Abstract
Objectives
To assess CT-attenuation of abdominal adipose tissue and psoas muscle as predictors of mortality in patients with sarcomas of the extremities.
Methods
Our study was IRB approved and HIPAA compliant. The study group comprised 135 patients with history of extremity sarcoma (mean age: 53 ± 17 years) who underwent whole body PET/CT. Abdominal subcutaneous adipose tissue (SAT), visceral adipose tissue (VAT), and psoas muscle attenuation (HU) was assessed on non-contrast, attenuation-correction CT. Clinical information including survival, tumour stage, sarcoma type, therapy and pre-existing comorbidities were recorded. Cox proportional hazard models were used to determine longitudinal associations between adipose tissue and muscle attenuation and mortality.
Results
There were 47 deaths over a mean follow-up period of 20 ± 17 months. Higher SAT and lower psoas attenuation were associated with increased mortality (p = 0.03 and p = 0.005, respectively), which remained significant after adjustment for age, BMI, sex, tumor stage, therapy, and comorbidities (p = 0.002 and p = 0.02, respectively). VAT attenuation was not associated with mortality.
Conclusion
Attenuation of SAT and psoas muscle, assessed on non-contrast CT, are predictors of mortality in patients with extremity sarcomas, independent of other established prognostic factors, suggesting that adipose tissue and muscle attenuation could serve as novel biomarkers for mortality in patients with sarcomas.
Key Points
• CT-attenuation of adipose tissue and muscle predict mortality in sarcoma patients
• CT-attenuation predicts mortality independent of established prognostic factors
• Patients with sarcomas often undergo CT for staging or surveillance
• Adipose tissue and muscle attenuation could serve as biomarkers for mortality
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sarcomas are a heterogeneous group of malignant neoplasms arising from mesenchymal cells throughout the body. They account for approximately 1 % of all adult malignancies [1]. About 50-60 % of sarcomas occur in the extremities [2, 3]. Established prognostic indicators of survival in patients with extremity sarcomas include histological tumour grade and tumour stage. However, the staging system for sarcomas is controversial. For example, the location of the tumour is not included in the staging system, and extremity, visceral, and retroperitoneal sarcomas are all combined, which precludes association of a particular stage with a uniform surgical approach [2]. Furthermore, biologic subtypes of sarcomas are not included in the staging system and tumour behaviour can vary considerably within a histological subtype [2]. Therefore, identification of additional predictors of survival is of clinical significance.
Recent studies have shown an important link between adipose tissue and tumour growth [4, 5]. Cancer cells can induce dedifferentiation of adipocytes, which secrete their lipids and can elicit systemic metabolic effects [4, 6]. On the other hand, dedifferentiated adipocytes can influence the growth of tumour cells [5, 7]. Furthermore, fatty infiltration of muscle is associated with sarcopenia and poor prognosis in cancer patients [8, 9].
Recent studies have suggested that the attenuation of abdominal adipose tissue, assessed on non-contrast computed tomography (CT), may serve as a biomarker for cardiovascular and cancer mortality [10, 11]. High attenuation of abdominal fat on CT has been found to be correlated with increased extracellular matrix fibrosis and smaller adipocytes on histology [10], findings that are also seen in cancer-associated wasting [12–14]. Furthermore, decreased muscle attenuation by CT has been found to serve as a predictor for all-cause mortality in older men and increased mortality in patients with carcinomas [15–18], and may reflect both quantitative and qualitative alterations of muscle tissue [19–21].
However, no studies have been performed on the use of these measures in patients with sarcomas.
Although MRI is the standard imaging modality for evaluating bone and soft tissue sarcomas, PET/CT is often used for staging and surveillance and adipose tissue and muscle attenuation, assessed on the non-contrast attenuation correction CT, and may serve as novel biomarkers predicting mortality in patients with sarcomas. The purpose of our study was to assess CT attenuation of abdominal adipose tissue and psoas muscle as predictors of mortality in patients with sarcomas of the extremities. We hypothesized that high adipose tissue attenuation and low muscle attenuation would be associated with higher mortality.
Materials and methods
This retrospective study was approved by the Institutional Review Board and complied with the Health Insurance Portability and Accountability Act (HIPAA) with exemption status for individual informed consent.
