Abstract
Purpose
We aimed to compare contrast-enhanced ultrasound (CEUS) with contrast-enhanced computed tomography (CECT) for evaluating the treatment response to transcatheter arterial chemoembolization (TACE) of hepatocellular carcinoma (HCC).
Materials and methods
Treatment responses of 130 patients who underwent TACE were evaluated by CEUS and CECT. We initially compared the abilities of CEUS and CECT to detect residual tumour, which were confirmed by histology or angiography. Then, we compared the tumour response to TACE assessed by CEUS and CECT, according to Modified Response Evaluation Criteria in Solid Tumours (mRECIST).
Results
The sensitivity and accuracy of detecting residual tumour by CEUS vs. CECT were 95.9 % vs. 76.2 % (p < 0.001) and 96.2 % vs. 77.7 % (p < 0.001), respectively. For target lesions, 13 patients were observed as complete response (CR) by CEUS, compared to 36 by CECT (p < 0.001). For nontarget lesions, 12 patients were observed as CR by CEUS, compared to 22 by CECT (p = 0.006). For overall response, eight patients were observed as CR by CEUS, compared to 31 by CECT (p < 0.001).
Conclusion
The diagnostic performance of CEUS was superior to CECT for detecting residual tumour after TACE. In clinical, CEUS should be recommended as an optional procedure for assessing the tumour response to TACE.
Key Points
• The mRECIST are widely applied for evaluating the response of HCC.
• Imaging method has been applied to assess the therapeutic response to TACE.
• The diagnostic performance of CEUS was superior to CECT for residual tumours.
• CEUS can be a valuable method for assessing tumour response to TACE.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Transarterial chemoembolization (TACE) is widely used to treat hepatocellular carcinoma (HCC), and is recommended as the standard treatment option for patients with large or multifocal tumours [1, 2]. In conventional TACE protocols, iodized oil is delivered intra-arterially to the liver tumour [3–6]. The modified Response Evaluation Criteria in Solid Tumours (mRECIST) are widely applied for evaluating the response of HCC to locoregional therapies [7]. Clinical studies have shown that the mRECIST are superior to other criteria, especially for patients treated with locoregional therapies [8–12].
Contrast-enhanced computed tomography (CECT) is an image acquisition modality recommended by mRECIST, and a weakness of it in detecting residual tumour has been observed in recent years. Shim et al. found, in CT-based evaluations, that lipiodol retention showed a weaker correlation with pathologic findings [13]. Bargellini et al. concluded that CT overestimated tumour response to TACE [14]. The complete response (CR) as assessed by CT does not reflect complete necrosis of the tumour pathologically [15–17].
In recent years, contrast-enhanced ultrasound (CEUS) has been applied to assess the therapeutic response to TACE [18]. Compared with CECT, the advantages of CEUS are less affected by lipiodol retention. Ultrasound with contrast agent is not affected by the original echogenicity of the lesion, or by the adjacent parenchyma on greyscale ultrasound [19, 20]. On CECT images, the iodine concentration in HCC tumour is usually higher than in the liver parenchyma. However, the lipiodol adopted in TACE also has a high intensity, which increases the difficulty of differentiating the arterial hypervascularization in the viable tumour from lipiodol deposition [4]. Salvaggio et al. found that CECT was less sensitive for detecting residual enhancement than CEUS [21]. In a study by Minami et al., nodules that were fully filled with lipiodol could be detected as incomplete responses by CEUS [22]. Numata et al. found that CEUS was useful for detecting viable tumour, and CECT could not evaluate lesions that accumulated massive lipiodol reliably [23].
The aim of this study was to compare CEUS with CECT for evaluating the treatment response of HCC to TACE according to mRECIST.
Materials and methods
Clinical data
This study was performed according to the guidelines of the Helsinki Declaration and was approved by our institutional ethics committee. Written informed consent was obtained from all patients prior to their participation. From June 2007 to December 2013, 130 patients (122 men, eight women; age range: 17–80 years, mean age: 53 years) with HCC, who underwent one procedure of TACE treatment, were enrolled retrospectively in this study. The inclusion criteria were as follows: (a) the patient was diagnosed with primary or recurrent HCC, on the basis of guidelines for HCC management [2]; (b) the HCC lesion was being treated with TACE for the first time; (c) the patient was categorized as Child-Pugh class A or B; (d) the patient’s platelet count was ≥ 50 × 109/L; and (e) CECT and CEUS were performed within 3 months after the first TACE for HCC. Patients treated with other systemic or local therapies after TACE and before the evaluation were excluded.
