Abstract
The South Georgia Shag Leucocarbo [atriceps] georgianus has breeding populations on the islands of South Georgia, the South Sandwich and South Orkney Islands. The South Orkney Islands are estimated to support ~ 18%–37% of the global population and South Georgia a further 37%–69%. Here, we examine changes in South Georgia Shag population size and productivity from Signy Island, South Orkney Islands, over a 43 year period (1978/1979 to 2020/2021) and from Bird Island, South Georgia, over a 32 year period (1989/1990 to 2020/2021). Analysis of total nesting pairs at Signy Island revealed an overall decline of 40.9% (− 1.3% per annum), with an increase during the 1980s, followed by a fluctuating decline from the 1990s to 2020/2021. Although the two Signy Island colonies showed correlated fluctuations in numbers of nesting pairs, over the whole time period these colonies showed markedly different population trajectories, indicating the limitations of using part-island counts to infer whole island trends, particularly given the low breeding-site fidelity in this species. Nest occupation in the larger colony (596 nests in 1978/1979) declined by 77.2% (− 3.5% per annum) whilst the smaller colony (50 nests in 1978/1979) exhibited an increase of 492% (+ 3.8% per annum). A decline in occupied nests of 58.3%, (− 2.8% per annum), has occurred in the Bird Island population since 1989/1990. Continuation of the significant decline in breeding numbers revealed in this study may be of important conservation concern, particularly as this trend has been mirrored at another site within the South Orkneys.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The South Georgia Shag (Leucocarbo [atriceps] georgianus) is a member of the cormorant family (Phalacrocoracidae) and part of the blue-eyed Shag taxonomic grouping of cormorant populations found between 30ºS and 70ºS (Schrimpf et al. 2018). There is still scientific debate as to the taxonomic status of shag populations within this complex, in particular whether or not the South Georgia Shag and Antarctic Shag (Leucocarbo bransfieldensis) are distinct species or subspecies (Kennedy and Spencer 2014). Both the South Georgia and Antarctic Shag are currently designated “Least Concern” by the International Union for Conservation of Nature (IUCN 2021). A recent population assessment of the Antarctic Shag, updated with census data from Ryder Bay, indicates that the global population is 11,684 breeding pairs (Schrimpf et al. 2018; Phillips et al. 2019). The overall population size and trend of the South Georgia Shag are unknown (IUCN 2021). The breeding range of the South Georgia Shag has previously been recognised as limited to the islands of the Scotia Sea, specifically South Georgia and its associated offshore islands, Shag Rocks and the South Sandwich and South Orkney Island archipelagos (Marchant and Higgins 1990). Studies of South Georgia Shag breeding populations have reported colonies made up of relatively small numbers of breeding pairs and/or declining trends in pair numbers (Croxall and Prince 1980; Casaux and Barrero-Oro 2006; Coria et al. 2011; Lynch et al. 2016). Considering the Signy Island population to be South Georgia Shags, as per recent studies referencing the South Orkney Islands shag population (Coria et al. 2011; Casaux and Barrera-Oro 2016; Schrimpf et al. 2018; Phillips et al. 2019), published historical data suggests a world population of between 5349 and 10,849 breeding pairs. This consists of an estimated ~ 2000–7500 pairs at South Georgia (Croxall and Prince 1980; Prince and Croxall 1983; Croxall et al. 1984), 365 pairs at the South Sandwich Islands (Convey et al. 1999), ~ 1000 pairs at Shag Rocks (Prince and Croxall 1983) and 1984 pairs (between ~ 18% and 37% of the total population) at the South Orkney Islands (Schrimpf et al. 2018). However, such values should be treated with considerable caution given the paucity of information regarding methods and timings of surveys, the lack of recent data from most breeding localities, the effects of intermittent breeding which can cause marked fluctuations in breeding numbers and the frequent movements of breeding birds between colonies (Creuwels et al. 2005; Lynch et al. 2008; Schrimpf et al. 2018). Nevertheless, previous analyses of long-term population data for both South Georgia and Antarctic Shags have suggested that breeding populations are changing across the West Antarctic Peninsula (WAP) and Scotia Arc, increasing at some locations (Casanovas et al. 2015; Phillips et al. 2019; Pfeifer et al. 2021) and declining at others (Lynch et al. 2008; Woehler et al. 2010; Coria et al. 2011; Casaux and Barrera-Oro 2016)
All members of the blue-eyed Shag taxonomic grouping feed on a variety of demersal fish and invertebrate species, obtained during dives as part of short foraging trips (Casaux and Barrero-Oro 2006). A number of studies have linked changes in the availability of known fish prey species to Antarctic and South Georgia Shag population declines at several locations (Casaux et al. 1997; Woehler et al. 2001; Casaux and Ramon 2002; Coria et al. 2011). Indeed, the impact of fisheries targeting specific demersal prey fish has been implicated in Antarctic Shag population declines at several sites in the South Shetland Islands (Casaux and Barrero-Oro 2006; Casaux and Barrera-Oro 2016). Following a comprehensive review of available Antarctic Shag population data, Schrimpf et al. (2018) suggested that there has been an environmentally driven spatial shift in population distribution across the WAP, concurrent with populations of sympatrically breeding pygoscelid penguins also identified as undergoing significant population changes (Hinke et al. 2007; Lynch et al. 2008, 2012; Trivelpiece et al. 2011; Lynch and LaRue 2014). Other significant penguin population changes, similar to those taking place on the WAP, have also been identified across much of the known Scotia Arc range of the South Georgia Shag, providing strong evidence that they are responding to the same environmental drivers (Forcada et al. 2006; Forcada and Trathan 2009; Coria et al. 2011; Dunn et al. 2016).