Subjects
A retrospective search was performed to identify patients with a history of extremity sarcoma who underwent FDG-PET/CT at our institution between April 2004 and December 2014. Inclusion criteria were age ≥18 years, diagnosis of sarcoma of the extremities, and presence of a diagnostic whole body attenuation correction non-contrast 18F-FDG-PET/CT. We selected patients with sarcomas of the extremity to avoid the influence of surgery or radiation therapy on abdominal adipose tissue and muscle attenuation measurements. Exclusion criteria were malignancy other than extremity sarcoma, abdominal surgery, radiation therapy to the abdomen or other pathology which could affect abdominal adipose tissue and muscle attenuation measurements.
Clinical data
The following clinical data were obtained from electronic medical records: age, BMI, smoking status, sarcoma type, sarcoma location, tumour size (cm), tumour grade and stage, type of treatment, presence and types of pre-existing comorbidities, final outcome (alive or deceased) at time of data collection, follow-up time since PET/CT, and date of death.
BMI was calculated as weight (kg) divided by height squared (m2). Smoking status was recorded according to the following subgroups: 1) never smoked, 2) quit ≥20 years ago, 3) quit <20 years ago, or 4) current smoker defined as having smoked at least one cigarette per day during the past year. Sarcoma type was categorized as bone or soft-tissue sarcoma and sarcoma location was categorized as upper or lower extremity. Sarcoma staging (stage I to IV) was performed using the TNM system of the American Joint Committee on Cancer (https://cancerstaging.org/references-tools/deskreferences/Documents/AJCC6thEdCancerStagingManualPart1.pdf). Type of treatment included surgery, radiation therapy, chemotherapy, or a combination. Pre-existing comorbidities were categorized as cardiovascular disease (coronary artery disease, aortic aneurysm, myocardial infarction, cardiomyopathy, congestive heart failure, cerebrovascular disease, cardiac arrhythmia, pulmonary embolism, hypertension), pulmonary disease (asthma, chronic bronchitis, emphysema), renal disease (nephropathy, chronic renal failure), type II diabetes mellitus (fasting blood sugar level of >126 mg/dL or HbA1c >6.5 %), or a combination of those.
Mortality was determined from medical records and death notes. Follow-up time in months between date of PET/CT and date of death was recorded.
CT attenuation measurements
All patients underwent whole body 18-F-FDG-PET/CT (Siemens Biograph 16 or 64, Siemens, Erlangen, Germany or GE Healthcare discovery, Milwaukee, Wisconsin, USA), per standard clinical protocol as previously described [22]. The CT scanners used in this study were tested on an annual basis according to American Association of Physicists in Medicine (AAPM) and American College of Radiology (ACR) guidelines (AAPM report #74 and #96 and ACR CT QC manual). The non-contrast, attenuation correction CT scans (slice thickness 5 mm; table feed per rotation, 18 mm; time per table rotation, 0.5 s; tube voltage, 120 kVp; tube current, 11 mAs; and field of view, 20 cm) were used for analyses. Standard clinical quality assurance measures were performed to assess for reproducibility of scans over time.
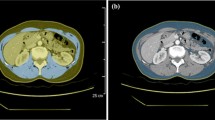
Adipose tissue and muscle measurements were performed in the abdomen, remote from the site of sarcoma, to avoid the influence of the primary tumour and treatment changes (surgery or radiation therapy) on attenuation measurements. Analyses were performed using semiautomated methods at the mid-portion of the fourth lumbar vertebra as this level has been shown to be correlate with whole body adiposity [23]. Analyses were performed using Osirix software version 3.2.1 (www.osirix-viewer.com/index.html). First, automated thresholding methods were applied to identify abdominal adipose tissue, using a threshold set for −50 to −250 Hounsfield units (HU) as described by Borkan et al. [24]. We then manually outlined the subcutaneous and visceral areas and the mean attenuation (HU) was determined for each adipose tissue depot. This has been shown to be a reliable method for adipose tissue attenuation measurements with inter-reader correlation coefficients (r) of 0.99 for VAT and SAT [25]. For muscle attenuation measurements, thresholds were set between -29 to 150 HU [16]. Muscle attenuation was measured within a region of interest of 1 cm2 in the centre of the right psoas muscle at the same level as the adipose tissue measurements, and mean attenuation (HU) was recorded (Supplemental Figure).