Table 1 presents the patients’ demographic and tumour characteristics.
TACE procedures
All TACE procedures were performed by experienced interventional radiologists from the Department of Interventional Radiology. After performing arteriography and portography to confirm the lesion size and location, a microcatheter was inserted into the feeding arteries. Each patient received oxaliplatin (Eloxatin®, Sanofi-Aventis, France). Then, an emulsion consisting of epirubicin (Pharmorubicin®, Pfizer, USA) and lipiodol (Xudong Pharmaceuticals, China) was injected through the microcatheter. The amounts of chemotherapy drugs and lipiodol were determined by the number, size, and vascularity of the tumours. Embolization was performed by gelatin sponge particles (Nanjing Jinling Pharmaceuticals, China).
CEUS examination
All patients underwent CEUS scanning within 1 month before TACE, and again within 0.5 to 3 months after TACE. CEUS was performed with one of three scanners: 26 patients were scanned by an Acuson Sequoia 512 scanner (Siemens Medical Solutions, Mountain View, CA), with a 1.0–4.0 MHz vector probe; 13 patients were scanned by an ALOKA α10 ultrasound scanner (ALOKA, Tokyo, Japan), with a 1.0–6.0 MHz convex array probe; and 91 patients were scanned by a Toshiba Aplio ultrasound scanner (Toshiba, Tokyo, Japan), with a 1.0–4.0 MHz convex array probe. The operator of CEUS was unaware of the CECT result. CEUS was performed with 2.4 mL of SonoVue (Bracco, Milan, Italy) as contrast agent. SonoVue was injected as a bolus via a 20-gauge intravenous cannula (Venflon; Becton Dickinson, Helsingborg, Sweden) placed in the left antecubital vein, followed by a 5-mL normal saline flush.
Each lesion was observed continuously for 6 minutes, including the arterial (7–30 seconds), portal (31–120 seconds), and late (121–360 seconds) phases throughout CEUS. During CEUS procedures, we tried to find new lesions in the late phase. If a lesion was found, then we performed the standard CEUS procedure to confirm this lesion.
In the present study, before undergoing TACE, 89 patients received additional administrations of SonoVue (range: 2–3 injections; median: 3 injections) for multiple lesions. Among them, 22 patients received two injections, and 67 patients received three injections. After TACE, 98 patients received additional administrations of SonoVue (range: 2–4 injections; median: 3 injections). Among them, 29 patients received two injections, 54 patients received three injections, and 15 patients received four injections. CEUS was performed by one of two authors (X.Y.X. or Z.F.X.), having 25 and 15 years of experience with CEUS, respectively.
CECT examination
Patients received CECT and CEUS contemporaneously. The interval between CECT and CEUS was less than 14 days (range: 0–14 days, median: 1 day). No treatments were performed during this interval. All patients were scanned before and after TACE by an Aquilion 64-slice helical CT machine (Toshiba, Tokyo, Japan) with parameters as follows: 0.5 mm × 64 mm collimation, 120 kV, and 150–200 mAs.
The standard multiphase scan procedure was used. An unenhanced helical sequence scan for the liver was performed. Then, 1.5 mL/kg of iopromide (Ultravist 370, Schering, Berlin, Germany) was administered at a rate of 4 mL/s via a 20-gauge intravenous cannula from the antecubital vein by using a power injector (Stellant D; Medrad, Indianola, PA). After administration of contrast material, CT sequences were obtained at 25–32 seconds (arterial phase), 60–75 seconds (portal venous phase), and 180 seconds (late phase).
Image analyses and response evaluation
The lesion number and size were established by analyzing both the pre-TACE CEUS and CECT images. Lipiodol retention was evaluated from the post-TACE unenhanced CT images. CECT images were interpreted in consensus by two authors (K.G.Z. or W.Q.Z.), both of whom had 25 years of experience with abdominal CT interpretation. CEUS images were interpreted in consensus by two authors (W.W. or M.X.L.), both of whom had 10 years of experience with CEUS. For each imaging modality, the two readers independently performed the interpretations, and they were blinded to the results of the other imaging modality. Figure 1 presents a flow diagram of the study population and response evaluation.