Here, we describe changes in South Georgia Shag population size at Signy Island, South Orkney Islands and Bird Island, South Georgia and also breeding success for an annually monitored shag colony at Signy Island. We present census records from two colonies which comprise the Signy Island population over a 43 year period since 1978/1979, and similar census data for Bird Island over a 32 year period since 1989/1990. We also present a 26 year individual colony dataset from Signy spanning the austral summers 1995/1996 to 2020/2021 and comprehensive total island population surveys during twelve seasons (1999/2000, 2009/2010 and 2011/2012 to 2020/2021). The breeding populations at the two Signy colonies were analysed for patterns of correlation between population size and productivity, and population variability at different time scales, to identify any evidence of shared environmental drivers. These data are compared with earlier historical Signy census data, and with colonies at Laurie Island, which is another location in the South Orkney archipelago. We evaluate variability and trends in these data for comparison with populations elsewhere.
Materials and methods
Study site and species
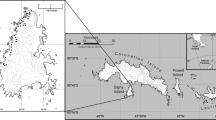
The study took place at Signy Island, South Orkney Islands (60° 42ʹ S, 45° 36ʹ W, Fig. 1a–c) and Bird Island, South Georgia (54° 00ʹ S, 38° 02ʹ W, Fig. 1a, d–e). The South Georgia Shag is a relatively long lived (~ 11 years), monogamous phalacrocoracid which lays two or three eggs per clutch with an incubation period of approximately 29 days; chicks fledge after approximately 65 days (Shaw 1984). The breeding population at Signy is limited to two colony areas: one at the most northerly point of the island (North Point) and the other, on a small offshore island less than 300 m in length lying off the southern coast of Signy (Shagnasty Island, Fig. 1c). The two colonies are separated from one another by an approximately 7 km straight-line distance across the island. However, shags rarely fly directly across the interior of the island, instead taking a coastal route of approximately 10 km between the two sites. At both locations, nests are found extending up cliff ledges and slopes and also on flat ground immediately adjacent to the top of cliffs. Shagnasty Island is only accessible at low tide, when it is possible to cross from Signy via a natural causeway of rock (a distance of approximately 50 m). At Bird Island, South Georgia Shags have been recorded as breeding on cliff ledges in 13 distinct areas, comprising of 19 separate sub-colonies, although numbers fluctuate considerably between them (Fig. 1e). All colonies are within < 2 km of a neighbouring breeding area and in most cases distances are < 1 km.
