Abstract
Objectives
To analyze the diagnostic accuracy of ultrasound-guided core-needle biopsy (CNB) of thyroid nodules.
Methods
Of 3517 CNBs performed using an 18G spring-loaded device in one institution, we retrospectively reviewed 676 nodules in 629 consecutive patients who underwent surgery. CNB and pathological examination were compared. CNB diagnosis was standardized in four categories: insufficient (I), benign (B), follicular lesion (FOL), and malignant (M). Main outcome measures were predictive positive values (PPV), false positives (FP), and false negatives (FN).
Results
CNB showed a low rate of insufficient and FOL diagnoses (5.8 % and 4.5 %). On surgery, there were eight FNs in 374 benign CNBs and three FPs in 148 malignant CNBs. The 154 nodules classified as FOL in CNB included, at surgery, 122 neoplasms; 28 of them malignant. PPV for malignancy of a malignant CNB was 98 %, and for a CNB diagnosis of FOL 18.2 %. Sensitivity for malignancy if CNB of FOL and M are considered positive was 95.6. Only one major complication was observed.
Conclusions
CNB is reliable, safe, and accurate to evaluate thyroid nodules and can be an alternative technique to FNA. It has low rate of non-diagnostic and undetermined cases, with high sensitivity and PPV.
Key Points
• Thyroid core-needle biopsy (CNB) has high sensitivity and PPV.
• Pitfalls of CNB are rare.
• Pitfalls are due to cystic cancer, histological heterogeneity, and mistakes in analysis.
• CNB is a reliable, safe, and accurate method to approach thyroid nodules.
• CNB can be used primarily or after insufficient or indeterminate FNA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fine-needle aspiration (FNA) with cytological evaluation has become a usual technique for screening thyroid cancer [1]. It has reduced the number of thyroid surgeries and increased the likelihood of detecting malignancy in patients undergoing thyroidectomy. Diagnostic performance of thyroid FNA, however, is hindered by non-diagnostic biopsies due to inadequate/insufficient samples or indeterminate cytological patterns, the latter including atypia of uncertain significance/follicular lesions of uncertain significance (AUS/FLUS), and follicular neoplasms/suspicious for follicular neoplasm (FN/SFN), third and fourth categories of Bethesda System for Reporting Thyroid Cytopathology [2]. Factors influencing indeterminate or insufficient FNA include the operator performing the procedure [3], the technique [4], and the experience of the cytologist [5], reaching up to 24 % in a recent multicenter review [6]. The recommended strategy to solve these drawbacks is repeating the FNA, which is diagnostic in 50 % of cases [7, 8]. Surgical follow-up of these groups yields a malignancy ratio that ranges from 6 % to 48 % in AUS/FLUS [9] and between 15 and 30 % in FN/SFN [2, 10].
Ultrasound (US) guided percutaneous thyroid nodule core needle biopsy (CNB) has proved to be useful in non-diagnostic FNA, and in nodules with AUS at FNA. The CNB allows obtaining a sample for histological study and thus a higher diagnostic accuracy than FNA, but this is still in debate [11–15]. Some authors report better results with a combination of both techniques [16–18]. Concerns regarding potential increase in patient pain and complications, as well as technical issues, have curtailed the spread of thyroid CNB.
Only a few reports about the accuracy of thyroid CNB can be found in the literature and, to our knowledge, there are no studies regarding performance evaluation as a primary tool and potential pitfalls of the technique. In our centre, after using CNB to improve the diagnostic performance of thyroid FNA, our preliminary results encouraged us to use this technique as primary diagnostic test over FNA [19].
The purposes of this study were to analyze the diagnostic accuracy of ultrasound-guided core-needle biopsy (CNB) of thyroid nodules using thyroidectomy specimens as a reference standard and to describe the main causes of its pitfalls.
Materials and methods
Study design
We performed a retrospective review of all CNB of thyroid nodules from October 1, 2005 to December 1, 2013 in our institution. Biopsies were requested for any nodule with sonographic criteria following the ATA guidelines [20]. We included in our study all the patients who had undergone thyroidectomy and had at least one previous CNB of one or more thyroid nodules. In our centre, surgery is indicated after a CNB result of malignancy or follicular proliferation, and in some nodules with benign CNB because of size, growth, local compressive symptoms, or patient’s choice.