Flow diagram of the study population and response evaluation. Abbreviations: HCC, hepatocellular carcinoma; TACE, transcatheter arterial chemoembolization; CECT, contrast-enhanced computed tomography; CEUS, contrast-enhanced ultrasound; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; IR, incomplete response
In step 1, for each patient, the largest pre-TACE lesion (with diameter ≥ 1 cm) that could be clearly visualized and that showed intratumoral arterial enhancement was selected. In this group of patients, all selected lesions showed intratumoral arterial enhancement. CEUS and CECT were used separately to determine whether a lesion was a residual tumour. The presence of residual tumour was confirmed by histological examination in 41 patients (surgical resection in 23 cases and biopsy in 18 cases) and by angiographic results in 89 patients. Residual tumour was defined as a lesion that had any viable portion remaining after TACE in angiography. These results were used as reference standards.
In step 2, the tumour response to TACE was separately assessed by CEUS and CECT, according to the mRECIST. Criteria included the target lesion response, nontarget lesion response, appearance of new lesion, and overall response. We chose up to a maximum of two lesions before TACE as target lesions [7]. Based on the mRECIST, the target lesion response was graded as follows: CR; partial response (PR); stable disease (SD); and progressive disease (PD). The nontarget lesion response was graded as CR, incomplete response (IR)/SD, or PD. If a new nodule had the typical vascular pattern of HCC and with diameter ≥ 1 cm, then it was classified as a new lesion. Overall response was established according to tumour responses in target and nontarget lesions with or without the appearance of new lesions.
Statistical analysis
The residual tumour size was compared by the paired-samples t-test. Comparisons between CEUS and CECT of detecting the residual tumour and new lesions were analyzed by the McNemar test. Comparisons of the target lesion, nontarget lesion, and overall responses were analyzed by the rank sum test. Agreement between the two imaging techniques was measured by a kappa statistic. A ƙ value of less than 0.20 was considered a poor agreement, ƙ = 0.21–0.40 was a fair agreement, ƙ = 0.41–0.60 was a moderate agreement, ƙ = 0.61–0.80 was a good agreement and ƙ = 0.81–1.00 was a very good agreement. Statistical calculations were performed by SPSS 19.0 (SPSS Inc., Chicago, USA). A p value < 0.05 was considered statistically significant.
Results
Step 1: Estimation of the residual tumour by CEUS and CECT
According to the reference standard, 122 lesions were residual tumours and eight lesions were completely necrotic tumours. Of the 130 tumours, two lesions were located in S1, 31 in the left liver, 84 in the right liver, and 13 lesions in both lobes of the liver. The mean size was 57.5 ± 41.9 mm (range: 10.0–214.0 mm). Lipiodol deposits occupied more than 50 % of the volume in 37 lesions and less than 50 % of the volume in 93 lesions. In the 122 residual tumours, 117 lesions and 93 lesions were detected by CEUS and CECT, respectively. The sensitivity, specificity, and accuracy of CEUS vs. CECT were 95.9 % (117/122) vs. 76.2 % (93/122) (p < 0.001), 100 % (8/8) vs. 100 % (8/8) (p = 1.000), and 96.2 % (125/130) vs. 77.7 % (101/130) (p < 0.001), respectively.
Nine lesions were observed as completely necrotic tumours by both CEUS and CECT (Fig. 2). Eighty-nine residual tumours were detected by CEUS and CECT simultaneously (Fig. 3). The mean size of the residual tumour portion was 50.8 ± 35.5 mm by CEUS and 48.0 ± 36.3 mm by CECT (p = 0.047). Twenty-eight lesions were observed as residual tumours by CEUS, but not by CECT (Fig. 4). Of them, seven lesions were located in the left liver, 17 in the right liver, and four lesions in both lobes of the liver. The mean lesion size was 47.0 ± 33.6 mm. All 28 lesions were masked by a complete retention of lipiodol. Four lesions were observed as residual tumours by CECT, but not by CEUS (Fig. 5). They were located in S2 (24 mm), S7 (21 mm), S6/7 (25 mm), and S7/8 (24 mm). When the combination of CEUS and CECT was used, one residual tumour was missed altogether. This lesion was located in S4, had a diameter of 14 mm, and exhibited complete retention of lipiodol, but angiography could visualize some tumour stain.