Survey methods
At Signy, population surveys were conducted between 1978/1979 and 2020/2021. Surveys at North Point were conducted in most years, whilst surveys at Shagnasty Island began in 1978/1979 and were annual from 2011/2012 to 2020/2021. In combination, surveys of North Point and Shagnasty provide a total-island count for Signy. Surveys at Bird Island began in 1989/1990 and were annual from 2016/2017 to 2020/2021. Two survey methodologies have been practiced on Signy: between 1978/1979 and 1988/1989 some nests were closely inspected to determine breeding pairs, noting nest contents (Shaw 1986; Cobley 1992) whilst other nests were recorded based on observations of presence/absence of adults (i.e. apparently occupied nests or AONs, defined as a pedestal nest on which an adult appeared to be incubating and providing an approximation of numbers of breeding pairs). Both are standard census methods employed when surveying Antarctic Shags (Schrimpf et al. 2018; Phillips et al. 2019). From 1996/1997 and 1989/1990 onwards at Signy and Bird islands, respectively, AONs were counted as a proxy for the number of breeding pairs (Creuwels et al. 2005), since many nests were unsafe or inaccessible to approach closely. All survey work was carried out either peripherally from each colony using a tally counter or, owing to the complexity of the nesting terrain (cliff tops, ledges, gullies and slopes), from surrounding vantage points at distances of up to 30–50 m using binoculars. To provide the most accurate assessment of breeding pairs, surveys were conducted as close as possible to known peak egg laying dates (Creuwels et al. 2005; Dunn et al. 2015; Braun et al. 2021). In the 2017/2018 season, multi-rotor, un-crewed aerial vehicles (UAVs) were used at Signy to take aerial images of each colony from a height of 50 m, in addition to the same standard AON counting methods employed in previous seasons. For full details of the UAV procedure see Dunn et al. (2021). At Bird Island, tally counters and binoculars were supplemented more recently with additional counts from hand-held digital camera photography. By employing a fixed counting method at Signy and Bird islands over successive seasons, consistency in data collection was maintained. A minimum of two experienced observers surveyed each colony at least twice on the same day and surveys were repeated until the count totals were within 10%, to ensure consistency. The mean count value was used in further analyses. During survey work, care was always taken to keep disturbance to a minimum: breeding birds rarely left their nests and those that did always returned immediately.
At Signy, optimal survey dates were determined based on the known egg laying dates for this species at this locality (Shaw 1986; Rootes 1988) and observed phenology each season. Following peak laying, all AONs were counted (between 13th November and 27th December) from 1996/1997 to 2020/2021 at the North Point colony. Data were also collected, using the same method, for 11 years within this period at the Shagnasty Island colony. At Bird Island, observed colony phenology each season was also used to determine optimal survey dates for AONs. Since empty nests were rarely observed during pair counts, it was assumed that few pairs had failed prior to surveys: consequently, no correction for earlier nest failure was made (Phillips et al. 2019).
The total number of chicks present at the North Point colony was counted between 6th January and 6th February in all seasons from 1996/1997 to 2020/2021. Since the fledging period for South Georgia shags at Signy spans January to February (Shaw 1984), at the time of annual counting, all chicks were sufficiently large to no longer be brooded or guarded by parent birds, facilitating easy counting. No chick counts took place at the Shagnasty colony (owing to difficulty of access) and at Bird Island. Annual breeding productivity was calculated as chicks per AON; percentage breeding success was expressed as numbers of chicks per AON relative to the maximum number of chicks (three) that could be produced per nest.
Data analyses
Relationships between time series can be confounded by the presence of correlated trends through time. To establish if trends were present, linear regressions were applied to log-transformed count data of AONs at all colonies (and total AONs across Signy) and North Point chicks. Since Signy nest-counting methodologies differed prior to 1996/1997, we examined North Point AONs over two timeframes; from 1978/1979, and from 1996/1997 to 2020/2021. Residual values were examined in Quantile–quantile (Q–Q) plots and tested for normality using the Shapiro–Wilk test. Consequently, log-transformed counts of AONs at all colonies (and total AONs across Signy) and North Point chicks were de-trended, using linear regression to extract residual values. To examine whether trends in Signy colony numbers were non-linear, piecewise regression analysis was conducted on all Signy log-transformed counts (AONs in all locations and North Point chick counts) and fitted using the segmented package in Program R (Muggeo 2003). The pseudo-score statistics test pscore.test was then used to test whether a single breakpoint was a better fit to the data than a linear regression (Muggeo 2016). Bird Island was not analysed in this way as it has a short time series.
A positive correlation in detrended values between Signy colonies might suggest similar environmental drivers influencing breeding pair abundance at both locations. We therefore used Kendall’s non-parametric tau correlation coefficient to examine patterns of correlation between log-transformed, detrended AON numbers at the two primary study sites (all years surveyed, and the most recent period 2009/2010 to 2020/2021).
