D, Dagher I, Koffron AJ, Thomas M, Gayet B, Han HS, Wakabayashi G, Belli G, Kaneko H, Ker CG, Scatton O, Laurent A, Abdalla EK, Chaudhury P, Dutson E, Gamblin C, D’Angelica M, Nagorney D, Testa G, Labow D, Manas D, Poon RT, Nelson H, Martin R, Clary B, Pinson WC, Martinie J, Vauthey JN, Goldstein R, Roayaie S, Barlet D, Espat J, Abecassis M, Rees M, Fong Y, McMasters KM, Broelsch C, Busuttil R, Belghiti J, Strasberg S, Chari RS, World Consensus Conference on Laparoscopic Surgery (2009) The international position on laparoscopic liver surgery: the Louisville Statement, 2008. Ann Surg 250(5):825–830
Acknowledgments
Christian Toso was supported by a Professorship from the Swiss National Science Foundation (PP00P3_139021). The work was supported by the Artères Foundation.
Disclosures
Drs. Giulio C. Vitali, Alexis Laurent, Sylvain Terraz, Pietro Majno, Nicolas C. Buchs, Laura Rubbia-Brandt, Alain Luciani, Julien Calderaro, Philippe Morel, Daniel Azoulay, and Christian Toso have no conflicts of interest or financial ties to disclose.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Vitali, G.C., Laurent, A., Terraz, S. et al. Minimally invasive surgery versus percutaneous radio frequency ablation for the treatment of single small (≤3 cm) hepatocellular carcinoma: a case–control study. Surg Endosc 30, 2301–2307 (2016). https://doi.org/10.1007/s00464-015-4295-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-015-4295-6