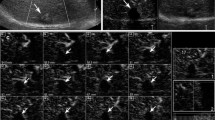
Accordance of contrast-enhanced ultrasound (CEUS) and contrast-enhanced computed tomography (CECT) for evaluation of complete response (CR) to transcatheter arterial chemoembolization (TACE). Completely homogeneous lipiodol retention in the whole tumour lesion in the arterial phase (A) and unenhanced CT (B), suggesting CR to TACE. CEUS visualized no enhancement of this lesion in the late arterial phase (C) and portal venous phase (D), consistent with the CECT findings
Accordance of contrast-enhanced ultrasound (CEUS) and contrast-enhanced computed tomography (CECT) for evaluation of residual tumour after transcatheter arterial chemoembolization (TACE). In the arterial phase of CECT (A), there was a small peripheral enhancing nodule in the anteromedial region of the treated lesion (black arrow), and the unenhanced CT scan showed a small mass of lipiodol inside the lesion (B), which suggested a residual portion. The arterial hyperenhancement of the whole lesion (black arrow) was observed on the CEUS images of the arterial phase (C) and with wash-out in the portal venous phase (D)
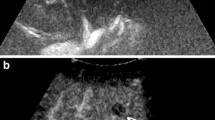
Arterial phase of contrast-enhanced computed tomography (CECT) scan (A) showing complete retention of lipiodol in the lesion without arterial enhancement compared to the nonenhanced CT (B), suggesting a complete response (CR) to transcatheter arterial chemoembolization (TACE). In the arterial phase of the contrast-enhanced ultrasound (CEUS) image (C), the same lesion shows a rim hyperenhancement (black arrow), with wash-out (black arrow) in the portal venous phase (D). This part is considered to be a residual tumour after TACE
In the arterial phase of contrast-enhanced computed tomography (CECT) (A), there was a small peripheral enhancing nodule in the upper region of the treated lesion (black arrow), with wash-out (black arrow) in the portal venous phase (B), this lesion was considered to be a residual tumour after transcatheter arterial chemoembolization (TACE). The same lesion with ill-defined margins (black arrow) was shown in the B-mode ultrasound (C), and with no enhancement (black arrow) in the late arterial phase of contrastenhanced ultrasound (CEUS) (D), suggesting a complete response (CR) to TACE
Step 2: Evaluation of tumour response to TACE by CEUS and CECT, according to mRECIST
The target lesion response to TACE assessed by CEUS and CECT is presented in Table 2 (p < 0.001, ƙ = 0.486). The CR rate was 10.0 % (13/130) by CEUS compared to 27.7 % (36/130) by CECT (p < 0.001). Significant differences between CEUS and CECT were found for the PR rates, but not for the SD and PD rate (p =0.024, 0.238, and 1.000, respectively). The kappa value indicated moderate agreement between CECT and CEUS for evaluation of the target lesion response.
Sixty-two (62/130, 47.7 %) patients had one or two lesions, and both were target lesions; 68/130 (52.3 %) patients had nontarget lesions by the combination of CEUS and CECT before TACE. The nontarget lesion response to TACE assessed by CEUS and CECT is presented in Table 3 (p = 0.003, ƙ = 0.474). The CR rate was 17.6 % (12/68) by CEUS compared to 36.8 % (25/68) by CECT (p = 0.001). CEUS and CECT differed significantly for the IR/SD rate, but not for the PD rate (p = 0.001 and 1.000, respectively). The kappa value indicated moderate agreement between CECT and CEUS for evaluation of the nontarget lesion response.
