Abstract
Objective
To determine the fat content of myxoid liposarcomas (MLS) on MRI and to identify any association between lipid content and survival.
Materials and methods
The fat percentage of MLS diagnosed between January 2006 and December 2016 at a single institution was assessed by two radiologists on preoperative MR images. A Cox proportional hazard model was used to determine any association between tumor fat percentage and survival time. Tumor fat percentage was the single predictor in the model. A significance level of 0.05 was used. The Kaplan–Meier estimator was also used to provide a nonparametric estimate of the survivor function within the entire sample and within two patient subgroups consists of lipid-rich and lipid-poor tumors. Lipid-rich tumors were defined as any tumors showing more than 20% of fat on MRI. A 20% cutoff was determined arbitrarily.
Results
Of the 43 cases identified through retrospective review, 8 tumors demonstrated ≥10% fat on MRI, and 4 tumors demonstrated ≥20% fat (highest fat percentage, 38%). There was no significant survival difference between patients with high tumor fat, which was defined as ≥20% fat, compared with those with little to no tumor fat.
Conclusion
Myxoid liposarcomas may demonstrate a higher fat content on MRI than has previously been reported in the literature. Increased tumor fat percentage in lipid-rich tumors was not found to be associated with increased risk of death. Radiologists must be aware of the existence of MLS lesions with higher fat content.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Liposarcoma is one of the most common soft-tissue sarcomas, representing 50% of retroperitoneal sarcomas and 25% of soft-tissue sarcomas of the extremities [1]. Initial classification of liposarcomas included three major separate groups: myxoid liposarcoma (MLS), pleomorphic liposarcoma, and well-differentiated liposarcoma by Evans in 1979 [2]. The most recent WHO classification also included dedifferentiated liposarcoma as the fourth major group in addition to previously described three major groups [3]. Several other subtypes that were not initially part of the WHO classification have been described in the literature and some of these subtypes can be difficult to diagnose [4, 5]. For instance, lipomatous and lipoblast-rich MLS may demonstrate predominantly fatty characteristics on biopsy specimens because of sampling errors; pathology series have shown that the fat content of such biopsy specimens can reach up to 80% [5]. A large series reporting the fat content and percentage of MLS on MRI is lacking. In the literature, it is suggested that the fat content of MLS is typically less than 10% on radiological imaging [6, 7]. Therefore, if a lesion shows an unusually large amount of fat on imaging, it may be erroneously identified by the radiologist as a well-differentiated liposarcoma\atypical lipomatous tumor (WDL/ALT) or dedifferentiated liposarcoma, and the sampling of fatty areas may then lead to further misdiagnosis. We measured the fat content of MLS to show if our center’s experience is compatible with the literature and if a fat content of more than 10%, which is typically reported in the literature, exists in these tumors. We also investigated the relation between fat content and survival. The increased amount of fat in these tumors may reflect a better differentiation and may offer a possible survival benefit; on the other hand, a round cell component commonly seen in the fatty component of these tumors may affect survival adversely.
Materials and methods
After institution review board approval, cases of MLSs diagnosed and archived in our institution’s anatomical pathology database between 1 January 2006, and 31 December 2016 were reviewed. Cases of recurrent and metastatic lesions and cases that did not have MR images available for review were excluded. From the database, a total of 51 cases were identified after elimination of recurrent or metastatic lesions (n = 11); 8 of these cases were excluded (4 were lacking preoperative MRI, 2 had MRI that did not meet the criteria for review, either not having T1-weighted fat-saturated/suppressed images (n = 1) or the available T1 fat-saturated/suppressed images did not include the whole tumor owing to data loss (n = 1), and 2 were lacking clinical information). The preoperative images of the remaining 43 cases were then reviewed independently by a fellowship-trained musculoskeletal radiologist and a musculoskeletal radiology fellow (Fig. 1).
T1-weighted and T1 fat-saturated/suppressed images were used for calculation of fat content. Maximum tumor margins and margins of areas showing T1 hyperintensity and suppressed signal intensity with fat-sensitive sequences (with either inversion recovery or frequency saturation) were measured using the freehand tool from each slice and multiplied by slice thickness for every slice that included the lesion. Slice thickness for T1-weighted images was 3 mm, without any skip. For fat-suppressed images all of the sequences were acquired with a slice thickness of 3 mm without any skip except for three studies that included short tau inversion recovery (STIR) sequences with a slice thickness of 5 and a skip thickness of 1 mm. For studies with a skip thickness, skip thickness was added to slice thickness in each slice measured (i.e., total thickness was calculated as 6 mm). For each case, two reviewers determined the fat percentage of the tumor. If the reviewers’ percentage assessments showed less than 5% disagreement, an arithmetic mean was calculated and accepted as the final fat percentage. If the reviewers’ percentage assessments showed more than 5% disagreement, both radiologists reviewed the images of both measurements and a consensus fat percentage value was measured This last consensus percentage was used for statistical analysis. The date of diagnosis and date of death or last follow-up were also recorded for each patient.
