Abstract
Objectives
Sarcopenia and changes in body composition following neoadjuvant chemotherapy (NAC) may affect clinical outcome. We assessed the associations between CT body composition changes following NAC and outcomes in oesophageal cancer.
Methods
A total of 35 patients who received NAC followed by oesophagectomy, and underwent CT assessment pre- and post-NAC were included. Fat mass (FM), fat-free mass (FFM), subcutaneous fat to muscle ratio (FMR) and visceral to subcutaneous adipose tissue ratio (VA/SA) were derived from CT. Changes in FM, FFM, FMR, VA/SA and sarcopenia were correlated to chemotherapy dose reductions, postoperative complications, length of hospital stay (LOS), circumferential resection margin (CRM), pathological chemotherapy response, disease-free survival (DFS) and overall survival (OS).
Results
Nine (26 %) patients were sarcopenic before NAC and this increased to 15 (43 %) after NAC. Average weight loss was 3.7 % ± 6.4 (SD) in comparison to FM index (−1.2 ± 4.2), FFM index (−4.6 ± 6.8), FMR (−1.2 ± 24.3) and VA/SA (−62.3 ± 12.7). Changes in FM index (p = 0.022), FMR (p = 0.028), VA/SA (p = 0.024) and weight (p = 0.007) were significant univariable factors for CRM status. There was no significant association between changes in body composition and survival.
Conclusions
Loss of FM, differential loss of VA/SA and skeletal muscle were associated with risk of CRM positivity.
Key Points
• Changes in CT body composition occur after neoadjuvant chemotherapy in oesophageal cancer.
• Sarcopenia was more prevalent after neoadjuvant chemotherapy.
• Fat mass, fat-free mass and weight decreased after neoadjuvant chemotherapy.
• Changes in body composition were associated with CRM positivity.
• Changes in body composition did not affect perioperative complications and survival.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The incidence of oesophageal cancer, and in particular adenocarcinoma, is increasing in the Western world owing to the higher prevalence of known predisposing factors to Barrett’s oesophagus, such as obesity and gastro-oesophageal reflux disease in this population [1]. However, the outcome of patients with oesophageal cancer remains poor with only 1 in 10 patients surviving beyond 10 years [2]. Even in those treated with curative intent, 5-year overall survival rarely exceeds 40 % [3].
The majority of patients with oesophageal cancer present with dysphagia and weight loss which could have an impact on their tolerance to neoadjuvant chemotherapy and surgery, and subsequent surgical and oncological outcomes. Although weight loss is commonly seen and easily assessed in these patients, a more objective assessment of changes in fat and lean body mass or fat-free mass may be a better prognostic tool as weight loss secondary to loss of fat and/or skeletal muscle may have different effects on clinical outcome. Sarcopenia is defined as skeletal muscle mass of less than 2 standard deviations (SD) below that of typical healthy adults [4] and is associated with increased morbidity and mortality in the non-oncological setting [5, 6]. Nutritional status and changes in body composition have been shown to affect perioperative surgical outcomes such as length of hospital stay (LOS) and complication rates [7, 8]. Sarcopenia is associated with increased toxicity to 5-fluorouracil (5FU) chemotherapy and its prodrug, capecitabine [9, 10], and early evidence suggests similar association with epirubicin as well [11]. In contrast, the association between sarcopenia and survival has not been consistently demonstrated in pancreatic, oesophageal, lung and colorectal cancers [10, 12–15]. The combination of sarcopenia and obesity appears to portend a poorer prognosis than the presence of either of these factors alone [13, 14].
There is increased effort to improve perioperative nutritional status in patients with oesophageal cancer owing to the recognition that weight loss and sarcopenia may be associated with inferior surgical and oncological outcome respectively [16]. Computed tomography (CT), magnetic resonance imaging (MRI) and dual energy X-ray absorptiometry (DEXA) are considered gold standards for evaluation of body composition in clinical practice. As diagnostic CT is performed in all patients with oesophageal cancer as part of the patient pathway, body composition analysis using CT images is readily achievable with no additional patient burden or cost, with wider clinical utility. We aimed to evaluate the changes in body composition due to neoadjuvant chemotherapy in patients with oesophageal cancer, and their association with the surgical and survival outcomes.