US pattern, localization, and size of the nodules, pathological diagnosis of both the biopsy and surgery, complications of CNB, and demographic issues were recorded. If necessary, a consensus was required between radiologist, pathologist, and surgeon. Surgical and pathological reports were recorded no more than 2 weeks after thyroidectomy. The interval between CNB and surgery was less than 12 months in all cases. Informed consent was obtained for all patients, and institutional review board approval was obtained for this study.
CNB procedure
CNB procedures were performed with real-time US guidance, using a 10-12 MHz linear probe in an IU-22 platform (Philips HC, Best, the Nederlands). Three radiologists with more than 20, 8, and 4 years of experience in thyroid biopsies performed all procedures. CNB were performed using a spring-loaded 18-gauge full-core Biopince biopsy needle (Angiotech, Vancouver, Canada). This needle has a variable stroke length of 13, 23, and 33 mm, thus allowing different sample sizes according to the volume and position of nodule, an excellent control of the reach of the biopsy, and allows obtaining good specimens with a relatively small gauge. Each patient was placed supine with the neck extended. After an initial US evaluation to identify the thyroid nodules of interest, 1 % lidocaine was injected in the path. The US probe was placed over the target, and the biopsy needle was advanced with US guidance to the edge of the nodule and then fired (Fig. 1). The approach was usually transisthmic (see Results), introducing the needle from the midline of the neck, trough the isthmus to the nodule. When this approach was not possible (i.e. nodules located close to the carotid artery) a lateral-to-midline approach was used. Usually, two CNB specimens of each thyroid nodule were taken. Cystic nodules were emptied using a 22-G needle before taken a CNB from the residual solid portion. Patients were discharged after a firm local compression of 5-10 min.
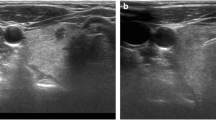
A 42-year-old woman was scheduled for biopsy of a left thyroid nodule of 2.5 cm (a). In the sonographic exploration before the biopsy, a small (3.5 mm) hypoechoic nodule was discovered in the right lobe (b). Both nodules underwent biopsy. In c, the needle (arrows) can be seen pointing to the small nodule. A transisthmic approach is used. In d, the needle (arrows) can be seen crossing the target after firing the spring-loaded device. The diagnosis of the biopsy of the bigger lesion was hyperplasia, and the smaller nodule was a papillary carcinoma
Specimen analysis
CNB samples were placed in cooled saline solution and immediately transported to the pathology laboratory. Refrigeration of the transport medium prevented tissue autolysis and contributed to maintain the samples in optimal conditions. Upon arrival in the laboratory, biopsy core samples were processed, fixed in formalin, and embedded in paraffin. Paraffin block slices were taken from the beginning with the microtome, keeping two or three unstained slides mounted for immunohistochemistry, if necessary. A board-certified attending staff pathologist reviewed each CNB specimen. We standardized four main diagnostic categories for the CNB results: insufficient (I); benign (B), including hyperplasic, inflammatory, and other benign lesions; follicular lesion (FOL), including follicular and oncocityc neoplasms; and malignant (M), papillary, medullary, or other thyroid cancer subtypes. Diagnosis of FOL versus hyperplasic lesions was defined according to previously described criteria [18], i.e. microfollicular pattern with scant or absent colloid, sometimes with minimal pleomorphism or discrete nuclear changes. This category could be equivalent to AUS/FLUS, and follicular neoplasms/suspicious for follicular neoplasm (FN/SFN), III and IV categories in Bethesda classification [2]. We do not consider suspicious of malignancy as a diagnostic category because the biopsy specimen allowed a more precise diagnosis.
After thyroidectomy, all surgery specimens were evaluated by the same pathologists who performed the CNB analysis. Definitive surgical diagnoses were categorized as follows: hyperplastic nodule, thyroiditis, other benign lesions, follicular adenoma, oncocytic adenoma, papillary carcinoma (PTC), follicular carcinoma (FTC), oncocytic carcinoma, medullary carcinoma (MTC), and other malignant tumours, based on classical histopathological criteria [19]. Surgical pathology diagnosis was considered the final diagnosis.