Twenty-three (23/130, 17.7 %) patients had at least one new lesion by both CEUS and CECT. Fourteen patients had three or more new lesions, two patients had two lesions, and seven patients had a single lesion. The mean size of new lesions was 18.8 ± 14.8 mm (10.0–31.0 mm). New lesions were identified in 26/130 (20.0 %) patients by CEUS compared to 27/130 (20.8 %) patients by CECT (p = 1.000). Three patients had new lesion(s) detected by CECT but missed by CEUS, located in S2 (25.0 mm, 11.0 mm), S5 (20.0 mm), and S8 (11.0 mm). Two patients had new lesion(s) detected by CEUS but missed by CECT, located in S1 (18.0 mm), and the right liver (multiple, 10.0–15.0 mm). In one patient with one new lesion in S1, the CECT image confused the lesion with a hepatic portal lymph node. In another patient with multiple new lesions, the new lesions were all smaller than 1 cm in size on the CECT image; therefore, these lesions could not be diagnosed as new HCC lesions.
The overall response to TACE assessed by CEUS and CECT is presented in Table 4 (p < 0.001, ƙ = 0.534). The CR rate by CEUS was 6.2 % (8/130) compared to 23.8 % (31/130) by CECT (p < 0.001). CEUS and CECT differed with respect to the PR rates, but not for the SD and PD rate (p = 0.014, 0.180, and 1.000, respectively). The kappa value indicated moderate agreement between CECT and CEUS for evaluation of the overall response.
Discussion
In the current study, CEUS detected significantly more residual tumours than CECT; these were verified by the reference standard. All of 28 residual tumours had massive accumulations of lipiodol. CEUS was sufficiently sensitive to detect the blood supply to these lesions, but CECT did not detect blood supply to these lesions. This finding is consistent with reports in previous studies [22–24]. Four residual tumours could not be detected by CEUS, but were detected by CECT. Among these lesions, two were in subcapsular locations covered by the diaphragm, an area that was difficult to access with the ultrasound transducer. Additionally, one lesion was considered to be a cirrhotic nodule because of its characteristic iso-enhancement in the three phases of CEUS. Another lesion was one of multiple HCC lesions and, although the contrast agents were repeatedly administered, the lesion did not show arterial enhancement. CEUS seemed to be more accurate to detect the blood supply of a tumour, even with massive lipiodol accumulations; however, CECT was superior to CEUS in detecting multiple lesions and lesions located in the subcapsular region.
According to mRECIST, the CR rate of the target lesion assessed by CECT was obviously higher than by CEUS, and the PR rates assessed by CECT were lower than by CEUS. The same results were observed for the nontarget lesions. On CECT imaging, if a target or nontarget lesion was completely filled with lipiodol, then it was considered as a CR. Given that lipiodol retention can affect the CECT results, as verified in the previous part of the current study and other studies [13–15], some PR cases may be misjudged as CR cases by CECT. Of the CR cases judged by CECT, 27 target lesions and 11 nontarget lesions could be visualized with some residual enhancement by CEUS. Thus, CECT underestimates the presence of persisting tumour after TACE with Lipiodol.
CEUS and CECT exhibited similar abilities to detect new lesions. However, CEUS still missed three patients with new lesions that were detected by CECT, whereas CECT missed two patients with new lesions that were detected by CEUS. These findings indicate that the combined use of CEUS and CECT to detect new lesions is superior to using either imaging approach alone.
Although CEUS and CECT showed equivalent detection rates of new lesions, the overall response rates were different, especially the CR rates. High CR rates for the target and nontarget lesions resulted in a high overall tumour response. In this group of patients, the CR rate of the overall response assessed by CECT was high; consequently, the need for additional treatments for patients was decreased. From the results of step 1, the main pitfall of CECT was misjudgment of residual tumour as a completely necrotic tumour (CR tumour), but CECT was still valuable for the assessment of PD cases.
CEUS is an operator-dependent imaging technology [25]. It has some other limitations, such as being affected by the tumour location and acoustic window [26, 27]. Additionally, ultrasound detection of liver lesions can be challenging in patients with lesions covered by the lung or diaphragm, and in patients with obesity, meteorism, or cirrhosis. In the present study, two patients had residual tumours located under the diaphragm that were undetected by CEUS. Furthermore, most patients had more than one lesion. They required additional doses of contrast agent to ensure that no lesions were missed and to clarify the character of each lesion. In earlier studies, lesion multiplicity was the main assessment challenge for CEUS [22, 27].