Methods and materials
Patients and clinical outcome
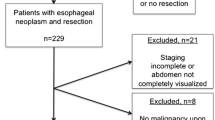
Following institutional review board waiver, we retrospectively identified 35 patients with primary oesophageal cancer treated with neoadjuvant chemotherapy followed by surgical resection from a prospectively managed institutional database and who had baseline and post-NAC treatment contrast-enhanced CT scans available for analysis on the institutional PACS system.
Medical notes were reviewed to extract the following information: patient’s height, baseline and post-chemotherapy weight, neoadjuvant chemotherapy regime, LOS, postoperative complications such as delayed wound healing, chyle leak and infection/sepsis. Body mass index (BMI) was computed and obesity was defined as BMI ≥ 30 kg/m2.
Pathological chemotherapy response was classified on the basis of the tumour regression grade (TRG) as described by Mandard et al. [17]: 1 = no residual cancer cells, 2 = rare residual cancer cells, 3 = fibrosis outgrowing residual cancer, 4 = residual cancer outgrowing fibrosis and 5 = absence of regressive changes. Patients with TRG 1–3 were grouped as responders whereas patients with TRG 4–5 were grouped as non-responders. Positive circumferential resection margin (CRM) was defined as per the Royal College of Pathologists criteria, as any tumour involvement or presence of tumour cells within 1 mm of the resection margin [18].
Overall survival (OS) and disease-free survival (DFS) were defined from the date of surgery to the date of death and date of disease relapse respectively. Patients who were alive were censored at the date of last clinical follow-up.
CT and image analysis
Baseline and post-chemotherapy CT of the thorax and abdomen [16–256 multidetector CT (Philips Healthcare, Best, Netherlands): 120–130 kV, 90–320 mAs, FOV 290–460 mm, matrix 256–512, reconstructed slice thickness 5 mm] were retrieved from the PACS system for image analysis. The top of the third lumbar vertebra (L3) which was defined as the first axial image which clearly showed the transverse processes was chosen as the standard landmark. Two consecutive images extending inferiorly from L3 vertebra were used to measure the fat and muscle cross-sectional areas (Fig. 1a–c). Image analysis was performed on a workstation (Syngo MultiModality Workplace, Siemens AG Healthcare, Forchheim, Germany). Automated specific tissue demarcation was performed using the following Hounsfield unit (HU) thresholds: −29 to +150 (skeletal muscle excluding visceral organs), −190 to −30 (subcutaneous and intramuscular adipose tissue) and −150 to −50 (visceral adipose tissue) as published previously [19–21]. Cross-sectional areas of skeletal muscle, visceral and subcutaneous adipose tissues were obtained.
Total body fat mass (FM) and fat-free mass (FFM) were estimated using the following regression equations [22]:
FM and FFM were normalised for stature to derive FM index and FFM index (kg/m2) respectively:
Subcutaneous fat to muscle ratio (FMR) and visceral to subcutaneous adipose tissue ratio (VA/SA) were calculated. The definition of sarcopenia was based on a previous study performed in patients with cancer by Prado et al. [13]: L3 skeletal muscle index (total L3 skeletal muscle mass normalised for stature) ≤38.5 cm2/m2 for women and ≤52.4 cm2/m2 for men.
Statistical analysis
Data were recorded as mean ± standard deviation (SD).
Percentage changes in FM, FFM, FM index, FFM index, FMR, VA/SA and weight after neoadjuvant chemotherapy were computed. Comparisons between baseline and post-treatment parameters were performed using the Wilcoxon signed-rank test. The correlations between changes in body composition parameters and weight loss or LOS were performed using Spearman’s rank correlation.