Statistical analysis
Positive predictive value (PPV) for malignancy was calculated for every category of CMB diagnosis. Also, as both FOL and M results lead to the recommendation of surgical management, sensitivity and PPV of CNB considering any of both diagnosis as positive, was calculated. Moreover, to test the diagnostic value of thyroid CNB to identify neoplasms, we calculated sensitivity and PPV of CNB for diagnosis of adenoma (follicular or oncocytic) or thyroid cancer considering CNB FOL and M results as positive. As we have only included patients who underwent surgery, calculation of predictive negative value (PNV) or specificity was not appropriate.
Analysis of variance (ANOVA) was used to test differences for age and size of the nodules. Chi-square test or Fisher’s exact test was used to compare frequencies for categorical variables such as nodular content, lobe, solitary or multinodular goiter and categorized size. A two-tailed p-value less than 5 % was considered significant. Statistical analysis was performed with the SPSS package software, version 19.0 (SPSS, Chicago, IL, USA).
Results
In total, 3517 thyroid CNB were performed with 204 (5.8 %) considered insufficient or inadequate in pathological evaluation. Of them, 54 (27 %) were biopsied again, and eight were surgically removed and thus included in this study. All of them were benign.
Only one major complication was observed: a recurrent nerve lesion after a direct puncture of the nerve (myelin was observed in the specimen) that caused a permanent dysphonia. After this case we favoured a transisthmic approach for the biopsy. Minor complications included 56 self-limited hematomas that did not require treatment. From all the 3313 diagnostic CNB, 158 samples were labelled as malignant (M), 160 were classified as FOL, and the remaining as benign (B) nodules. Out of them, 684 nodules in 637 patients had surgery (in 47 patients, two different nodules were biopsied). Eight of them (1.2 %) had unsatisfactory or insufficient tissue to make a diagnosis, all of them benign on surgery.
In 676 nodules undergoing surgery, CNB diagnosis was benign in 374, malignant in 148, and FOL in 154 (Table 1). Demographic and sonographic characteristics of these groups are shown in Table 2. After surgery, 181 nodules were malignant (154 PTC, 16 FTC, five MTC, three anaplastic carcinomas, two poorly differentiated carcinoma, one mucoepidermoid carcinoma) and 495 nodules benign (380 hyperplasic lesions, 60 follicular adenomas, 41 oncocytic adenomas, 10 lymphocytic thyroiditis, one fibrotic nodule, one cavernous hemangioma, one amiloid goiter, and one necrotic cyst). The correlation between CB results and histopathological results is shown in Table 3. In 614 cases (90.8 %) CB diagnosis and final diagnosis were coincident and in 62 (9.2 %) not (Fig. 2).
Incidental papillary microcarcinomas were discovered on surgery in 56 patients of the study group, 38 of them in patients with CB diagnosis of hyperplasia and 18 in diagnosis of FOL. These cases were not considered false negatives, and the incidental lesions were not included in the study because they were located in non-biopsied areas, distant from the biopsied nodules.
Discrepancies and causes
Discrepant results are shown in Table 4. A benign CNB corresponded to a malignant final diagnosis in 2.1 % of cases (8/374). The causes of false negative were:
a. Cystic PTC with small solid component in four cases measuring 12 to 14 mm. One of them revealed their malignancy when simultaneous FNA was performed to empty the cyst.
b. Sampling error in three cases: two well-differentiated follicular variants of PTC with scattered milimetric tumour foci into nodules bigger than 30 mm, and one multifocal PTC with six foci from 2 to 18 mm, none of them sampled on CNB.
c. Error in pathological interpretation. One follicular variant PTC of 40 mm with evident diagnosis of malignancy in the revision of CNB specimen after surgical diagnosis.
False positive patients included two hyperplasic lesions with partial nuclear changes that had been identified as suspicious of PTC in CNB, and one hyperplasic nodule with squamous metaplasia classified as squamous carcinoma in CNB, as previously reported [21]. A diagnosis of malignancy in CNB had a false positive rate of 2 % (3/148). One PTC, diffuse sclerosing variant, was diagnosed as MTC in CNB. Revision after surgical histopathologic analysis, showed an error in pathological interpretation, with negative immunohistochemistry for calcitonin in CNB specimen.
CNB and surgical diagnosis of follicular and oncocytic neoplasms
Follicular non-oncocytic tumours at CNB (n = 89) included 72 truly follicular tumours when operated on: 55 follicular adenomas (one oncocytic variant), nine follicular carcinomas, and eight PTC (seven follicular variant and one cribiform morular). The other lesions were 16 hyperplasic lesions and one MTC.
