Abstract
Purpose
To evaluate the limitations and the feasibility of contrast-enhanced ultrasonography (CEUS) for the assessment of tumor response shortly after transarterial chemoembolization (TACE).
Materials and methods
Fifty seven patients (41 patients with hepatomas, 16 patients with metastases) were studied with CEUS before, 1 day after, and 30 days after TACE. A CEUS-efficiency score (CEUS-ES) was calculated, which evaluated: (a) the completeness of visualization of the target tumor(s) (2: good, 1: adequate, 0: poor) and (b) the quality of delineation of post-TACE necroses (2: good, 1: adequate, 0: poor). A CEUS study was considered as “diagnostic,” if each of the aforementioned parameters was associated with grade 1 or 2.
Results
CEUS studies were “diagnostic” in 36/57 patients (63.1%). Patients with hepatomas were more likely to undergo “diagnostic” CEUS than patients with metastases (70.7% vs. 43.7%, P = 0.0728). Lesions’ multiplicity, deep location, hypoenhancement on pretreatment CEUS, and diffuse growth had a statistically significant (P < 0.05) negative impact on CEUS-ES. Hyperechogenicity on pre-treatment, unenhanced US had a non-statistically significant (P = 0.176) negative impact. Differences between “diagnostic” CEUS studies and CT/MR regarding detection of residual tumor were insignificant (P = 0.8178).
Conclusion
The percentage of lesions which are unsuitable for post-TACE evaluation with CEUS is not negligible. For the rest, the respective role of CEUS is promising.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
During the last 10 years, contrast-enhanced ultrasonography (CEUS) has been used with promising results for the study of malignant liver tumors (especially hepatocellular carcinomas, HCCs) after transarterial chemoembolization (TACE) [1–5]. With the introduction of second generation echo-enhancers, along with contrast-specific software and low-mechanical-index imaging modes, detailed study of tumor vascularity is feasible in real time, and high accuracy in the detection of necrotic and residual viable neoplastic tissue has been achieved [6, 7]. Tumor necrosis, demonstrated as disappearance of tumoral enhancement on dynamic imaging studies, is the major determinant of treatment efficacy after locoregional therapies [8] and extensive TACE-induced necrosis is associated with improved survival, in patients with unresectable HCC [9].
However, in some studies which underscored the advantages of CEUS as a post-TACE imaging modality, observations were also made regarding limitations of CEUS. For example, multiple or deep-seated lesions were considered difficult to evaluate [3, 4]; transient, TACE-induced hyperechogenicity of liver tumors, also impaired detection of residual tumor enhancement [6].
In our institution we routinely use CEUS as a complementary modality for the study of liver tumors after TACE. Based on this experience, we herein attempt to study and analyze the aforementioned and some additional limitations; we also try to assess the impact of these limitations on the role of CEUS, as a tool for the short-term evaluation of the effectiveness of TACE.
Materials and methods
Patients-tumors
Fifty seven patients (41 men, 16 women; mean age: 67.6 years; range: 30–84) with a total of 111 target tumors were included in this retrospective study. Forty one patients were affected by hepatocellular carcinoma (HCC), and 16 patients were affected by liver metastases. The maximum diameter of the lesions was 1.9–16 cm (mean: 5.47 ± 2.97 cm). 30/57 patients (52.6%) underwent TACE for solitary tumors. Twenty six of these patients were affected by solitary HCCs and four by solitary metastases. 27/57 patients (47.4%) had two or more target lesions, as follows: two lesions in 12 patients, three lesions in nine patients, four lesions in two patients, five lesions in two patients, six lesions in two patients. Multifocal (two or more lesions) liver involvement was caused by HCC in 15 patients and by metastases in 12 patients. Target lesions’ distribution was unilobar in 49 patients (right liver lobe: 41 patients, left liver lobe: 8 patients) and bilobar in eight patients. Tumor involvement of the liver exceeded 50% in 6/57 patients (11.5%).
Diagnosis of HCC was confirmed by biopsy in all cases. Liver metastases were diagnosed on the basis of typical imaging findings, combined with marked elevation of tumor markers and appropriate history. Fifty five patients of this study were unsuitable for surgery or radiofrequency (RF) ablation because of the size and/or location of their tumors, coexisting cardiopulmonary pathology, intolerance to RF, or combinations of the aforementioned causes. Two patients finally underwent a right hepatectomy for the removal of solitary hepatomas, approximately 2 months after the 3rd and 4th session (respectively) of TACE.