To identify patterns of periodicity in breeding pair abundance at each colony, we examined patterns of autocorrelation and partial autocorrelation of detrended residuals, using the acf and pacf functions in Program R. North Point AONs were analysed from 1996/1997 to 2020/2021. Since the time series’ from Shagnasty and Bird Island contain missing data prior to 2011, these data were analysed from 2011/2012 and 2012/2013, respectively.
To test whether population changes correlate with prior productivity changes, for example as chicks hatched at year 0 become old enough to breed (the main age range of first breeding at Signy is 3–5 years, (Cobley 1992)), we also examined the cross-correlation pattern between log-transformed, de-trended counts of AONs and chicks within the North Point colony over lag periods of t-1 to t-6 years (cor.test in Program R, R Core Team 2016).
Results
Population trends
Numbers of AONs/breeding pairs varied considerably between years and overall there was a strong (492%) increase at North Point between 1978/1979 (50 AONs) and 2020/2021 (246 AONs, + 3.8% per annum, Fig. 2, Table 1). Over the entire time period including all historical data (1948/1949 to 2020/2021), an increase in breeding pairs at North Point of + 4.6% per annum was calculated (Online Resource 1, Dunn 2020). However, there is little information on historical survey effort or rigour, so any conclusions about longer-term population trends should be treated cautiously. The number of AONs recorded in the Shagnasty Island colony decreased by 72.7% (− 6.2% per annum) between 1999/2000 and 2020/2021, and declined by 77.2% (− 3.5% per annum) between 1978/1979 and 2020/2021 (Fig. 2; Table 1). As with the North Point colony, there was substantial inter-annual variability in the nest counts (Table 1). AON numbers at Bird Island, South Georgia, showed an overall decline of 58.3%, spanning 32 years from 1989/1990 to 2020/2021 (− 2.8% per annum, Fig. 2; Table 2), from 343 to 143 pairs. In two periods there was a substantial decline in the population: AON numbers declined by 50.4% (− 14% per annum) between 1989/1990 and 1994/1995, and by 26.3% (− 3.8% per annum) between 2012/2013 and 2020/2021.
Linear regressions of log-transformed AON count data over time were strongly significant for all Signy datasets, with adjusted R2 values of 0.74 for North Point (1978/1979 onwards), 0.71 for North Point (1996/1997 onwards), 0.74 for Shagnasty, 0.45 for North Point and Shagnasty (Signy island-wide), 0.47 for chicks at North Point and 0.72 for Bird Island AONs. Shapiro–Wilk tests did not reject a null hypothesis of normality for nearly all datasets, with the exception of the North Point AONs from 1996/1997 onwards (W = 0.917, p = 0.0438). Piecewise regression showed a good fit to all datasets with adjusted R2 values of 0.80 for North Point (1978/1979 onwards), 0.73 for North Point (1996/1997 onwards), 0.78 for North Point and Shagnasty (Signy island-wide), 0.46 for chicks at North Point and 0.55 for Bird Island. However, piecewise regression was only a significant improvement on linear regression for the North Point AON counts from 1978/1979 (observed value from two-sided test = 2.827, df = 30, p < 0.0080). This suggested North Point AONs underwent a step change in population trend in 1994 (standard error, SE = 4.146 years, n = 30, Online Resource 2).
Between 1999/2000 and 2020/2021 there was a decrease of 35.8% (− 2.1% per annum) in the total South Georgia Shag breeding population on Signy, from 595 to 382 AONs (Fig. 2; Table 2). Analysis of counts from North Point and Shagnasty colonies combined showed an overall decline in AONs/breeding pair numbers across Signy from 1978/1979 to 2020/2021 of 40.9% (− 1.3% per annum, from 646 to 382 pairs (Fig. 2; Table 2)). Log-transformed AON counts at North Point and Shagnasty were significantly negatively correlated from 1978/1979 to present (t = -4.758, df = 17, p = 0.0002), confirming a contrasting abundance trend, but no significant correlation was detected in the most recent period between 2009/2010 and 2020/2021 (when Shagnasty AONs were counted annually). In contrast, de-trended, log-transformed AON counts at North Point and Shagnasty were not significantly correlated over all survey years, but were significantly positively correlated between 2009/2010 and 2020/2021 (t = 2.880, df = 9, p = 0.0182), indicating similar drivers in pair numbers may have been operating at both sites in the last decade.