At surgery, the 65 oncocytic tumours in CNB, resulted in 47 truly oncocytic lesions (40 adenomas and seven oncocytic carcinomas), two PTC oncocytic variant, 15 hyperplasic nodules (all with oncocytic transformation), and one poorly differentiated carcinoma.
Diagnostic performance of CNB
Positive predictive value of a CNB diagnosis of malignancy is 98 % (Table 3). The sensitivity for malignancy if CNB diagnosis of FOL or M are considered positive was 95.6 % (173 of 181) and PPV 57.9 % (173/299). The sensitivity for diagnosis of thyroid neoplasm (adenoma or carcinoma) if CNB diagnosis of FOL an M are considered positive was 95 % (268/282) and PPV 88.7 % (268/302).
Discussion
FNA is an excellent diagnostic tool and the reference standard in evaluating nodular thyroid disease, but limited by insufficient or indeterminate results [22]. Repeated FNA, second opinion diagnosis and group consensus review of cytological specimens may reduce uncertainty, but results are inconclusive in a significant number of cases. In addition, the indeterminate AUS/FLUS category has a high inter-observer variability and the probability of malignancy in these nodules remains unclear [8, 23–27].
CNB offers an alternative that could improve the accuracy of diagnosis in these challenging nodules [14–16, 28–30]. Recently, some authors have published good results when using this technique in combination with FNA [18] or after insufficient, non–diagnostic, or suspicious results in FNA, as in nodules with suspicious US features and benign cytology [11, 12, 14–16, 31–33]. The results suggest higher diagnostic accuracy for CNB, mainly because it can offer a bigger and a better sample than FNA, as has been demonstrated with samples acquired from surgical specimens [34]. However, since CNB is a seldom used technique, its actual diagnostic power as a primary indication has not been studied so thoroughly as that of the FNA, with only a few articles studying its accuracy, and these reports include selected cases [35].
Only a low number of series compares the results from CNB with that of pathology after surgery. Most of them use instead a stable clinical course and the lack of sonographic changes to consider the nodule as benign, without a definitive histological diagnosis. This approach potentially misses up to 10 % carcinomas, particularly in large thyroid nodules, due to histological heterogeneity [36], also observed in two of our cases. To our knowledge, ours is the biggest published series of thyroid CNB with surgical correlation. Moreover, we have included consecutive thyroidectomy confirmed nodules; regardless if a prior FNA had been performed, thus not selected by previous indeterminate or failed FNA as in other published series [11, 12, 14, 15, 29, 37].
Higher economical cost, technical requirements, time consumption, and concerns about potential complications, have limited the use of CNB. However, we have observed a low rate of insufficient samples for CNB (5.8 %), thus additional procedures are less frequently required. Also, we have observed only one major complication: a neurogenic lesion, also reported with FNA [38], and, in our experience, the procedure, in experienced hands, is similarly as time consuming as FNA cytology. In addition, FNA diagnostic categories show great inter-operator variability owing to technical aspects, application of criteria and subjective interpretation of each cytologist [9]. Our proposed four main diagnostic categories for CNB could avoid these limitations of FNA.
The main problem of FNA in thyroid nodules is the indeterminate cytological patterns, the third and fourth groups of diagnostic categories in the Bethesda system: AUS/FLUS (with a risk of malignancy of 5–15 %), and FN/ SFN (with 15–30 % risk of malignancy) [2]. When these cytomorphologic features appear, the usual practice is repeating FNA for AUS/FLUS or a surgical procedure for FN/SFN. Recommended rate of insufficient FNA in Bethesda’s guidelines [2] are less than 10 % and 7 % for diagnosis of AUS/FLUS. There is no recommended ratio for FN/SFN, but usually is slightly higher than malignant [9]. This means that more than 20 % of FNA do not have a reliable diagnosis. CNB is also affected by this limitation, but insufficient and FOL cases include only 10.3 % of all the biopsies. Almost nine of ten CNB obtained a definitive diagnosis in a single session. In the group labelled as FOL, 79.7 % of the nodules were neoplasms, 18.2 % malignant, similar to reported in undetermined cases with FNA.
Histology is the reference standard diagnostic test in the evaluation of follicular neoplasms. Numerous tissue blocks have to be prepared and analysed to demonstrate or exclude vascular invasion and/or capsular breakthrough as decisive hallmarks of malignancy. CNB that includes the nodule capsule for preoperative diagnosis of indeterminate follicular neoplasms for detecting malignancy has been proposed in a short series [39].