Limitations of the study design included its retrospective nature, dependency of operator skill and the need for additional patient follow-up to provide long-term survival data. We selected only patients with positive hyper-enhancing CEUS, and this limitation made some patient selection bias. Furthermore, in this study, the most common reference standard was angiography; however, angiography has relatively low sensitivity for detecting small residual tumour after TACE [28]. However, in the literature, angiography is generally used as a reference standard in HCC treated with TACE [21, 29].
In conclusion, the diagnostic performance of CEUS was superior to CECT, and CEUS seemed to be more sensitive and accurate to detect residual tumour after TACE, especially for the tumour completely filled with lipiodol. As CEUS is a radiation-free, well-tolerated and convenient method, it should be recommended as an optional procedure for assessing the tumour response to TACE in clinical. Further studies with larger samples are needed to validate these findings.
Abbreviations
- CEUS:
-
Contrast-enhanced ultrasound
- CECT:
-
Contrast-enhanced computed tomography
- TACE:
-
Transcatheter arterial chemoembolization
- HCC:
-
Hepatocellular carcinoma
- mRECIST:
-
Modified Response Evaluation Criteria in Solid Tumours
- CR:
-
Complete response
- PR:
-
Partial response
- SD:
-
Stable disease
- PD:
-
Progressive disease
- IR:
-
Incomplete response
References
Han KH, Kudo M, Ye SL et al (2011) Asian consensus workshop report: expert consensus guideline for the management of intermediate and advanced hepatocellular carcinoma in Asia. Oncol Basel 81:158–164
Bruix J, Sherman M (2011) Management of hepatocellular carcinoma: an update. Hepatology 53:1020–1022
Gadaleta CD, Ranieri G (2011) Trans-arterial chemoembolization as a therapy for liver tumours: new clinical developments and suggestions for combination with angiogenesis inhibitors. Crit Rev Oncol Hematol 80:40–53
Huppert P (2011) Current concepts in transarterial chemoembolization of hepatocellular carcinoma. Abdom Imaging 36:677–683
Sieghart W, Pinter M, Reisegger M et al (2012) Conventional transarterial chemoembolisation in combination with sorafenib for patients with hepatocellular carcinoma: a pilot study. Eur Radiol 22:1214–1223
Bouvier A, Ozenne V, Aube C et al (2011) Transarterial chemoembolisation: effect of selectivity on tolerance, tumour response and survival. Eur Radiol 21:1719–1726
Lencioni R, Llovet JM (2010) Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis 30:52–60
Prajapati HJ, Spivey JR, Hanish SI et al (2013) mRECIST and EASL responses at early time point by contrast-enhanced dynamic MRI predict survival in patients with unresectable hepatocellular carcinoma (HCC) treated by doxorubicin drug-eluting beads transarterial chemoembolization (DEB TACE). Ann Oncol 24:965–973
Shim JH, Lee HC, Kim SO et al (2012) Which response criteria best help predict survival of patients with hepatocellular carcinoma following chemoembolization? A validation study of old and new models. Radiology 262:708–718
Gillmore R, Stuart S, Kirkwood A et al (2011) EASL and mRECIST responses are independent prognostic factors for survival in hepatocellular cancer patients treated with transarterial embolization. J Hepatol 55:1309–1316
Kim BK, Kim KA, Park JY et al (2013) Prospective comparison of prognostic values of modified Response Evaluation Criteria in Solid Tumours with European Association for the Study of the Liver criteria in hepatocellular carcinoma following chemoembolisation. Eur J Cancer 49:826–834
Edeline J, Boucher E, Rolland Y et al (2012) Comparison of tumor response by Response Evaluation Criteria in Solid Tumors (RECIST) and modified RECIST in patients treated with sorafenib for hepatocellular carcinoma. Cancer 118:147–156
Shim JH, Han S, Shin YM et al (2013) Optimal measurement modality and method for evaluation of responses to transarterial chemoembolization of hepatocellular carcinoma based on enhancement criteria. J Vasc Interv Radiol 24:316–325
Bargellini I, Bozzi E, Campani D et al (2013) Modified RECIST to assess tumor response after transarterial chemoembolization of hepatocellular carcinoma: CT-pathologic correlation in 178 liver explants. Eur J Radiol 82:e212–e218