At North Point, log-transformed residual values (1996/1997 to 2020/2021) showed a significant, negative autocorrelation pattern at t + 3 (Pearson’s r = − 0.459). When accounting for serial autocorrelation, partial autocorrelation analysis revealed a significant negative correlation at t + 2 (correlation = − 0.421, Online Resource 3). Autocorrelation and partial autocorrelation analysis of residuals for Shagnasty (2011/2012 to 2020/2021) showed a significant negative correlation at t + 2 (correlation = − 0.683 and – 0.703, respectively, Online Resource 3). No significant autocorrelation patterns were detected in the Bird Island data.
Breeding success
At Signy, breeding success varied substantially between 16 and 73.3% (0.47–2.2 productivity) in the North Point shag colony over the period from 1996/1997 to 2020/2021, characterised by two distinct periods of high (> 40%, > 1.2 chicks/AON) and low (< 25%, < 0.75 chicks/AON) productivity (Fig. 3; Table 1). Despite inter-annual variation, breeding productivity underwent a steep decline during the latter part of the 1990s and was lowest for an extended period between 1998/1999 and 2003/2004. In the following years (2004/2005 to 2008/2009) breeding productivity recovered, followed by a second period of relatively low productivity from 2012/2013 to 2015/2016 (Fig. 3; Table 1). No significant correlations were found between log-transformed North Point chick counts compared to AON numbers in subsequent years. Detrended residuals of chick counts also did not show any significant autocorrelation patterns over time, nor any significant correlations with detrended residuals of North Point AONs, between years.
Discussion
Population trends
This study indicates a clear decline of ~ 36% in South Georgia Shag AONs at Signy Island between 1999/2000 and 2020/2021. We also found a similar pattern at Bird Island, South Georgia, showing a ~ 60% decline in AONs over a 32 year period (1989/1990 to 2020/2021). Interestingly, at Signy the decline in AONs has been confined to the Shagnasty colony, where numbers declined by over 70% between 1999/2000 and 2020/2021. Conversely, the initially smaller North Point colony experienced a 2.5-fold increase in AONs over the same period. Indeed, historical data (Dunn 2020; Woehler et al. 2001) indicates an even longer period of population increase in shag breeding pairs at North Point of ~ 5% per annum between 1948/1949 and 2020/2021. Comparison with historical data also shows the recent declining trend of the total Signy shag population over the past 22 years extends that found in earlier studies (Shaw 1984; Cobley 1986; Rootes 1988; Woehler et al. 2001). These earlier studies indicate a much longer declining trend between 1978/1979 and 1998/1999 equating to an overall Signy population decrease of > 40%, from 646 to 382 pairs, stretching across a 43 year period from the late 1970s to 2020/2021. However, a lack of information on historical survey effort or rigour in some years prior to 1996 means any conclusions about longer-term population trends and comparisons with this study should be treated cautiously. These historical data nevertheless indicate a prolonged and substantial population change for the shag population at Signy, which has continued to the present.
Annual nest counts carried out at the North Point colony since 1978/1979 revealed an increase in numbers of nesting pairs (Rootes 1988; Woehler et al. 2001) with a steady increase in population size beginning in 1994. Surveys of the less accessible Shagnasty colony were infrequent until recently, so the longer-term trend in this population is less well resolved. However, AON numbers in North Point and Shagnasty colonies fluctuated with similar regularity (~ 4 year periodicity). These correlated population dynamics suggest that similar environmental drivers may be impacting both colonies. Breeding productivity at Signy broadly increased at the North Point colony over the period 1996/1997 to 2019/2020, which may explain the trend of fluctuating yet broadly increasing pair numbers observed in this colony. However, the significant annual variation makes it difficult to distinguish a clear trajectory or to confirm natal recruitment as the main driver, and no temporal correlations between North Point AONs and chicks could be deduced in the data to point to direct recruitment.