In addition, some groups suggest that FTC may develop from follicular adenoma due to the accumulation of genetic alterations [40]. This prospect would question the nonsurgical option if the adenomatous lineage of the neoplasm could be preoperatively demonstrated.
CNB, in our study, is a highly reliable test, with a disagreement ratio of 9.2 %, lower than 15.3 % reported by Yang et al in a series of 4703 FNA in 2007 [41]. Only in a small number of cases the result of CNB has lead to a mistaken clinical decision. Our results show a very high PPV (98 %) for a malignant CNB in preoperative diagnosis of malignancy of thyroid nodules. Pitfalls of CNB include cystic PTC. So, it should be stressed the importance to drain the cystic component of any cystic lesion before the CNB and to carry out a cytological analysis of the fluid in such cases. Other causes of false negatives are infrequent: sampling error due to histological heterogeneity in non-homogeneous neoplasms with undefined pathologic foci, and mistakes in pathological analysis. False positives are even more infrequent (2 %) and also related to pathological interpretation.
Sensitivity of a CNB diagnosis of malignancy is very high (94.8 %). Thus, a CNB diagnosis of malignancy and a benign diagnosis should be both regarded as highly reliable. PPV for malignancy of a CNB diagnosis of FOL is 18.2 %, similar to Bethesda IV [2] lesions. Moreover, PPV of CNB diagnosis of neoplasm (FOL or M) for diagnosis of thyroid neoplasm (adenoma or carcinoma) is 88.7 %. Difference in PPV between a CNB diagnosis of follicular neoplasm and oncocytic neoplasm is low.
This study demonstrates the potential of CNB as primary diagnostic test for patients with thyroid nodules. Using a robust volume of patients the study confirms that one diagnostic CNB correlates to subsequent pathological study, making unnecessary studies in CNB labelled as benign.
Selection of the type of biopsy needle is, in our opinion, a very important technical item to achieve good results with thyroid CNB. The use of end-cut biopsy needles allows obtaining larger specimens with needles of small bore. Side-cut needles, the other type of CNB needles, have also the inconvenience of having a non-sampling distal end that can cause undesired damage to structures located beyond the biopsied nodule.
Our study has some limitations. It is a retrospective study. The surgical diagnoses were not reviewed by an external pathology expert or a central panel of pathologists, as should be done in studies evaluating accuracy in clinical trials designed for the development of diagnostic test performance. However, the pathologists evaluating the CNB and surgical specimens have an extensive experience. The job was carried out in a single centre with a high volume of cases, and the results cannot be applied to other centre with lesser experience. Given that we have included only cases with diagnostic CNB and surgical correlation, we cannot calculate specificity or PNV of the technique. Also, despite the guidelines of our centre, some cases with M or FOL diagnosis have not been operated on, including five thyroid lymphomas treated without surgery, three invasive anaplastic thyroid cancers, two metastatic carcinomas and six CNB diagnosed as FOL not operated because the patient refused surgery. However, the potential impact of these cases in the measured parameters is low given our big sample size and the high accuracy observed for CNB.
We conclude that CNB is a reliable, safe, and accurate method to approach thyroid nodules in the clinical practice. Our results show high sensitivity and PPV and a low rate of non-diagnostic cases. Pitfalls of CNB are scarce and related to cystic PTC, sampling error due to histological heterogeneity and mistakes in pathological analysis. US-guided CNB can be an alternative technique to FNA in selected nodules, in addition to a technique to be used after insufficient samples or indeterminate cytological patterns in FNA instead of repeating FNA or surgical lobectomy.