Kim YS, Rhim H, Lim HK et al (2006) Completeness of treatment in hepatocellular carcinomas treated with image-guided tumor therapies: evaluation of positive predictive value of contrast-enhanced CT with histopathologic correlation in the explanted liver specimen. J Comput Assist Tomogr 30:578–582
Choi BI, Kim HC, Han JK et al (1992) Therapeutic effect of transcatheter oily chemoembolization therapy for encapsulated nodular hepatocellular carcinoma: CT and pathologic findings. Radiology 182:709–713
Imaeda T, Yamawaki Y, Seki M et al (1993) Lipiodol retention and massive necrosis after lipiodol-chemoembolization of hepatocellular carcinoma: correlation between computed tomography and histopathology. Cardiovasc Intervent Radiol 16:209–213
Cioni D, Lencioni R, Bartolozzi C (2000) Therapeutic effect of transcatheter arterial chemoembolization on hepatocellular carcinoma: evaluation with contrast-enhanced harmonic power Doppler ultrasound. Eur Radiol 10:1570–1575
Alzaraa A, Gravante G, Chung WY et al (2013) Contrast-enhanced ultrasound in the preoperative, intraoperative and postoperative assessment of liver lesions. Hepatol Res 43:809–819
Yanagisawa K, Moriyasu F, Miyahara T, Yuki M, Iijima H (2007) Phagocytosis of ultrasound contrast agent microbubbles by Kupffer cells. Ultrasound Med Biol 33:318–325
Salvaggio G, Campisi A, Lo GV, Cannella I, Meloni MF, Caruso G (2010) Evaluation of posttreatment response of hepatocellular carcinoma: comparison of ultrasonography with second-generation ultrasound contrast agent and multidetector CT. Abdom Imaging 35:447–453
Minami Y, Kudo M, Kawasaki T et al (2003) Transcatheter arterial chemoembolization of hepatocellular carcinoma: usefulness of coded phase-inversion harmonic sonography. AJR Am J Roentgenol 180:703–708
Numata K, Tanaka K, Kiba T et al (2001) Using contrast-enhanced sonography to assess the effectiveness of transcatheter arterial embolization for hepatocellular carcinoma. AJR Am J Roentgenol 176:1199–1205
Youk JH, Lee JM, Kim CS (2003) Therapeutic response evaluation of malignant hepatic masses treated by interventional procedures with contrast-enhanced agent detection imaging. J Ultrasound Med 22:911–920
Leen E, Averkiou M, Arditi M et al (2012) Dynamic contrast enhanced ultrasound assessment of the vascular effects of novel therapeutics in early stage trials. Eur Radiol 22:1442–1450
Ding H, Kudo M, Onda H et al (2001) Evaluation of posttreatment response of hepatocellular carcinoma with contrast-enhanced coded phase-inversion harmonic US: comparison with dynamic CT. Radiology 221:721–730
Moschouris H, Malagari K, Papadaki MG et al (2011) Short-term evaluation of liver tumors after transarterial chemoembolization: limitations and feasibility of contrast-enhanced ultrasonography. Abdom Imaging 36:718–728
Lee JK, Chung YH, Song BC et al (2002) Recurrences of hepatocellular carcinoma following initial remission by transcatheter arterial chemoembolization. J Gastroenterol Hepatol 17:52–58
Kono Y, Lucidarme O, Choi SH et al (2007) Contrast-enhanced ultrasound as a predictor of treatment efficacy within 2 weeks after transarterial chemoembolization of hepatocellular carcinoma. J Vasc Interv Radiol 18:57–65
Acknowledgments
The scientific guarantor of this publication is Xiao-yan Xie. The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article. The authors state that this work has not received any funding. No complex statistical methods were necessary for this paper. Institutional Review Board approval was obtained. Written informed consent was obtained from all subjects (patients) in this study. Methodology: retrospective, diagnostic or prognostic study, performed at one institution.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, M., Lin, Mx., Lu, Md. et al. Comparison of contrast-enhanced ultrasound and contrast-enhanced computed tomography in evaluating the treatment response to transcatheter arterial chemoembolization of hepatocellular carcinoma using modified RECIST. Eur Radiol 25, 2502–2511 (2015). https://doi.org/10.1007/s00330-015-3611-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-015-3611-9