The declining total number of South Georgia Shag breeding pairs at Signy and fluctuating annual breeding success at the North Point colony is intriguing, particularly since a similar contrast between pair numbers and breeding success has also been shown in the declining populations of sympatrically nesting Adélie (Pygoscelis adéliae) and Chinstrap (Pygoscelis antarctica) Penguins at Signy over the same time period (Dunn et al. 2016). Human disturbance was minimal at each colony, suggesting that population changes are likely driven by environmental factors. It may be that the same environmental factors influencing the decline of the neighbouring Adélie and Chinstrap Penguin populations at Signy, and more widely across the WAP and Scotia Arc (Woehler and Croxall 1997; Forcada et al. 2006; Lynch et al. 2008, 2012; Forcada and Trathan 2009) may be affecting the South Georgia Shag population in the South Orkney Islands. The variable but broadly increasing numbers of chicks recorded from one of the two Signy colonies suggests a factor other than breeding productivity to be causing the continuing decline in population size, at least at a local “colony-scale”. The lack of correlation between North Point chicks and adult pairs suggests chicks may not necessarily recruit as adults back to the same colony. Indeed, the movement of breeding-age birds between the North Point and Shagnasty colonies over multiple seasons has previously been recorded, with more birds moving from North Point to Shagnasty Island than vice versa (Shaw 1984; Cobley 1986), though only at a low rate of exchange (Cobley 1992). Nevertheless, the overall island-wide pair decline cannot be explained by breeding birds relocating from one Signy colony to another. It is possible the population decline on Signy may be due to individual shags moving to breeding sites on other nearby islands. For example, shag colonies are known to exist on Southern Powell Island, Atriceps Island and the Inaccessible Islands, distances from Signy of approximately 35 km, 25 km and 62 km, respectively (Harris et al. 2015).
The difference in magnitude of decline in shag AONs at Signy between the Shagnasty colony, the all-island survey totals and the opposing trend of the North Point colony shows the limitations of inferring absolute abundance, or even trends on a larger scale, from local-scale counts alone. This has an important implication for monitoring methodologies in general, and highlights the importance of supplementing regular local-scale surveys with equally rigorous wider-scale population censuses (Lynch et al. 2012; Trathan et al. 2012; Dunn et al. 2016). The limitations of the Signy shag survey should also be acknowledged: despite best efforts to carry out AON and chick surveys at the optimum time, some failed breeders and non-breeding birds are nevertheless likely to be included in the AON counts, resulting in a possible population overestimate. Any chick mortalities following our chick surveys are not accounted for and as such, realised breeding success in some years may be overestimated. Identifying and quantifying drivers of breeding success is challenged by variation between study sites, making identification of specific drivers of population change and how their impacts equate to actual population changes difficult to discern (Carlini et al. 2007; Micol and Jouventin 2001; Trathan et al. 2015; Coetzee and Chown 2016). For example, whilst we did not identify any inter-annual correlations between numbers of chicks and subsequent numbers of AONs (indicating recruitment back to the colony), if for example age of first breeding is variable, such recruitment may not have been detected with this approach. A future expansion of the shag monitoring programme at Signy to include the collection of annual survival and recruitment rates, as previously carried out during the 1980s, would be desirable to facilitate a better understanding of population change, productivity patterns across the whole island, and to allow a means of measuring emigration and immigration at the North Point and Shagnasty Island colonies (Shaw 1984, 1986; Cobley 1986, 1992). Uncrewed aerial vehicles (UAVs) have recently been successfully trialled at Signy to collect large-scale penguin and shag population data, including from the North Point and Shagnasty Island shag colonies (Dunn et al. 2021) This study found the UAV AON census total was shown to vary in the case of individual colonies between < 1% (Shagnasty Island) and 14.5% (North Point) from a simultaneous AON ground count of the two colonies (Dunn et al. 2021). Such technology offers an ideal means of regularly collecting productivity data from the difficult-to-access Shagnasty Island, including individual nest survival data, and offers the potential of expanding future surveys to include shag colonies on other islands in the South Orkney archipelago.
Long-term population trends for South Georgia Shags breeding at colonies at a second study site in the South Orkney Islands, Laurie Island, (approximately 50 km from Signy Island), indicate a similar pattern of decline to that of Signy Island. Between 1994/1995 and 2005/2006 the number of breeding pairs decreased by 48.2% (− 4.4% per annum), (Coria et al. 2011). More recent data from Laurie Island would be useful in determining whether this decline has continued to the present. Given the global importance of the South Orkney shag population, surveying all other known sites within the archipelago (Shaw 1984) should be a priority, to determine whether or not the declines at Signy and Laurie islands have taken place elsewhere. By using comparable standard survey methods, such data would provide a wider geographic context to understand the changes taking place at Signy and Laurie. The previously published figure of 1984 pairs of South Georgia Shags breeding in the South Orkney Islands (Schrimpf et al. 2018) should be updated to 1849 pairs to take into account the most recent population data from Signy.