References
Martin HE, Ellis EB (1930) Biopsy by needle puncture and aspiration. Ann Surg 92:169–181
Cibas ES, Ali SZ (2009) The Bethesda system for reporting thyroid cytopathology. Thyroid 19:1159–1165
Singh N, Ryan D, Berney D, Calaminici M, Sheaff MT, Wells CA (2003) Inadequate rates are lower when FNAC samples are taken by cytopathologists. Cytopathology 14:327–331
Tublin ME, Martin JA, Rollin LJ, Pealer K, Kurs-Lasky M, Ohori NP (2007) Ultrasound-guided fine-needle aspiration versus fine-needle capillary sampling biopsy of thyroid nodules: does technique matter? J Ultrasound Med 26:1697–1701
Sebo TJ (2012) What are the keys to successful thyroid FNA interpretation? Clin Endocrinol 77:13–17
Lewis CM, Chang K-P, Pitman M, Faquin WC, Randolph W (2009) Thyroid fine-needle aspiration biopsy: variability in reporting. Thyroid 19:717–723
Alexander EK, Heering JP, Benson CB et al (2002) Assessment of nondiagnostic ultrasound-guided fine needle aspirations of thyroid nodules. J Clin Endocrinol Metab 87:4924–4927
VanderLaan PA, Marqusee E, Krause JF (2011) Clinical outcome for atypia of undetermined significance in thyroid fine-nedle aspirations: should repeated FNA be the preferred initial approach? Am J Clin Pathol 135:770–775
Ohori NP, Schoedel KE (2011) Variability in the Atypia of Undetermined Significance/Follicular Lesion of Undetermined Significance Diagnosis in the Bethesda System for Reporting Thyroid Cytopathology: Sources and recommendations. Acta Cytol 55:492–498
Deandrea M, Ragazonni F, Molla M et al (2010) Diagnostic value of a cytomorphological subclassification of follicular patterned thyroid lesions: a study of 927 consecutive cases with histological correlation. Thyroid 20:1077–1083
Samir AE, Vij A, Seale MK et al (2012) Ultrasound-guided percutaneous thyroid nodule core biopsy: clinical utility in patients with prior nondiagnostic fine-needle aspirate. Thyroid 22:461–467
Na DG, Kim JH, Sung JY et al (2012) Core-Needle Biopsy Is More Useful Than Repeat Fine-Needle Aspiration in Thyroid Nodules Read as Nondiagnostic or Atypia of Undetermined Significance by the Bethesda System for Reporting Thyroid Cytopathology. Thyroid 22:468–475
Renshaw AA, Pinnar N (2007) Comparison of thyroid fine-needle aspiration and core needle biopsy. Am J Clin Pathol 128:370–374
Ha EJ, Baek JH, Lee JH et al (2014) Core needle biopsy can minimise the non-diagnostic results and need for diagnostic surgery in patients with calcified thyroid nodules. Eur Radiol 24:1403–1409
Choi SH, Baek JH, Lee JH et al (2014) Thyroid nodules with initially non-diagnostic, fine-needle aspiration results: comparison of core-needle biopsy and repeated fine-needle aspiration. Eur Radiol 24:2819–2826
Quinn SF, Nelson HA, Demlow TA (1994) Thyroid biopsies: fine-needle aspiration biopsy versus spring-activated core biopsy needle in 102 patients. J Vasc Interv Radiol 5:619–623
Oppenheimer JD, Kasuganti D, Nayar R et al (2010) How to Interpret Thyroid Biopsy Results: A Three-Year Retrospective Interventional Radiology Experience. Cardiovasc Interv Radiol 33:800–805
Sung JY, Na DG, Kim KS et al (2012) Diagnostic accuracy of fine-needle aspiration versus core-needle biopsy for the diagnosis of thyroid malignancy in a clinical cohort. Eur Radiol 22:1564–1572
López JI, Zabala R, del Cura JL (2013) Histological diagnosis of thyroid disease using ultrasound-guided core-biopsies. Eur Thyroid J 2:29–36
Cooper DS, Doherty GM, Haugen BR et al (2009) Revised American Thyroid Association Management Guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 19:1167–1214
Pellicer DL, Sadow PM, Stephen A, Faquin WC (2013) Atypical squamous metaplasia in a benign cystic thyroid nodule mimicking high-grade carcinoma. Diagn Cytopathol 41:706–709
Sakorafas GH (2010) Thyroid nodules; interpretation and importance of fine-needle aspiration (FNA) for the clinician. Practical considerations. Surg Oncol 19:e130–e139
Gerhard R, Boerner SL (2014) The value of second opinion in thyroid cytology. Cancer Cytopathol. doi:10.1002/cncy.21436
Jing X, Knoepp SM, Roh MH et al (2012) Group consensus review minimizes the diagnosis of “Follicular lesion of undetermined significance” and improves cytohistologic correlation. Diagn Cytopathol 40:1037–1042