Statistical analysis
Statistical analysis was performed using JMP statistical software version 11.0 (SAS Institute Inc., Cary, NC.). The Shapiro-Wilk test was used to test normality of distribution. In case data were not normally distributed, non-parametric tests were performed. Data in Table 1 are shown as mean ± standard deviation (SD) for normally distributed parameters and median ± interquartile range for not normally distributed data. The primary outcome of interest was mortality. Cox proportional hazard models were used to explore the longitudinal association between abdominal adipose tissue and muscle attenuation and mortality. Univariate and multivariate analyses of survival were performed using Cox’s regression models, and the results are presented as hazard ratios (HR) with 95 % confidence interval (CI). Multivariate analysis was performed by subsequently adding five different covariate models, which included known risk factors for mortality [10, 11, 16]. The first model adjusted for age, sex, and BMI. Model 2 included the first model plus smoking status, whereas model 3 consisted of model 2 plus the patients’ number of pre-existing comorbidities. Model 4 included model 3 plus sarcoma stage, whereas the fifth model included model 4 plus therapy type. For all analyses, a two-sided P-value of <0.05 was considered statistically significant.
Results
Patient characteristics
Patient characteristics are shown in Table 1. We identified 135 patients who met inclusion criteria (mean age at time of PET/CT: 52.5 ± 17.4 years, range 18 – 101 years). Of the 135 patients, 49 were women (mean age 52.3 ± 17.9 years, range 20 – 101 years) and 86 were men (mean age 52.7 ± 17.3 years, range 18 – 89 years). There were 47 deaths over a mean follow-up period of 20.4 ± 16.6 months (range of follow-up 0.46 and 65.9 months).
Adipose tissue attenuation and mortality
Mean SAT attenuation was -98.7 ± 8.2 HU and mean VAT attenuation was -89.2 ± 9.8 HU. A positive association between SAT attenuation and mortality was found (Table 2). An increase in SAT HU was associated with increased mortality showing an uncorrected HR of 1.05 (95 % CI 1.01-1.10, p = 0.03). Correcting for age, sex, and BMI did not alter this relationship (HR 1.07, 95 % CI 1.01-1.12, p = 0.02). Adjusting for smoking status enhanced this association (HR 1.09, 95 % CI 1.03-1.15, p = 0.003), as did the amount of pre-existing comorbidities (HR 1.10, 95 % CI 1.04-1.16, p = 0.001). The relationship remained significant after additional adjustment for sarcoma stage (HR 1.10, 95 % CI 1.04-1.16, p = 0.002) and therapy type (HR 1.10, 95 % CI 1.04-1.17, p = 0.002).
VAT attenuation was not associated with mortality in any of the models (Table 2).
In patients with soft tissue sarcomas only (n = 116) SAT attenuation remained a positive predictor of mortality in the uncorrected and corrected models, while VAT density was not associated with mortality (Table 3).
Muscle attenuation and mortality
Mean psoas attenuation was 44.5 ± 14.7 HU. A positive association between decreased psoas attenuation and mortality was found (Table 2). A decrease in psoas HU was associated with increased mortality showing an uncorrected HR of 0.96 (95 % CI 0.94-0.99, p = 0.005). Correcting for age, sex, and BMI enhanced this relationship (HR 0.95, 95 % CI 0.92-0.98, p = 0.0005). The relationship remained significant after additional adjustment for smoking status (HR 0.94, 95 % CI 0.91-0.98, p = 0.0009), amount of pre-existing comorbidities (HR 0.94, 95 % CI 0.91-0.97, p = 0.0006), sarcoma stage (HR 0.93, 95 % CI 0.89-0.97, p = 0.0005), and therapy type (HR 0.95, 95 % CI 0.90-0.99, P = 0.02).
When psoas and SAT attenuation were entered into the same model, the HR of the model was HR 1.10 , with 95 % CI 1.04 – 1.16.
In patients with soft tissue sarcomas only (n = 116), decreased muscle attenuation remained a predictor of mortality in the uncorrected and corrected models (Table 3).
Discussion
Our study shows that higher abdominal SAT attenuation and lower psoas muscle attenuation, assessed on non-contrast CT, are positive predictors of mortality in patients with extremity sarcomas, independent of other established prognostic factors, such as age, BMI, tumour stage, and comorbidities. These data suggest that adipose tissue and muscle attenuation could serve as novel biomarkers for mortality in patients with sarcomas, who often undergo CT as part of staging or surveillance.