For each patient, only one session of TACE was studied. Twenty six patients had received TACE, systemic chemotherapy, or both, 2 or more months prior to the studied session of TACE. This study did not include subjects who had received RF ablation or antiangiogenetic agents before, or shortly after TACE. Also excluded were patients unsuitable for a technically adequate baseline US scan (due to unfavorable anatomy, overlying bowel gas, or poor cooperation). Demographic and clinical data of the patients are provided in Table 1 and in 2nd–5th column of Table 2.
Written informed consent was obtained from all patients prior to treatment. This study was approved by the institutional review board of our hospital.
Chemoembolization
Selection criteria (fulfilled by all patients) as well as technical details of chemoembolization were similar to those described in previous work [10, 11]. Fifty three patients were treated with subsegmental, segmental, or multisegmental TACE. Four patients (two patients with multiple metastases and two patients with bulky hepatomas) were treated with lobar TACE.
Chemoembolization was performed with drug-eluting beads (DC-Beads Biocompatibles Ltd, Surrey, UK). Each patient received 3–4 mL of DC beads (diameters: 100–300 μm and 300–500 μm). For TACE of HCC, DC Beads had been loaded with doxorubicin (Adriblastina, Pfizer Italia S.r.L., Nerviano, Milano, Italy) at a dose of 25–37.5 mg drug/mL of hydrated beads. In 12 patients, additional embolization with microspheres with diameters of 40, 100, and 400 μm (Embozene, CeloNova BioSciences Inc., Newnan, Georgia, USA) was performed. Five patients with HCC underwent bland embolization with Embozene microspheres (40/75/100/400 μm). In this study and for the sake of simplicity, these patients were also considered as having undergone TACE. For TACE of metastases, DC Beads had been loaded with irinotecan (Campto, Aventis Pharma, Dagenham, UK) at a dose of 50 mg drug/mL of hydrated beads.
Sonography
Liver lesions were first studied by conventional, B-mode ultrasonography (US). The positions of the probe and of the patient that ensured the best quality of imaging and the most complete scanning of the treated tumor(s) were defined during this phase. CEUS was performed 3 times in each patient: 1–3 days before TACE, 1 day post-TACE and approximately 30 (28–35) days post-TACE.
Conventional US and CEUS were performed either with an Esaote Megas GPX (Esaote, Genoa, Italy) or with a Philips HD11 XE (Philips Ultrasound, Andover, MA, USA) ultrasononographic unit with convex, 2.5–5 MHz probes. For CEUS, a second generation ultrasound contrast agent (suspension of microbubbles of sulfur hexafluoride, SonoVue, Bracco, Milan Italy) was used. The total dose of each vial of SonoVue (4.8 mL) was injected as a bolus in a forearm vein, followed by a flush of 10 mL of normal saline. A dedicated, contrast specific, continuous scanning, low mechanical index technique was utilized (Mechanical Index = 0.08–0.11). A high MI “flash,” causing destruction of the microbubbles at the imaging plane, was occasionally applied. The scanning position(s) that had been defined by the baseline examination was preferentially used. The aim was to visualize the entire tumor(s), when the contrast between the viable (enhancing) and necrotic (non-enhancing) tumoral components was greatest. This occurred in the arterial phase but (particularly in tumors with delayed wash-out) the portal phase was also evaluated. A second (but not a third) dose of 4.8 mL of SonoVue was administered when deemed necessary. Each CEUS examination was transferred and stored as a digital video clip in a personal computer.
All the sonographic studies were performed by the same consultant radiologist (HM) with 12 years’ experience in abdominal sonography and 6 years’ experience in CEUS. This radiologist was aware of the findings of pretreatment CT/MR. He was also aware of the results of TACE (i.e. which lesions were embolized), so that patients with multifocal disease underwent a post-interventional CEUS focused on the target lesions.