Bird Island is located approximately 860 km from the South Orkney Islands. As such, the declining Bird Island shag population identified in this study—when considered alongside the known declines in the South Orkney Islands—may indicate a wider-scale reduction in the South Georgia Shag breeding population. Shag population declines similar to those identified in this study have been recorded elsewhere in recent decades for other members of the blue-eyed Shag taxonomic grouping. The Crozet Island Shag (P. [atriceps] melanogenis) has declined on Marion and Edward Islands (Crawford et al. 2003; Crawford et al. 2014; Ryan et al. 2003), several colonies of Antarctic Shag have declined in the South Shetland Islands and Antarctic Peninsula, (Casaux and Barrera-Oro 2016; Schrimpf et al. 2018), and the Heard Island Shag (P. [atriceps] nivalis) has also declined (Green et al. 1998). Drivers of the Bird Island decline are at present unclear, and further comparative surveys carried out in shag colonies on the neighbouring island of South Georgia would provide a much-needed context. Wanless et al. (1995) suggested the Bird Island population could be particularly sensitive to changes in food availability owing to the predominance of deep, long-duration feeding dives made by individuals from this colony, significantly limiting the length of available daily foraging time. Emigration from Bird Island to other colonies on South Georgia (a distance of just a few km) is certainly possible. However, the current declining trend at Bird Island, given that South Georgia hosts between 37% and 69% of the historically estimated world population of the South Georgia Shag (Convey et al. 1999; Croxall and Prince 1980; Schrimpf et al. 2018), nevertheless poses a conservation concern.
Population drivers
Decreasing abundance of demersal fish prey (Crawford et al. 2003, 2014; Ryan et al. 2003; Casaux and Barrera-Oro 2016) and/or climate change (Casanovas et al. 2015; Schrimpf et al. 2018) have been suggested as possible causes of shag population declines in the Antarctic and sub-Antarctic. Woehler et al. (2001) previously speculated that changes in abundance of juvenile year-classes of fish prey species may be a contributing factor in shag population declines at Signy. Coria et al. (2011) suggested that the decline in shag breeding pairs at Laurie Island may be due to changes in the abundance of certain notothenioid fish species, based upon data published by Casaux and Ramon (2002). A number of studies have identified several demersal-benthic fish species to be the primary prey of both the Antarctic and South Georgia Shag (Orta 1992; Casaux and Barrero-Oro 2006; Casaux and Barrera-Oro 2016; Beltran et al. 2017; Bertolin and Casaux 2019) and analyses of pellets from South Georgia Shags nesting at Laurie Island between 1995/1996 and 1999/2000 revealed demersal-benthic fish as the principal prey items, followed by molluscs and polychaetes (Casaux and Ramon 2002). A similar diet composition was previously reported for South Georgia Shags breeding at Signy and Bird Island (Shaw 1984; Wanless et al. 1992). The diet of demersal fish species consumed by shags, such as Notothenia coriiceps, has previously been shown at Signy, and elsewhere, to be dominated by crustacean prey such as amphipods and euphausiids (Coggan 1997). As such, the declining shag populations at Signy and Laurie islands may be symptomatic of reduced survival or recruitment in these fish, driven in turn by localised stock depletion due to shag predation and/or a localised decline in euphausiids, amphipods and other crustacean prey. More recent diet data from the Signy shag population would be useful in determining current prey composition for comparison.
Stochastic environmental factors such as air temperature and snow coverage can have spatial and temporal impacts on seabird colonies, often at local scales (Van Franeker et al. 2001; Southwell et al. 2010; Dunn et al. 2019). In particular, adverse climatic conditions are often identified as having a significant negative effect on breeding seabirds (Van Franeker et al. 2001; Jenouvrier et al. 2003; Massom et al. 2006; Forcada and Trathan 2009). Previous research carried out at Signy from 1986 to 1987 indicated that survival rates of juvenile South Georgia Shags during their first year following fledging were negatively correlated to increased sea ice and low air temperatures, particularly during April (Cobley 1992). It was surmised that the early formation of sea ice around Signy and associated low temperatures was indicative of the arrival of winter conditions and specifically pack ice, itself likely to restrict foraging by the local shag population with potential adverse effects on survival, particularly in juveniles (Cobley 1992). Consequently, determining environmental parameters such as snow cover extent and duration and also air temperature, would be highly desirable at Signy as a future means of identifying possible relationships between seasonally adverse weather conditions and shag population dynamics.