Two recent large community-based cohort studies have suggested that increased adipose tissue attenuation on non-contrast CT might serve as a biomarker for all-cause mortality, including cancer mortality [10, 11]. In addition, a large longitudinal community-based cohort study in older men [17] and studies in patients with hepatocellular [16], pancreatic [15], and gastro-oesophageal [18] carcinoma found low muscle attenuation to be predictive of mortality. However, no study has been performed to assess the predictive value of tissue attenuation by CT on mortality in patients with sarcomas.
Sarcomas are relatively rare but challenging neoplasms with an incidence of about 1.5 per 100,000 [1]. They arise from mesenchymal cells, encompass multiple histological subtypes, and can occur at any anatomic site. In a large multicenter European study, analyzing 76 cancer registries, 84 % of sarcomas were soft tissue sarcomas and 14 % were bone sarcomas [1]. In contrast to the biological behaviour of carcinomas, which depends largely on the site and cell type of origin, the management and outcome of sarcomas is based primarily on the anatomic location [2]. However, the biological behaviour and prognosis also depend on the histological tumour grade and tumour stage. Therefore, the complex relationship between anatomic site and histology as well as the overall rarity of sarcomas adds to the challenges in managing patients with these neoplasms [1, 2]. Established prognostic factors of mortality in extremity sarcomas include tumour stage, therapy response, age, and associated comorbidities [2, 26–28]; however, these factors do not always correlate with outcome [29, 30].
In our study, increased SAT attenuation and decreased psoas attenuation were predictors of mortality, independent of prognostic factors such as tumour stage, age, sex, therapy, BMI, and comorbidities. Patients with sarcomas often undergo PET/CT for staging and surveillance [31, 32] and the non-contrast attenuation correction CT can be used to determine tissue attenuation. Adipose tissue and muscle attenuation can be easily measured on any clinical workstation by placing a ROI in the subcutaneous abdominal tissues and the psoas muscle and these measurements could be included on routine scans to provide additional information on prognosis. Furthermore, these biomarkers might help in identifying patients with muscle wasting and impaired nutritional status, who might benefit from intensive nutritional/exercise support.
There is an important link between adipose tissue and tumour growth [5]. Cancer cells can cause dedifferentiation of adipocytes and reprogramming into cancer-associated adipocytes [4]. During this process adipocytes secrete their lipids and these reprogrammed lipid-poor adipocytes secrete adipokines, which stimulate invasion of tumour cells and can cause systemic metabolic effects [4, 6]. In addition, cancer-associated cachexia results in adipose tissue atrophy and increased lipolysis and inability to store triacylglycerol [33]. Conversely, the tissue microenvironment can influence the growth of tumour cells, supporting tumorigenesis and metastases [5, 7]. Biopsy studies in animals have shown that high attenuation adipose tissue by CT corresponds to smaller adipocytes with lower lipid content and increased extracellular matrix fibrosis [10]. Our finding of increased adipose tissue attenuation in patients with increased mortality may reflect reprogrammed adipocytes with decreased lipid content and inability to store lipids. Alternatively, increased adipose tissue attenuation may reflect an adipocyte microenvironment promoting tumour cell growth. There is a potential systemic effect of chemo- or radiation therapy on adipose tissue. However, we controlled for the type of therapy in and our analyses, and this did not adversely affect our results.
Our findings of an independent association between low psoas muscle attenuation and increased mortality is consistent with a study by Fujiwara et al., which demonstrated an inverse association between muscle attenuation and mortality in patients with hepatocellular carcinoma [16], and a study by Miljkovic et al., which found low muscle attenuation to be a predictor of all-cause and cardiovascular mortality in older men [17]. Low muscle attenuation by CT reflects increased lipid content [34] and is associated with sarcopenia [8] and poor prognosis in cancer patients[9, 35]. In addition, low muscle attenuation has been identified as a risk factor for insulin resistance [36], decreased muscle strength [37], and increased fracture risk [38]. An additional mechanism for increased mortality in sarcoma patients with muscle fatty infiltration may be the secretion of pro-inflammatory cytokines from adipocytes surrounding muscle fibres [19, 20]. Increased secretion of pro-inflammatory cytokines has been found to promote tumorigenesis [21].
Our study had several limitations. First, the retrospective study design limits our ability to infer causality. Second, we did not obtain adipose tissue or muscle biopsies to investigate potential mechanisms linking adipose tissue and muscle attenuation to mortality, and we did not have laboratory data, such as serum albumin available, which has been associated with mortality [39]. Third, we combined sarcoma subtypes in the analyses. The strengths of our study include the large number of patients with extremity sarcomas and detailed measures of tissue attenuation on non-contrast CTs. Moreover, we controlled our analyses for a wide variety of covariates.