Image analysis. Evaluation and scoring of the efficiency of CEUS
Two consultant radiologists (HM, MGP) experienced in abdominal imaging and in CEUS reviewed in consensus pre- and 30 days post-treatment US and CEUS studies. The following features of the treated tumors were recorded (Table 2): Number: in patients with multiple lesions, only the treated ones were registered. Maximum diameter: in cases with multiple lesions, the average of the maximum diameters of the treated lesions was recorded. Distance from the skin: this was defined as the distance from the center of the lesion to the skin, at the scanning position which provided the best acoustic window. In cases with multiple lesions, the most deep-seated lesion was registered. Echogenicity at fundamental (non-enhanced) pretreatment US: if a lesion had higher echogenicity than that of normal liver it was characterized as “hyperechoic,” otherwise as “non-hyperechoic.” Multifocal liver involvement by lesions of different echogenicity was considered as “hyperechoic,” even if only one of the lesions was hyperechoic. The rationale was that, if even one of multiple lesions was hyperechoic, and if the hyperechogenicity of this lesion precluded the assessment of its enhancement, this would render non-diagnostic the entire CEUS examination. Enhancement at pretreatment CEUS: if the viable portion of a lesion enhanced to a lesser degree than non-tumorus liver, this lesion was characterized as “hypoenhancing,” otherwise as “non-hypoenhancing.” In the material of this study, multiple lesions in the same patient did not differ regarding their enhancement on pretreatment CEUS. Growth pattern: lesions with indistinct borders and with diffuse infiltration of liver parenchyma were considered as having a “diffuse growth pattern.” Diffusely infiltrating tumors were arbitrarily considered as solitary lesions.
To assess diagnostic performance of CEUS, a scoring system was applied, which evaluated the following two parameters: (a) if all treated lesions were completely visualized, both in pre- and in post-treatment CEUS and (b) if post-TACE necroses could be clearly and confidently detected within treated lesions. Disappearance of enhancement in a part of a tumor after TACE was defined as post-TACE necrosis. Regarding parameter (a), the following scale was used: 2 good visualization of the treated tumor(s) with no special effort from the examiner, or the patient. 1 Adequate visualization of the treated tumor(s). Deep breath and changes in patient’s position or in acoustic window were required in order to obtain a complete “sweep” through all the treated lesions. 0 Inadequate visualization of the treated tumor(s). Regarding parameter (b), the following scale was used: 2 Definite and detailed delineation of post TACE necroses as enhancement defects. A quantitative evaluation of post TACE necrosis could be achieved. For example, a percentage of necroses could be calculated by measuring and comparing the extent of non-enhancing (necrotic) components with the extent of the entire tumor (necrotic and viable) at representative CEUS sections, as described in previous work [5]. 1 Adequate detection of post TACE necroses, although not as clear and detailed as in grade 2. A rough estimation of post TACE necrosis (< 50%, > 50% and complete necrosis) could be made. 0 Poor delineation of post TACE necroses. The latter could not be safely differentiated from residual viable tumor. If multiple lesions in the same patient differed in the clarity of depiction of necrosis, the lowest score was recorded.
For each patient, a CEUS efficiency score (CEUS-ES) was calculated. CEUS-ES was defined as the sum of the parameters (a) and (b). A CEUS examination (comprising pre- and 30 days post-TACE scans) was considered “diagnostic,” if both parameters, (a) and (b), were associated with a grade 1 or 2.
The two reviewers also examined the CEUS studies performed 1 day post-TACE, in order to identify additional factors that could reduce the effectiveness of this early post-interventional evaluation.
Other investigations and correlations
All patients underwent pretreatment imaging (no more than 10 days prior to TACE) with CT (20 patients) or MR (37 patients). CT scans were performed on 4-slice multi-detector scanners (typical parameters: 2.5 mm collimation; 15 mm per rotation table feed; 4 mm reconstruction interval) or single-slice helical CT scanners (typical parameters: 5 mm collimation; 8 mm per rotation table feed, 4 mm reconstruction interval) during a single breath-hold helical acquisition. The hepatic arterial, portal venous, and delayed phases were scanned 30, 60, and 180 s, respectively, after the start of the intravenous injection of nonionic iodinated contrast material (concentration: 300 mg iodine per milliliter, dose: 2 mL per kg of bodyweight), by power injector at a rate of 3 mL/s. MRI was performed with various types of MR scanners at 1.5 Tesla and included T1 fat-suppressed images, T2 fat-suppressed sequences, and dynamic fat-suppressed T1 sequences obtained after intravenous injection of gadolinium (0.1 mmol gadolinium chelate per kg of body weight). 5–8 weeks post TACE, each patient underwent follow-up imaging with the same contrast-enhanced imaging modality and with similar parameters with those used for pre-treatment imaging.