The associations between changes in body composition or weight change and pathological chemotherapy response, presence of postoperative complications and CRM status were analysed using the Mann–Whitney U test. The associations between presence of sarcopenia, sarcopenic obesity or obesity and pathological chemotherapy response, presence of postoperative complications and CRM status were evaluated using Fisher’s exact test. Significant univariable factors were included in a multivariate logistic regression analysis to adjust for other confounding factors such as age and/or tumour stage.
Median values were used to dichotomise each parameter. The associations between changes in body composition parameters and OS or DFS were performed using the Kaplan–Meier method. Comparisons between groups were performed using the log-rank test. A p value less than 0.05 was considered statistically significant. All analyses were performed using SPSS 21 statistical package (IBM).
Results
Patient characteristics
Patient and treatment characteristics are summarised in Table 1. Median follow-up for the entire cohort was 24 months. All patients received at least one cycle of neoadjuvant chemotherapy. The median interval between baseline CT and start of chemotherapy was 37 days (interquartile range (IQR) 24–45) whereas the interval between post-treatment CT and end of chemotherapy was 19 days (IQR 10–27). Patients were reviewed by the dietetics team and received oral nutritional supplements. Two patients received enteral feeding.
Changes in body composition
There were 9 patients (26 %) who were sarcopenic prior to treatment and this number increased to 15 (43 %) after chemotherapy. Eight patients who were sarcopenic prior to chemotherapy remained so after treatment. However, there were only 1 (3 %) and 2 (6 %) patients who had sarcopenic obesity before and after treatment respectively (Table 1).
Table 2 shows the changes in the body composition parameters after neoadjuvant chemotherapy. Analyses using baseline weight and BMI were limited to 32 patients as baseline weight was not available for 3 patients. There were 6 (19 %) and 5 (14 %) patients who were classified as obese before and after NAC, respectively (Table 1). Overall, there was a significant decrease in FM and FFM indices, VA/SA, weight and BMI after neoadjuvant chemotherapy. Average weight loss was 3.7 % ± 6.4 in comparison to FM index (−1.7 % ± 4.2), FFM index (−4.6 % ± 6.8), VA/SA (−62.3 % ± 12.7) and BMI (−3.3 % ± 6.2).
There were significant correlations between changes in FM index (r = 0.786, p <0.001) and FMR (r = 0.671, p <0.001) after treatment, and changes in weight (Fig. 2a, b).
Association with outcomes
Surgical
Nine patients (26 %) had postoperative complications but there was no perioperative mortality (Table 1). Changes in FM index (p = 0.022), FMR (p = 0.028), VA/SA (p = 0.024), and weight (p = 0.007) were significant univariable factors for CRM status (Electronic Supplementary Material). There were no significant associations between changes in FM index, FFM index, FMR or VA/SA and LOS, pathological chemotherapy response or presence of postoperative complications. The presence of sarcopenia was not associated with CRM status, chemotherapy response or postoperative complications. As there were very few patients with sarcopenic obesity, no further analysis was performed in this subgroup.
As changes in FM index and FMR were significantly correlated with weight change, the former two variables, changes in VA/SA and clinical tumour stage were included in the subsequent multivariate logistic regression analysis to assess for the risk of positive CRM. No significant multivariable factors were found but changes in VA/SA showed a trend towards significance as a predictor of CRM status (p = 0.072) (Table 3).
Chemotherapy
Weight change following chemotherapy showed a trend towards significance with the need for dose reduction during neoadjuvant chemotherapy (p = 0.054) but changes in FM index (p = 0.876), FFM index (p = 0.407), FMR (p = 0.678) and VA/SA (p = 0.708) did not. The presence of sarcopenia prior to treatment was not associated with chemotherapy dose reduction (p = 0.268).