A Cox proportional hazards model was used to determine any association between tumor fat percentage and survival time. Tumor fat percentage (as a continuous variable) was the single predictor in the model. A significance level of 0.05 was used. The Kaplan–Meier estimator was also used to provide a nonparametric estimate of the survivor function within the entire sample and within lipid-rich and lipid-poor patient subgroups.
Results
In the 43 cases included in the analysis, the percentage of intralesional tumor fat on MRI ranged from 0 to 38% (mean: 5.2%, median: 0%). Approximately half of the patients had a tumor with no discernible fat on MRI (n = 22), whereas the other half had a tumor with at least some fat (n = 21). Eight patients had tumors with fat content ≥10%, and 4 patients had tumors with fat content ≥20%. Fat percentages of 2 patients with the highest fat percentage reached as high as 26% (Fig. 2) and 38% (Figs. 3, 4) on consensus measurements.
a T1-weighted MRI of the left thigh lesion showing a large, heterogeneous mass containing a large nodule medially (thin arrow) and several smaller nodules laterally (thick arrow) showing T1 hyperintensity. b Short tau inversion recovery (STIR) image of the same level with suppression of the T1 hyperintense signal of large nodule medially (thin arrow) and several smaller nodules laterally (thick arrow), proving the fatty characteristics. c H&E stain photomicrographs at medium power demonstrates an almost pure round cell liposarcoma with scattered adipocytes. d 400× H&E photomicrograph shows areas of an almost pure round cell liposarcoma in detail with large nuclei in addition to scattered mature-looking adipocytes
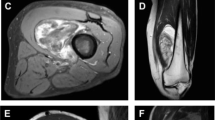
a Axial T1-weighted MRI of a lower posterior thigh lesion showing a large, lobulated mass containing areas of T1 hyperintensity centrally (thin arrows). b STIR images with suppression of the T1 hyperintense signal in the area of interest (thin arrow), signifying fatty characteristics. c Coronal T1-weighted MRI of the same lesion showing a central area corresponding to the axial image (white star) in addition to areas continuing with the central part more superiorly and showing the same signal characteristics (thick arrow). d Signal loss with STIR images showing fatty characteristics of the same areas in C (thick arrow and white star)
a H&E stain photomicrographs at low power demonstrate that most of the biopsy had a lipomatous appearance reminiscent of lipoma or atypical lipomatous tumor. b ×400 H&E photomicrograph showing well-differentiated lipoma-like/atypical lipoma-like morphology. c Low power photo of the area of transition from more lipoma-like (upper right corner) to more myxoid liposarcomatous areas (lower right). d Medium power view of the area showing pure myxoid liposarcomatous areas
For 93% of patients (40 out of 43), the tumor fat percentage values from the two reviewers were within 5% of each other, demonstrating good interobserver agreement (Fig. 5); however, the amount of disagreement between the reviewers increased as the tumor’s fat percentage increased.
Tumors were grouped into two: those with fat percentages higher than 20% and those with lower. A cutoff of 20% was determined arbitrarily. Increases in tumor fat percentage were found to be associated with increases in the risk of death (hazard ratio for each 1% increase in tumor fat: 1.07), but this result was not statistically significant (p = 0.073; 95% confidence interval: 0.94, 1.14). Figure 6a shows an estimate of the probability of survival as a function of time after diagnosis across the entire sample. In subgroup analysis, survival was worse among patients with tumor fat >20% (n = 4) than among patients with tumor fat ≤20% (n = 39; Fig. 6b).
a Kaplan–Meier estimate of the survivor function among 43 patients with myxoid liposarcomas (solid line). Censored observations are marked by vertical bars on the curve. Dotted lines represent 95% confidence intervals. b Kaplan–Meier estimate of the survivor function among 39 patients with tumor fat <20% (gray line) and 4 patients with tumor fat ≥20% (black line). Censored observations are marked by vertical bars on the curves
Discussion
Myxoid liposarcoma is one of the most common adult soft-tissue sarcomas and represents one-third to one-half of all liposarcoma cases [8]. MLS has a predilection for the lower extremity and most frequently manifests as a multinodular gelatinous mass [9, 10]. The most recent World Health Organization classification of soft-tissue liposarcomas described four major groups: well-differentiated, dedifferentiated, myxoid, and pleomorphic [3, 4].