Post-TACE results of CT and MR were also compared with those of the corresponding “diagnostic” CEUS examination, regarding the presence and distribution of residual tumor. For patients with “non-diagnostic” CEUS studies, post-interventional CT or MR scans were also evaluated, for the presence of any signs of tumor response.
Histologic sections of the two removed tumors were examined by a pathologist (unaware of the imaging findings), who evaluated the extent of tumoral necrosis. His report was compared with the corresponding imaging studies.
Statistical analysis
Numerical data were expressed as mean ± standard deviation. Differences in the means of numerical data between subgroups were evaluated with t-test. The degree of concordance between CEUS and CT/MR was assessed with Fisher’s exact test. In order to assess the effect of various potential limitations on the efficiency of CEUS-ES, multivariate analysis was performed. Statistic calculations were performed by dedicated software (“data analysis” tool of Microsoft Excel 2007 [Microsoft Corp., Redmond, WA, USA]). A P value of <0.05 was considered significant.
Results
In all patients TACE resulted in complete or partial devascularization of the treated lesions.
“Diagnostic” CEUS studies (comprising pre- and 30 days post TACE scans) were achieved in 36/57 patients (63.1%). A “diagnostic” CEUS was achieved more frequently in patients with HCC (29/41) than in patients with metastases (7/16) (70.7% vs. 43.7%, P = 0.0728). Moreover, CEUS-ES was averagely higher in patients with HCC than in patients with metastases (2.68 ± 1.33 vs. 1.62 ± 1.15, P = 0.0071).
Multiplicity, deep location of the treated lesions, hypoenhancement on pretreatment CEUS, and diffuse growth pattern were identified as independent factors with a statistically significant (P < 0.05) negative effect on the efficiency of CEUS. In two patients, marked lesional hyperechogenicity on unenhanced US (both pre- and post-TACE) compromised detection of tumoral enhancement, but overall the adverse effect of tumor hyperechogenicity on CEUS-ES was not statistically significant (P = 0.176). Lesion hypovascularity on pre-treatment CEUS, with or without multiplicity, accounted for the “non-diagnostic” CEUS studies of metastases. Deep location and diffuse growth pattern accounted for most of “non-diagnostic” CEUS studies of hepatomas (Fig. 1).
Bar graph comparing the numbers of “diagnostic” and “non-diagnostic” CEUS studies (white and shadowed bars, respectively) and illustrating the contribution of various limitations to the latter. Bars of the first row: patients with HCC. Bars of the second row: patients with metastases. DL deep location, DG diffuse growth, HE hyperechogenicity on unenhanced US, M multiplicity, HY hypoenhancement on CEUS prior to TACE. Numbers of the patients of each category are in parentheses.
A “diagnostic” CEUS could be achieved in 23/27 (85.2%) of patients treated for nodular, solitary tumors, in 7/12 (58.3%) of patients with two treated lesions and in 6/9 (66.6%) of patients with 3 treated lesions. On the other hand, no patient with 4 or more target lesions and no patient with diffusely growing tumor proved suitable for a “diagnostic” CEUS study.
One day post-TACE CEUS assessment was impaired by markedly increased echogenicity, which occupied the largest part of the treated tumors in 8/57 patients (14%). This finding had resolved when patients were re-evaluated 30 days post TACE (Fig. 2).
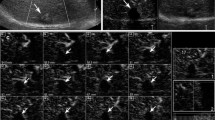
Limitation of first day post-TACE evaluation. CEUS image (A) of a liver metastasis prior to TACE shows an ovoid hypoenhancing lesion. Unenhanced US image (B) of the same lesion 1 day post-TACE shows high level echoes occupying the largest part of the lesion (arrows). This feature impaired detection of residual enhancement at the same day. On CEUS 30 days post-TACE (C) high level echoes have disappeared and complete lack of enhancement can be diagnosed. Correlation with axial, enhanced T1 weighted MR images prior to (D), and 6 weeks post-TACE (E).