Survival
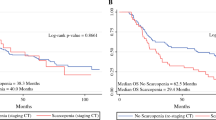
None of the parameters (changes in FM index, FFM index, FMR, VA/SA) were significant prognostic factors for OS and DFS, although a change greater than −5.1 % in FFM index showed a trend as a prognostic factor for DFS (median DFS not reached vs. 27.9 months, p = 0.076) (Fig. 3). Patients who were sarcopenic after chemotherapy had inferior overall survival although this did not reach statistical significance (median OS 25.6 months vs. not reached, p = 0.063) (Fig. 4).
Discussion
Our study showed that patients with oesophageal cancer had decreased fat mass and fat-free mass with resultant weight loss after neoadjuvant chemotherapy. However, the magnitude of these changes was not large and the average differences of these body composition indices varied between −1 and −5 %. Of interest, 19 % of our patients were considered obese and 26 % were sarcopenic prior to the start of neoadjuvant chemotherapy. However, there were only one and two patients with sarcopenic obesity prior to and after neoadjuvant treatment respectively. Our findings were similar to a study by Awad et al. who found a low number of patients (3–6 %) with sarcopenic obesity in those treated with neoadjuvant chemotherapy for gastro-oesophageal (GOJ) cancer [15]. These results suggest that sarcopenic obesity as a potential negative prognostic index may not be useful in this patient population compared to other malignancies where this phenomenon is more prevalent [13, 14].
Our study also showed that changes in FM index, FMR, VA/SA and weight after chemotherapy were associated with CRM status, although these factors did not reach statistical significance in multivariate analysis. This hypothesis-generating finding seems to suggest that fat mass, and perhaps the differential amount of visceral and subcutaneous adipose tissues, may affect the resectability of primary tumour. Another potential reason for this could be that patients with fat and/or weight loss had more advanced and bulky tumours which resulted in higher rates of CRM positivity. As a positive CRM is a poor prognostic factor in oesophageal cancer, this finding may deserve further evaluation in future studies.
Weight loss has been associated with inferior outcome in oesophageal cancer. Although weight loss could be easily assessed in the clinics, it could be attributed to a differential loss of adipose tissue and/or skeletal muscle which may have different clinical implications. Firstly, the distribution of chemotherapeutic agents is dependent on the patient’s body composition as hydrophilic drugs will distribute into the lean compartment whereas lipophilic drugs will distribute to the fat compartment. Thus, changes in fat mass and fat-free mass could lead to increased incidence and severity of chemotherapy toxicities. However, we did not find any significant association between baseline body composition parameters (Electronic Supplementary Material) or changes in these parameters and the need for chemotherapy dose reductions. All of our patients received 5-fluoropyrimidine-based chemotherapy. This differed from previous studies which showed that FFM or sarcopenia was predictive of toxicities to 5-FU and capecitabine chemotherapy [9, 10]. In addition, visceral and subcutaneous adipose tissues have different metabolic and prognostic significance [23]. Visceral adipose tissue is more insulin-resistant compared to subcutaneous adipose tissue [23], and is associated with increased all-cause mortality risk [24]. Visceral and subcutaneous adipose tissues were also found to be significant prognostic factors for survival outcomes in colorectal cancer [25, 26]. In contrast, we did not find similar associations between adipose tissues and survival in our cohort of patients with oesophageal cancer.
The clinical impact of body composition in oesophageal cancer has been investigated in a few studies with variable conclusions. In one retrospective study, lower CT-derived lean psoas muscle area had a negative impact on OS and DFS in patients with oesophageal cancer who underwent transhiatal oesophagectomy without neoadjuvant therapy [27]. In another study, Awad et al. included 47 patients with GOJ cancers, of which a third had gastric primary and were treated with neoadjuvant chemotherapy [15]. Both baseline and post-treatment CT were analysed in this study. The authors found that 57 % of patients were sarcopenic prior to chemotherapy and this increased to 79 % after completion of treatment. Although they found a significant reduction in FM and FFM during chemotherapy, these parameters were not associated with non-completion of chemotherapy, increased hospital stay and mortality, which were not too dissimilar to our findings. In a separate case-controlled study, low FFM and FFM index, which were obtained using bioelectrical impedance analysis (BIA), were shown to be associated with increased LOS in patients admitted to the medical and surgical wards [7]. However, cancer patients made up less than 6 % of all patients studied. FFM and FFM index were found to be more sensitive than weight loss and BMI in predicting LOS.