The typical histological appearance of MLS is spindle cells in a myxoid stroma with a prominent vascular pattern [2, 5]. Several other morphological types with different histological patterns have been described, including lipomatous, lipoblast-rich, traditional round cell, pseudoacinar, stromal hyalinization, cord-like, nested, chondroid metaplasia, and, hemangiopericytoma-like [5]. MLS can also display a wide range of fat maturation, ranging from lipoblasts to mature fat [2]. The presence of some amount of fat is quite variable in MLS, occurring in 42–95% of cases [10,11,12]. Because of this, biopsy specimens from the lipid-rich parts of the tumor may be misdiagnosed as atypical lipomatous tumors, which could delay the correct diagnosis, surgery, and treatment [5]. The fat content of MLS on MRI studies has been reported only in small case series and case reports; previous studies have suggested that the fat content of these tumors on imaging studies should be less than 10% of the total tumor volume subjectively [6, 7].
Magnetic resonance imaging is the preferred imaging modality for cases of MLS [13, 14]. On MRI, the tumor appears as a well-defined multilobulated heterogeneous mass showing varying components with differing imaging characteristics consistent with its pathology [15]. Its myxoid component appears low density on computed tomography (CT) and T2 hyperintense and T1 hypointense on MRI; this component enhances on contrast-enhanced studies [13, 16]. The fatty component appears T1 hyperintense with suppressed signal on fat-suppressed sequences. Fat content is not always visible on MRI even when present in biopsy material [13]. In our series, 18.6% of the all cases (8 out of 43) showed fat content more than or equal to 10% of the total volume and in addition 9.3% of cases (4 out of 43) showed fat content more than or equal to 20% and these values are higher than those typically reported in the literature. These lipid-rich liposarcomas may be common, as suggested in the literature. Some radiological signs such as large tumor size (> 10 cm), deep location, irregular margins, lack of lobulations, and presence of thick septa (> 2 mm) are reported to be associated with high-grade MLS. Some specific features were reported to signify a worse prognosis [17]. Fat content and its effect on survival, however, was not previously reported in MLS.
Imaging characteristics reported in the literature that signify survival favorably include the presence of thin septa and tumor capsule, and the absence of pronounced enhancement. Pronounced peripheral enhancement and globular or nodular enhancement were associated with a higher rate of malignancy and poor survival [17, 18]. The latter enhancement patterns are believed to depict the round-cell, high-grade component of the tumors [17]. The presence of >5% nonfatty, nonmyxoid enhancing components has also been shown to be a good predictor of high-grade liposarcoma, correlating with round-cell clusters on histopathological analysis [19]. Our study showed that a higher fat content on MRI in these tumors may predict poorer survival; however, this finding did not reach statistical significance.
Mature fat content could be taught to represent better differentiation and as such better prognosis in MLS tumors and better survival with a higher fat component may appear more plausible; however, there could be several explanations for a possible worse prognosis in these patients. Although some tumors could be lipid-rich, it is important to know that the fat component does not represent largest part of the lesion. Areas showing fat characteristics on MRI may contain lipoblasts and perivascular round cells, which was previously associated with more aggressive behavior [17]. Also, we should keep in mind that previously, myxoid and round-cell liposarcomas were considered distinct histological subtypes. However, the WHO Classification of Soft-Tissue Tumors has now combined these lesions as MLS, representing a continuum of these previous two subtypes [4]. The latter histology, round-cell liposarcoma, could be a prominent component in lipid-rich tumors, explaining the poorer survival.
The main limitation of this study is the retrospective nature of the study. Also because of the retrospective nature of the study, there was no standardization across the MRI studies of all cases of MLS. We tried to overcome this by reviewing each study for quality. The limited number of lipid-rich tumors is another limitation; however, this limitation can be addressed by multicenter studies in the future.
In conclusion, the fat content of MLS on MRI could be greater than that typically reported in the literature. Radiologists must be aware of the existence of lipid-rich MLS lesions and should include MLS in their differential diagnosis of lipid-rich tumors so that they can avoid misdiagnosis.