Results of “diagnostic” CEUS regarding the presence and distribution of residual tumor enhancement post TACE correlated well with those of CT/MR (Fig. 3). “Diagnostic” CEUS revealed 44 lesions with residual enhancement after TACE and 11 lesions with complete necrosis. On corresponding CT/MR evaluation, 42 lesions were diagnosed with residual enhancement and 13 lesions with complete lack of enhancement. In one patient, CEUS identified a small marginal tumor residue which was overlooked by CT. In a second case, detection of residual tumor enhancement with MR was impaired by T1 hyperintensity (already present prior to gadolinium injection), whereas CEUS showed definite residual tumor enhancement. The differences between “diagnostic” CEUS studies and CT/MR regarding detection of residual tumor were not significant (P = 0.8178).
Correlation of imaging and pathologic findings after TACE. CEUS image (A) of a solitary HCC 1 month after the fourth session of TACE shows two small peripheral enhancing nodules (arrows), indicative of residual disease. Contrast-enhanced CT section (B) of the same lesion shows similar amount and distribution of residual enhancement (arrows). Pathology specimen of right hepatectomy (C) shows the tumor (asterisk) with a yellowish cut surface, indicative of extensive coagulative necrosis. Histologic section (hematoxylin-eosin stain, original magnification ×10) of the same lesion (D) shows necrosis (N), coexisting with viable tumor tissue (T). A few drug-eluting beads are also depicted at the left upper part of the picture.
For 19/21 patients with “non-diagnostic” CEUS, follow-up with CT or MR revealed varying degrees of response in the form of reduction in enhancement (Fig. 4), or tumor shrinkage (Fig. 5). For 2/21 patients, CT/MR failed to detect any change compared to pre-TACE studies. This could be attributed to a poor TACE result (i.e. ineffective TACE) and not to a limitation of CT/MR. However, the consideration of these two cases as “non-diagnostic” was not changed, because their CEUS imaging was anyway suboptimal. The categorization and correlations of CEUS studies are summarized in Fig. 6.
Limitation of CEUS in assessing strongly hyperechoic lesions. Unenhanced US image (A) in a patient with multinodular HCC 1 month post-TACE shows three hyperechoic tumors. Similar appearance was observed prior to TACE (not shown). On comparable CEUS section 1 month after TACE (B), the high level echoes of these 3 lesions interfere with detection of enhancement. Axial, enhanced T1 weighted MR image 6 weeks post-TACE (C) clearly shows intralesional necroses as enhancement defects (arrows).
Limitation of CEUS for short-term post-TACE evaluation of hypovascular tumors. CEUS image (A) of a small, hypovascular metastasis prior to TACE shows subtle rim lesional enhancement. No significant change in lesion enhancement is noted on CEUS performed 29 days post-TACE (B), despite successful closure of the afferent vessels (not shown). Comparison of axial enhanced T1 weighted MR images prior to (C) and 8 weeks post TACE (D) of the same lesion (arrows) indicates a definite decrease in lesion’s size, although the enhancement pattern is unchanged.
No significant differences regarding the size of the lesions were observed between pre- and 30 days post-TACE CEUS studies. Moreover, the effect of tumor size on CEUS-ES was not statistically significant (P = 0.64). However, the extent and the distribution of neoplastic liver involvement had an impact on the efficiency of CEUS. None of the patients with a tumor burden of more than 50% and only 3/8 patients with bilobar tumor distribution proved suitable for “diagnostic” CEUS evaluation.
All CEUS examinations were well tolerated and no side effects were observed. A second injection of echo-enhancer was required in 24 patients (42.1%). In 20 of those patients, the second dose was used to facilitate the evaluation of two or more lesions. The other four cases were solitary lesions. In three of them the second dose was used to elucidate small areas of marginal enhancement in order to differentiate total from subtotal necrosis. The last lesion was a solitary hypoenhancing metastasis, in which the second injection facilitated differentiation of hypoenhancing (but still viable) tissue from non-enhancing (necrotic) tissue.
Discussion
The results of this study indicate that several features of malignant liver lesions may affect the efficiency of CEUS, when the latter is used for short-term evaluation of the response of these lesions to TACE.