Changes in body composition in patients with oesophageal cancer could have different clinical implications. Loss of weight, adipose and skeletal tissues could be indicative of poor nutritional intake secondary to patient and tumour factors. Worsening dysphagia due to tumour progression, or anorexia, nausea and vomiting due to chemotherapy are potential causes and warrant prompt clinical evaluation and intervention which could alter the treatment course. Furthermore, changes in body composition could also affect tolerability to systemic or local therapy, possibly resulting in inferior locoregional control and survival, although our results did not support this hypothesis.
Overall, our study benefitted from a similar treatment regime as all patients were treated according to a standard institutional protocol and received similar nutritional support, thus minimising any treatment-related confounding factors. In addition, a single observer performed all the image analysis which removed the element of interobserver variability. However, our study has several limitations. Firstly, our study was based on a small sample size and was not adequately powered to detect differences in survival. The results of this study should be validated in a larger study. In addition, the retrospective nature of this analysis limited the amount of information we could obtain from medical records regarding chemotherapy toxicity and intraoperative complications. We were unable to analyse the differences in body composition and clinical outcomes according to gender as the majority of patients in our study were men. We also did not evaluate the role of systemic inflammatory response or other nutritional markers such as albumin, as our primary study aim was to investigate the changes in body composition after neoadjuvant chemotherapy. Nonetheless, the prognostic role of gender and the addition of these serum biomarkers to body composition indices could be evaluated in future studies.
In conclusion, in our patient cohort, the proportion of sarcopenic patients increased following neoadjuvant chemotherapy, with a reduction in fat mass and fat-free mass. However, changes in CT body composition parameters did not affect perioperative or survival outcomes although changes in fat composition could be related to CRM status.
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2011) Global cancer statistics. CA Cancer J Clin 61:69–90
Cancer Research UK (2012) Oesophageal cancer survival statistics, Cancer Research UK, London. http://www.cancerresearchuk.org/cancer-info/cancerstats/types/oesophagus/survival/. Accessed 2 Sept 2013
Berger B, Belka C (2009) Evidence-based radiation oncology: oesophagus. Radiother Oncol 92:276–290
Baumgartner RN, Koehler KM, Gallagher D et al (1998) Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol 147:755–763
Janssen I, Heymsfield SB, Ross R (2002) Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc 50:889–896
Cosqueric G, Sebag A, Ducolombier C, Thomas C, Piette F, Weill-Engerer S (2006) Sarcopenia is predictive of nosocomial infection in care of the elderly. Br J Nutr 96:895–901
Pichard C, Kyle UG, Morabia A, Perrier A, Vermeulen B, Unger P (2004) Nutritional assessment: lean body mass depletion at hospital admission is associated with an increased length of stay. Am J Clin Nutr 79:613–618
McClave SA, Snider HL, Spain DA (1999) Preoperative issues in clinical nutrition. Chest 115:64S–70S
Prado CM, Baracos VE, McCargar LJ et al (2007) Body composition as an independent determinant of 5-fluorouracil-based chemotherapy toxicity. Clin Cancer Res 13:3264–3268
Prado CM, Baracos VE, McCargar LJ et al (2009) Sarcopenia as a determinant of chemotherapy toxicity and time to tumor progression in metastatic breast cancer patients receiving capecitabine treatment. Clin Cancer Res 15:2920–2926
Prado CM, Lima IS, Baracos VE et al (2011) An exploratory study of body composition as a determinant of epirubicin pharmacokinetics and toxicity. Cancer Chemother Pharmacol 67:93–101