McElroy MK, Sepi Mahooti S, Hasteh F (2014) A single institution experience with the new Bethesda system for reporting thyroid cytopathology: Correlation with existing cytologic, clinical, and histological data. Diagn Cytopathol 42:564–569
Ho AS, Sarti EE, Jain KS et al (2014) Malignancy Rate in Thyroid Nodules Classified as Bethesda Category III (AUS/FLUS). Thyroid 24:832–839
Tepeoğlu M, Bilezikçi B, Bayraktar SG (2014) A histological assessment of the Bethesda system for reporting thyroid cytopathology (2010) abnormal categories: a series of 219 consecutive cases. Cytopathology 25:39–44
Harvey JN, Parker D, De P, Shrimati RK, Otter M (2003) Sonographically guided core guided biopsy in the assessment of thyroid nodules. J Clin Ultrasound 33:57–62
Taki S, Kakuda K, Kakuma K et al (1997) Thyroid nodules: evaluation with US-guided core biopsy with an automated biopsy gun. Radiology 202:874–877
Screaton NJ, Berman LH, Grant JW (2003) US-guided core-needle biopsy of the thyroid gland. Radiology 226:827–832
Zhang S, Ivanovic M, Nemcek AA Jr, Defrias DV, Lucas E, Nayar R (2008) Thin core needle biopsy crush preparations in conjunction with fine needle aspiration for the evaluation of thyroid nodules: a complementary approach. Cancer 114:512–518
Choi YJ, Baek JH, Ha EJ et al (2014) Different risk of malignancy and management recommendations in subcategories of thyroid nodules with atypia of undetermined significance (AUS) or follicular lesion of undetermined significance (FLUS): The role of US-guided core-needle biopsy (CNB). Thyroid 24:494–501
Ha EJ, Baek JH, Lee JH et al (2013) Sonographically suspicious thyroid nodules with initially benign cytologic results. The role of a core needle biopsy. Thyroid 23:703–708
Hakala T, Kholová I, Sand J, Saaristo R, Kellokumpu-Lehtinen P (2013) A core needle biopsy provides more malignancy-specific results than fine-needle aspiration biopsy in thyroid nodules suspicious for malignancy. J Clin Pathol 66:1046–1050
Trimboli P, Nasrollah N, Guidobaldi L et al (2014) The use of core needle biopsy as first-line in diagnosis of thyroid nodules reduces false negative and inconclusive data reported by fine-needle aspiration. World J Surg Oncol 12:61
McCoy KL, Jabbour N, Ogilvie JB, Ohori NP, Carty SE, Yim JH (2007) The incidence of cancer and rate of false-negative cytology in thyroid nodules greater than or equal to 4 cm in size. Surgery 142:837–844
Carpi A, Naccarato AG, Iervasi G et al (2006) Large needle aspiration biopsy and galectin-3 determination in selected thyroid nodules with indeterminate FNA-cytology. Br J Cancer 95:204–209
Polyzos SA, Anastasilakis AD (2009) Clinical complications following thyroid fine-needle biopsy: a systematic review. Clin Endocrinol (Oxf) 71:157–165
Nasrollah N, Trimboli P, Cicciarela Modica DD et al (2013) Thin core biopsy should help to discriminate thyroid nodules cytologically classified as indeterminate. A new sampling technique. Endocrine 43:659–665
Schmid KW, Farid NR (2006) How to define follicular thyroid carcinoma? Virchow’s Arch 448:385–393
Yang J, Schnadig V, Logrono R, Wasserman PG (2007) Fine needle aspiration of thyroid nodules: a study of 4703 patients with histologic and clinical correlations. Cancer Cytopathol 111:306–315
Acknowledgments
The scientific guarantor of this publication is Jose Luis del Cura. Jose Luis del Cura declares relationships as advisor of CIVCO. The remaining authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article. The authors state that this work has not received any funding. Jose Luis del Cura provided statistical advice for this manuscript. Institutional Review Board approval was obtained. Written informed consent was obtained from all subjects (patients) in this study.
Methodology: retrospective, diagnostic study / observational, performed at one institution.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Paja, M., del Cura, J.L., Zabala, R. et al. Ultrasound-guided core-needle biopsy in thyroid nodules. A study of 676 consecutive cases with surgical correlation. Eur Radiol 26, 1–8 (2016). https://doi.org/10.1007/s00330-015-3821-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-015-3821-1