Lesion multiplicity may cause difficulties to CEUS evaluation, since each lesion has to be studied separately in order to detect changes in its enhancement. This drawback has already been reported previously [3]. In our series, CEUS could not reliably evaluate livers with 4 or more lesions. On the contrary, CEUS proved efficient for the evaluation of a significant proportion (13/21, 61.9%) of patients with 2–3 lesions. In the absence of other limitations, and with two injections of echo-enhancer, two lesions can be separately studied. Three lesions can also be evaluated, under favorable imaging conditions, particularly if at least two of the lesions are located close to each other (for example, in the case of a dominant HCC with 2 smaller satellites). In this study, we administered a full dose (4.8 mL) of SonoVue in each injection; this decreased significantly the cost-effectiveness of CEUS. With more advanced equipment and with increased experience, a diagnostic CEUS examination can be achieved with only a part (1.2 mL or 2.4 mL) of the total dose of the echo-enhancer [12].
Deep location of the treated tumors is another limitation for CEUS assessment. Similar observations were made in two older studies [1, 4], in which tumor response was studied with a 1st generation echo-enhancer (Levovist). Detection of lesion enhancement was found to be severely impaired at a distance of 8 or 10 cm from the skin. In our study, deep location was a major limitation in 6/13 (46.1%) of the patients with lesions located deeper than 11 cm from the skin. In a study [13], which utilized SonoVue for the diagnosis (not for post-treatment evaluation) of liver lesions, difficulties were reported in analyzing contrast uptake in lesions located more than 12 cm below the skin surface. Although a detailed comparison is not feasible, the increased depth compared to the former studies with Levovist might indicate an advantage of the 2nd generation echo-enhancers over their 1st generation counterparts. CEUS is clearly superior to unenhanced power-Doppler sonography, the latter being reportedly unreliable for detection of residual vascularity in tumors located at a depth of 5 cm or more [14].
In the absence of other limitations, detection of TACE-induced necroses seems straightforward in tumors, which are hyper- or isoenhancing prior to treatment. On the contrary, there was difficulty in detecting TACE-induced necroses in originally hypoenhancing lesions, because of the minimal contrast between non-enhancing necroses and hypoenhancing tumor tissue. We have encountered a similar problem in a few renal tumors, in which we failed to differentiate histologically proven, spontaneous, tumoral necrosis from viable, hypoenhancing neoplastic tissue [15]. Ding et al. [1] also reported of a hepatoma unsuitable for post-TACE CEUS evaluation, because of inherent hypovascularity. We experienced this limitation with metastases, which were originally hypovascular (occasionally with the exception of an enhancing rim), or their vascularity had been reduced after intravenous chemotherapy. This problem could probably be overcome by means of dedicated software, which could quantify lesional enhancement and detect subtle differences with higher sensitivity than visual assessment. Of note, neither CT nor MR is devoid of this limitation [16, 17]. The assessment of hypovascular tumors’ response has probably to incorporate RECIST, or other size-based criteria. Additional evaluation with PET, or dedicated CT and MR techniques may also be of value [16, 18].
Similar to the reported limitations in detection and characterization of diffusely infiltrating hepatomas [19], our small experience with these tumors indicates that they also pose significant difficulties regarding their post-TACE CEUS evaluation, being unsuitable, both for complete visualization and for detailed evaluation of necrosis.
In accordance to another report [20], increased lesion echogenicity at baseline (non-enhanced) US did not have a statistically significant effect on CEUS-ES. However, a few, strongly hyperechoic tumors are associated with high-signal intensity artifacts on low-MI imaging, which may interfere with detection of tumor enhancement [13]. CEUS evaluation of such lesions may be problematic both prior to, and after TACE. A particular form of intralesional hyperechogenicity is observed immediately after, until a few days after TACE and is attributed to post-necrotic gas formation, or to gas-bubbles trapped in the embolic material [21, 22]. Depending on its extent, this finding may render non-diagnostic a CEUS study performed immediately after, or 1–2 days after TACE [6, 21]. Post-embolization hyperechogenicity resolves almost completely a week after TACE.