Balentine CJ, Enriquez J, Fisher W et al (2010) Intra-abdominal fat predicts survival in pancreatic cancer. J Gastrointest Surg 14:1832–1837
Prado CM, Lieffers JR, McCargar LJ et al (2008) Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol 9:629–635
Tan BH, Birdsell LA, Martin L, Baracos VE, Fearon KC (2009) Sarcopenia in an overweight or obese patient is an adverse prognostic factor in pancreatic cancer. Clin Cancer Res 15:6973–6979
Awad S, Tan BH, Cui H et al (2012) Marked changes in body composition following neoadjuvant chemotherapy for oesophagogastric cancer. Clin Nutr 31:74–77
Bower MR, Martin RC 2nd (2009) Nutritional management during neoadjuvant therapy for esophageal cancer. J Surg Oncol 100:82–87
Mandard AM, Dalibard F, Mandard JC et al (1994) Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer 73:2680–2686
Mapstone NP (2007) Dataset for the histopathological reporting of oesophageal carcinoma, 2nd edn. Royal College of Pathologists, London
Mitsiopoulos N, Baumgartner RN, Heymsfield SB, Lyons W, Gallagher D, Ross R (1998) Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol 85:115–122
Kvist H, Sjostrom L, Tylen U (1986) Adipose tissue volume determinations in women by computed tomography: technical considerations. Int J Obes 10:53–67
Vehmas T, Kairemo KJ, Taavitsainen MJ (1996) Measuring visceral adipose tissue content from contrast enhanced computed tomography. Int J Obes Relat Metab Disord 20:570–573
Mourtzakis M, Prado CM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE (2008) A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab 33:997–1006
Ibrahim MM (2010) Subcutaneous and visceral adipose tissue: structural and functional differences. Obes Rev 11:11–18
Kuk JL, Katzmarzyk PT, Nichaman MZ, Church TS, Blair SN, Ross R (2006) Visceral fat is an independent predictor of all-cause mortality in men. Obesity 14:336–341
Moon HG, Ju YT, Jeong CY et al (2008) Visceral obesity may affect oncologic outcome in patients with colorectal cancer. Ann Surg Oncol 15:1918–1922
Clark W, Siegel EM, Chen YA et al (2013) Quantitative measures of visceral adiposity and body mass index in predicting rectal cancer outcomes after neoadjuvant chemoradiation. J Am Coll Surg 216:1070–1081
Sheetz KH, Zhao L, Holcombe SA et al (2013) Decreased core muscle size is associated with worse patient survival following esophagectomy for cancer. Dis Esophagus 26:716–722
Acknowledgements
The scientific guarantor of this publication is Prof. Vicky Goh. The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article. This study has received funding by the Department of Health via the National Institute of Health Research Biomedical Research Centre award to Guy’s and St Thomas’ NHS Foundation Trust in partnership with King’s College London and King’s College Hospital NHS Foundation Trust; and from the Comprehensive Cancer Imaging Centre, funded by the Cancer Research UK and Engineering and Physical Sciences Research Council in association with the Medical Research Council and Department of Health. Dr. Connie Yip receives funding support from the National Medical Research Council, Singapore. No complex statistical methods were necessary for this paper. Institutional review board approval was obtained. Written informed consent was not required for this study because anonymised patient data was used with no intervention or patient contact. Methodology: retrospective, diagnostic or prognostic study, performed at one institution.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 16 kb)
Rights and permissions
About this article
Cite this article
Yip, C., Goh, V., Davies, A. et al. Assessment of sarcopenia and changes in body composition after neoadjuvant chemotherapy and associations with clinical outcomes in oesophageal cancer. Eur Radiol 24, 998–1005 (2014). https://doi.org/10.1007/s00330-014-3110-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-014-3110-4