In conclusion, our study shows that high adipose tissue attenuation and low muscle attenuation are positive predictors of mortality in patients with extremity sarcomas, independent of established prognostic factors. These results suggest that adipose tissue and muscle attenuation may serve as novel biomarkers of mortality in sarcoma patients.
References
Stiller CA, Trama A, Serraino D et al (2013) Descriptive epidemiology of sarcomas in Europe: report from the RARECARE project. Eur J Cancer 49:684–695
Morrison BA (2003) Soft tissue sarcomas of the extremities. Proc (Baylor Univ Med Cent) 16:285–290
Tzeng CW, Smith JK, Heslin MJ (2007) Soft tissue sarcoma: preoperative and postoperative imaging for staging. Surg Oncol Clin N Am 16:389–402
Dirat B, Bochet L, Dabek M et al (2011) Cancer-associated adipocytes exhibit an activated phenotype and contribute to breast cancer invasion. Cancer Res 71:2455–2465
Nieman KM, Romero IL, Van Houten B, Lengyel E (1831) Adipose tissue and adipocytes support tumorigenesis and metastasis. Biochim Biophys Acta 2013:1533–1541
Andarawewa KL, Motrescu ER, Chenard MP et al (2005) Stromelysin-3 is a potent negative regulator of adipogenesis participating to cancer cell-adipocyte interaction/crosstalk at the tumor invasive front. Cancer Res 65:10862–10871
Martinez-Outschoorn UE, Sotgia F, Lisanti MP (2012) Power surge: supporting cells "fuel" cancer cell mitochondria. Cell Metab 15:4–5
Boutin RD, Yao L, Canter RJ, Lenchik L (2015) Sarcopenia: current concepts and imaging implications. AJR Am J Roentgenol 205:W255–W266
Martin L, Birdsell L, Macdonald N et al (2013) Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol 31:1539–1547
Murphy RA, Register TC, Shively CA et al (2014) Adipose tissue density, a novel biomarker predicting mortality risk in older adults. J Gerontol A Biol Sci Med Sci 69:109–117
Rosenquist KJ, Massaro JM, Pedley A et al (2015) Fat quality and incident cardiovascular disease, all-cause mortality and cancer mortality. J Clin Endocrinol Metab 100:227–234
Dahlman I, Mejhert N, Linder K et al (2010) Adipose tissue pathways involved in weight loss of cancer cachexia. Br J Cancer 102:1541–1548
Mracek T, Stephens NA, Gao D et al (2011) Enhanced ZAG production by subcutaneous adipose tissue is linked to weight loss in gastrointestinal cancer patients. Br J Cancer 104:441–447
Rolland Y, Abellan van Kan G, Gillette-Guyonnet S, Vellas B (2011) Cachexia versus sarcopenia. Curr Opin Clin Nutr Metab Care 14:15–21
Di Sebastiano KM, Yang L, Zbuk K et al (2013) Accelerated muscle and adipose tissue loss may predict survival in pancreatic cancer patients: the relationship with diabetes and anaemia. Br J Nutr 109:302–312
Fujiwara N, Nakagawa H, Kudo Y et al (2015) Sarcopenia, intramuscular fat deposition, and visceral adiposity independently predict the outcomes of hepatocellular carcinoma. J Hepatol 63:131–140
Miljkovic I, Kuipers AL, Cauley JA et al (2015) Greater skeletal muscle fat infiltration is associated with higher all-cause and cardiovascular mortality in older men. J Gerontol A Biol Sci Med Sci 70:1133–1140
Tamandl D, Paireder M, Asari R et al () Markers of sarcopenia quantified by computed tomography predict adverse long-term outcome in patients with resected oesophageal or gastro-oesophageal junction cancer. Eur Radiol
Vettor R, Milan G, Franzin C et al (2009) The origin of intermuscular adipose tissue and its pathophysiological implications. Am J Physiol Endocrinol Metab 297:E987–E998
Zoico E, Rossi A, Di Francesco V et al (2010) Adipose tissue infiltration in skeletal muscle of healthy elderly men: relationships with body composition, insulin resistance, and inflammation at the systemic and tissue level. J Gerontol A Biol Sci Med Sci 65:295–299
Fujiki H, Sueoka E, Suganuma M (2013) Tumor promoters: from chemicals to inflammatory proteins. J Cancer Res Clin Oncol 139:1603–1614