In cases free of the aforementioned limitations, CEUS could be a valuable tool for post-TACE assessment, as indicated by the high degree of concordance, between “diagnostic” CEUS” and post-TACE CT/MR. CEUS could partially substitute for CT or MR in patients with superficially located, solitary hepatomas treated with sequential TACE. Moreover, the combination of CEUS with CT/MR could increase diagnostic accuracy in challenging cases, in which detection of the smallest enhancing focus (for differentiation of total from subtotal necrosis) has a great impact on patient management. Post-interventional CEUS could also be applied, when detection of enhancement is impaired in the other dynamic studies (for example, by lipiodol artifacts in CT, or by TACE-related increased signal at unenhanced T1 sequences in MR).
Liver metastases treated with TACE are probably less suitable than hepatomas for post-treatment evaluation with CEUS, and respective experience is limited. However, CEUS could be adequate for the evaluation of solitary secondaries, if they exhibit even a brief arterial hyperenhancement. Clearly, CEUS cannot compete with the global evaluation provided by CT/MR in cases of multifocal metastatic involvement of the liver. Furthermore, despite some encouraging data, the role of CEUS for the detection of new lesions, as a part of the long-term follow-up, has not been established [23].
Several weaknesses are associated with the present study: First, our results regarding CEUS performance are probably affected by the ultrasonographic equipment which was not very advanced, or very modern. Second, we did not take into account other factors, (such as diffuse liver steatosis) which were likely to impair CEUS efficiency [24]. Although we did observe some cases of fatty infiltration, this was mild to moderate and its effect on the quality of CEUS was considered insignificant. The type of embolization might also be related to the clarity of delineation of TACE-induced necrosis. Segmental or subsegmental embolization is expected to cause a more focused and well-defined devascularization (thus more readily detectable on CEUS, as enhancement defect) than lobar embolization. Finally, CT and MR, which were used for the comparison with CEUS, are imperfect reference standards [25].
In conclusion, we reviewed some factors which affect diagnostic performance of CEUS, when this modality is used to evaluate tumor response shortly after liver TACE. Our observations are not novel, however, they are based on a relatively large number of patients and they could serve as additional evidence on the practical application of CEUS for monitoring intraarterial locoregional treatments in the liver. CEUS is not likely to replace CT or MR, as the primary modality for imaging liver tumors after TACE; however, under favorable conditions, CEUS could reduce the number of CT/MR scans required for this purpose. CEUS could also be combined with the aforementioned modalities, in order to increase diagnostic confidence in difficult cases.
References
Ding H, Kudo M, Onda H, et al. (2001) Evaluation of posttreatment response of hepatocellular carcinoma with contrast-enhanced coded phase-inversion harmonic US: comparison with dynamic CT. Radiology 221:721–730
Numata K, Tanaka K, Kiba T, et al. (2001) Using contrast-enhanced sonography to assess the effectiveness of transcatheter arterial embolization for hepatocellular carcinoma. Am J Roentgenol 176:1199–1205
Minami Y, Kudo M, Kawasaki T, et al. (2003) Transcatheter arterial chemoembolization of hepatocellular carcinoma: usefulness of coded phase-inversion harmonic sonography. Am J Roentgenol 180:703–708
Youk JH, Lee JM, Kim CS (2003) Therapeutic response evaluation of malignant hepatic masses treated by interventional procedures with contrast-enhanced agent detection imaging. J Ultrasound Med 22:911–920
Moschouris H, Malagari K, Papadaki MG, Kornezos I, Matsaidonis D (2010) Contrast-enhanced ultrasonography of hepatocellular carcinoma after chemoembolisation using drug-eluting beads: a pilot study focused on sustained tumor necrosis. Cardiovasc Intervent Radiol 33:1022–1027
Kono Y, Lucidarme O, Choi SH, et al. (2007) Contrast-enhanced ultrasound as a predictor of treatment efficacy within 2 weeks after transarterial chemoembolization of hepatocellular carcinoma. J Vasc Interv Radiol 18:57–65