Abbreviations
- MLS:
-
Myxoid liposarcoma
- WDL:
-
Well-differentiated liposarcoma
- ALT:
-
Atypical lipomatous tumor
References
Crago AM, Brennan MF. Principles in management of soft tissue sarcoma. Adv Surg. 2015;49(1):107.
Evans HL. Liposarcoma A study of 55 cases with a reassessment of its classification. Am J Surg Pathol. 1979;3(6):507–24.
Fletcher C, Bridge J, Hogendoorn P, Mertens F. WHO classification of tumours of soft tissue and bone. Pathology and genetics of tumours of soft tissue and bone. 4th ed. Lyon: ARC Press; 2013.
Jo VY, Fletcher CD. WHO classification of soft tissue tumours: an update based on the 2013 (4th) edition. Pathology. 2014;46(2):95–104.
Fritchie KJ, Goldblum JR, Tubbs RR, Sun Y, Carver P, Billings SD, et al. The expanded histologic spectrum of myxoid liposarcoma with an emphasis on newly described patterns: implications for diagnosis on small biopsy specimens. Am J Clin Pathol. 2012;137(2):229–39.
Miettinen M. Diagnostic soft tissue pathology. Diagn Cytopathol. 2005;33(2):140.
Petscavage-Thomas JM, Walker EA, Logie CI, Clarke LE, Duryea DM, Murphey MD. Soft-tissue myxomatous lesions: review of salient imaging features with pathologic comparison. Radiographics. 2014;34(4):964–80.
Alaggio R, Coffin CM, Weiss SW, Bridge JA, Issakov J, Oliveira AM, et al. Liposarcomas in young patients: a study of 82 cases occurring in patients younger than 22 years of age. Am J Surg Pathol. 2009;33(5):645–58.
Mentzel T, Fletcher CD. Dedifferentiated myxoid liposarcoma: a clinicopathological study suggesting a closer relationship between myxoid and well-differentiated liposarcoma. Histopathology. 1997;30(5):457–63.
Murphey MD, Flemming DJ, Jelinek JS, Temple HT, Levine A, Torop AH. Imaging of higher grade liposarcoma with pathologic correlation. Radiology; 1997;205:813–813.
Jelinek JS, Kransdorf MJ, Shmookler BM, Aboulafia AJ, Malawer MM. Liposarcoma of the extremities: MR and CT findings in the histologic subtypes. Radiology. 1993;186(2):455–9.
Sung MS, Kang HS, Suh JS, Lee JH, Park JM, Kim JY, et al. Myxoid liposarcoma: appearance at MR imaging with histologic correlation. Radiographics. 2000;20(4):1007–19.
Van Vliet M, Kliffen M, Krestin GP, van Dijke CF. Soft tissue sarcomas at a glance: clinical, histological, and MR imaging features of malignant extremity soft tissue tumors. Eur Radiol. 2009;19(6):1499–511.
Crago AM, Dickson MA. Liposarcoma: multimodality management and future targeted therapies. Surg Oncol Clin N Am. 2016;25(4):761–73.
Murphey MD, Arcara LK, Fanburg-Smith J. From the archives of the AFIP: imaging of musculoskeletal liposarcoma with radiologic-pathologic correlation. Radiographics. 2005;25(5):1371–95.
Baheti AD, Tirumani SH, Rosenthal MH, Howard SA, Shinagare AB, Ramaiya NH, et al. Myxoid soft-tissue neoplasms: comprehensive update of the taxonomy and MRI features. AJR Am J Roentgenol. 2015;204(2):374–85.
Tateishi U, Hasegawa T, Beppu Y, Kawai A, Satake M, Moriyama N. Prognostic significance of MRI findings in patients with myxoid–round cell liposarcoma. Am J Roentgenol. 2004;182(3):725–31.
Peterson JJ, Kransdorf MJ, Bancroft LW, O’Connor MI. Malignant fatty tumors: classification, clinical course, imaging appearance and treatment. Skeletal Radiol. 2003;32(9):493–503.
Lowenthal D, Zeile M, Niederhagen M, Fehlberg S, Schnapauff D, Pink D, et al. Differentiation of myxoid liposarcoma by magnetic resonance imaging: a histopathologic correlation. Acta Radiol. 2014;55(8):952–60.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Kuyumcu, G., Rubin, B.P., Bullen, J. et al. Quantification of fat content in lipid-rich myxoid liposarcomas with MRI: a single-center experience with survival analysis. Skeletal Radiol 47, 1411–1417 (2018). https://doi.org/10.1007/s00256-018-2974-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-018-2974-9