Bredella MA, Gill CM, Rosen CJ et al (2014) Positive effects of brown adipose tissue on femoral bone structure. Bone 58:55–58
Shen W, Punyanitya M, Wang Z et al (2004) Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. J Appl Physiol 97:2333–2338
Borkan GA, Gerzof SG, Robbins AH et al (1982) Assessment of abdominal fat content by computed tomography. Am J Clin Nutr 36:172–177
Maurovich-Horvat P, Massaro J, Fox CS et al (2007) Comparison of anthropometric, area- and volume-based assessment of abdominal subcutaneous and visceral adipose tissue volumes using multi-detector computed tomography. Int J Obes (Lond) 31:500–506
Bramer JA, van Linge JH, Grimer RJ, Scholten RJ (2009) Prognostic factors in localized extremity osteosarcoma: a systematic review. Eur J Surg Oncol 35:1030–1036
Kang S, Kim HS, Kim W et al (2015) Comorbidity is independently associated with poor outcome in extremity soft tissue sarcoma. Clin Orthop Surg 7:120–130
Pakos EE, Nearchou AD, Grimer RJ et al (2009) Prognostic factors and outcomes for osteosarcoma: an international collaboration. Eur J Cancer 45:2367–2375
Brennan MF, Antonescu CR, Moraco N, Singer S (2014) Lessons learned from the study of 10,000 patients with soft tissue sarcoma. Ann Surg 260:416–421, discussion 421-412
Mullen JT, Hornicek FJ, Harmon DC et al (2014) Prognostic significance of treatment-induced pathologic necrosis in extremity and truncal soft tissue sarcoma after neoadjuvant chemoradiotherapy. Cancer 120:3676–3682
Choi YY, Kim JY, Yang SO (2014) PET/CT in benign and malignant musculoskeletal tumors and tumor-like conditions. Semin Musculoskelet Radiol 18:133–148
Sheikhbahaei S, Marcus C, Hafezi-Nejad N et al (2015) Value of FDG PET/CT in patient management and outcome of skeletal and soft tissue sarcomas. PET Clin 10:375–393
Tisdale MJ (2002) Cachexia in cancer patients. Nat Rev Cancer 2:862–871
Goodpaster BH, Kelley DE, Thaete FL et al (2000) Skeletal muscle attenuation determined by computed tomography is associated with skeletal muscle lipid content. J Appl Physiol (1985) 89:104–110
Yip C, Goh V, Davies A et al (2014) Assessment of sarcopenia and changes in body composition after neoadjuvant chemotherapy and associations with clinical outcomes in oesophageal cancer. Eur Radiol 24:998–1005
Kelley DE, Goodpaster BH (2001) Skeletal muscle triglyceride. An aspect of regional adiposity and insulin resistance. Diabetes Care 24:933–941
Goodpaster BH, Carlson CL, Visser M et al (2001) Attenuation of skeletal muscle and strength in the elderly: the health ABC study. J Appl Physiol (1985) 90:2157–2165
Lang T, Cauley JA, Tylavsky F et al (2010) Computed tomographic measurements of thigh muscle cross-sectional area and attenuation coefficient predict hip fracture: the health, aging, and body composition study. J Bone Miner Res 25:513–519
Gupta D, Lis CG (2010) Pretreatment serum albumin as a predictor of cancer survival: a systematic review of the epidemiological literature. Nutr J 9:69
Acknowledgments
The scientific guarantor of this publication is Miriam A. Bredella. The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article. The authors state that this work has not received any funding. Elkan F. Halpern, Director of Statistics of the Department of Radiology at MGH and co-author on the study kindly provided statistical advice for this manuscript. Institutional Review Board approval was obtained. Written informed consent was waived by the Institutional Review Board. Methodology: retrospective, observational, performed at one institution.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental Figure
DOCX 184 kb)
Rights and permissions
About this article
Cite this article
Veld, J., Vossen, J.A., De Amorim Bernstein, K. et al. Adipose tissue and muscle attenuation as novel biomarkers predicting mortality in patients with extremity sarcomas. Eur Radiol 26, 4649–4655 (2016). https://doi.org/10.1007/s00330-016-4306-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-016-4306-6