Salvaggio G, Campisi A, Lo Greco V, et al. (2010) Evaluation of posttreatment response of hepatocellular carcinoma: comparison of ultrasonography with second-generation ultrasound contrast agent and multidetector CT. Abdom Imaging 35:447–453
Gervais DA, Kalva S, Thabet A (2009) Percutaneous image-guided therapy of intra-abdominal malignancy: imaging evaluation of treatment response. Abdom Imaging 34:593–609
Ebied OM, Federle MP, Carr BI, et al. (2003) Evaluation of responses to chemoembolization in patients with unresectable hepatocellular carcinoma. Cancer 97:1042–1050
Malagari K, Alexopoulou E, Chatzimichail K, et al. (2008) Transcatheter chemoembolization in the treatment of HCC in patients not eligible for curative treatments: midterm results of doxorubicin-loaded DC bead. Abdom Imaging 33:512–519
Malagari K, Pomoni M, Kelekis A, et al. (2010) Prospective randomized comparison of chemoembolization with doxorubicin-eluting beads and bland embolization with beadblock for hepatocellular carcinoma. Cardiovasc Intervent Radiol 33:541–551
von Herbay A, Westendorff J, Gregor M (2010) Contrast-enhanced ultrasound with SonoVue: differentiation between benign and malignant focal liver lesions in 317 patients. J Clin Ultrasound 38:1–9
Chami L, Lassau N, Malka D, et al. (2008) Benefits of contrast-enhanced sonography for the detection of liver lesions: comparison with histologic findings. Am J Roentgenol 190:683–690
Kubota K, Hisa N, Nishikawa T, et al. (2001) Evaluation of hepatocellular carcinoma after treatment with transcatheter arterial chemoembolization: comparison of Lipiodol-CT, power Doppler sonography, and dynamic MRI. Abdom Imaging 26:184–190
Moschouris H, Stamatiou K, Kornezos I (2010) Re: Xu et al.: “Renal cell carcinoma: real-time contrast-enhanced ultrasound findings”. Abdom Imaging 35:507–509
Miller FH, Keppke AL, Reddy D, et al. (2007) Response of liver metastases after treatment with yttrium-90 microspheres: role of size, necrosis, and PET. Am J Roentgenol 188:776–783
Riaz A, Lewandowski RJ, Kulik L, et al. (2010) Radiologic-pathologic correlation of hepatocellular carcinoma treated with chemoembolization. Cardiovasc Intervent Radiol 33:1143–1152.
Tochetto SM, Rezai P, Rezvani M, et al. (2010) Does multidetector CT attenuation change in colon cancer liver metastases treated with 90Y help predict metabolic activity at FDG PET? Radiology 255(1):164–172
Jakate S, Yabes A, Giusto D, et al. (2010) Diffuse cirrhosis-like hepatocellular carcinoma: a clinically and radiographically undetected variant mimicking cirrhosis. Am J Surg Pathol 34:935–941
Liu GJ, Xu HX, Xie XY, et al. (2009) Does the echogenicity of focal liver lesions on baseline gray-scale ultrasound interfere with the diagnostic performance of contrast-enhanced ultrasound? Eur Radiol 19(5):1214–1222
Moschouris H, Malagari K, Kornezos I, et al. (2010) Unenhanced and contrast-enhanced ultrasonography during hepatic transarterial embolization and chemoembolization with drug-eluting beads. Cardiovasc Intervent Radiol 33:1215–1222
Ohishi H, Uchida H, Kurosaki Y, et al. (1983) Evaluation by ultrasonography of arterial embolization therapy for hepatocellular carcinoma. Cardiovasc Intervent Radiol 6:104–108
Cabassa P, Bipat S, Longaretti L, Morone M, Maroldi R (2010) Liver metastases: sulphur hexafluoride-enhanced ultrasonography for lesion detection: a systematic review. Ultrasound Med Biol 36:1561–1567
Bartolotta TV, Taibbi A, Midiri M, Lagalla R (2009) Focal liver lesions: contrast-enhanced ultrasound. Abdom Imaging 34:193–209
Hunt SJ, Yu W, Weintraub J, Prince MR, Kothary N (2009) Radiologic monitoring of hepatocellular carcinoma tumor viability after transhepatic arterial chemoembolization: estimating the accuracy of contrast-enhanced cross-sectional imaging with histopathologic correlation. J Vasc Interv Radiol 20:30–38
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Moschouris, H., Malagari, K., Papadaki, M.G. et al. Short-term evaluation of liver tumors after transarterial chemoembolization: limitations and feasibility of contrast-enhanced ultrasonography. Abdom Imaging 36, 718–728 (2011). https://doi.org/10.1007/s00261-011-9690-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-011-9690-4