Given the limited and in many cases outdated population size estimates currently available for South Georgia Shags of a maximum of ~ 10,850 pairs worldwide (Croxall and Prince 1980; Convey et al. 1999; Casaux and Ramon 2002; Coria et al. 2011) it is vitally important to gain up-to-date, comprehensive data on world-wide population size, to facilitate accurate assessment for the IUCN Red List. Considering the South Orkney Islands were previously estimated as home to potentially ~ 18%–37% of the world population of South Georgia Shags, continued declines in the Signy and Laurie Island shag populations, particularly if mirrored at other breeding sites within the South Orkneys, at Bird Island and elsewhere, would be of considerable conservation significance. Continued rigorous monitoring of the Signy, Bird and Laurie islands breeding populations is therefore needed, together with comprehensive future surveys of other breeding populations across the South Orkney Islands and also at South Georgia, Shag Rocks and the South Sandwich Islands. Only by continuing to collect such long-term data are we likely to accurately discern current and future population trends and the processes driving them.
Code availability
Not applicable.
References
Beltran M, Tavella G, Casaux R (2017) The diet of post-breeding Antarctic shags Phalacrocorax bransfieldensis at Cockburn Island, Antarctic Peninsula. Mar Ornithol 45:17–19
Bertolin ML, Casaux R (2019) Diet overlap among top predators at the South Orkney Islands, Antarctica. Polar Biol 42:371–383. https://doi.org/10.1007/s00300-018-2428-9
Braun C, Esefeld J, Savelieva L, Peter HU (2021) Population decline of the cape petrel (Daption capense) on King George Island, South Shetland Islands, Antarctica. Polar Biol 44:1795–1801. https://doi.org/10.1007/s00300-021-02914-4
Carlini AR, Coria NR, Santos MM, Libertelli MM, Donini G (2007) Breeding success and population trends in Adelie penguins in areas with low and high levels of human disturbance. Polar Biol 30:917–924. https://doi.org/10.1007/s00300-006-0251-1
Casanovas P, Naveen R, Forrest S, Poncet J, Lynch HJ (2015) A comprehensive coastal seabird survey maps out the front lines of ecological change on the western Antarctic Peninsula. Polar Biol 38:927–940. https://doi.org/10.1007/s00300-015-1651-x
Casaux R, Barrera-Oro E (2016) Linking population trneds of Antarctic shag (Phalacrocorax barnsfieldensis) and fish at Nelson Island, South Shetland Islands (Antarctica). Polar Biol 39:1491–1497. https://doi.org/10.1007/s00300-015-1850-5
Casaux R, Barrero-Oro E (2006) Shags in Antarctica: their feeding behaviour and ecological role in the marine food web. Antarct Sci 18:3–14. https://doi.org/10.1017/S0954102006000010
Casaux R, Coria N, Barrero-Oro E (1997) Fish in the diet of the Antarctic shag Phalacrocorax bransfieldensis at Laurie Island, South Orkney Islands. Polar Biol 18:219–222. https://doi.org/10.1007/s003000050179
Casaux R, Ramon A (2002) The diet of the South Georgia shag Phalacrocorax georgianus at South Orkney Islands in five consecutive years. Polar Biol 25:557–561. https://doi.org/10.1007/s00300-002-0389-4
Cobley N (1986) Influence of age on the biology of the blue-eyed shag Phalacrocorax atriceps bransfieldensis (Murphy). British Antarctic Survey. Report available on request from the British Antarctic Survey Archives. H/1986/N10.
Cobley N (1992) Aspects of the population dynamics of Antarctic blue-eyed shags Phalacrocorax atriceps king. PhD, Durham University. http://etheses.dur.ac.uk/6267/.
Coetzee BWT, Chown SL (2016) A meta-analysis of human disturbance impacts on Antarctic wildlife. Biol Rev 91:578–596. https://doi.org/10.1111/brv.12184
Coggan R (1997) Seasonal and annual growth rates in the Antarctic fish Notothenia coriiceps Richardson. J Exp Mar Biol Ecol 213:215–229
Convey P, Morton A, Poncet J (1999) Survey of marine birds and mammals of the South Sandwich Islands. Polar Rec 35:107–124. https://doi.org/10.1017/S0032247400026450
Coria NR, Montalti D, Rombola EF, Santos MM, Garcia Betono MI, Juares MA (2011) Birds at Laurie Island, South Orkney islands, Antarctica: breeding species and their distribution. Mar Ornithol 39:207–213
Crawford RJM et al (2003) Population, Breeding, Diet and Conservation of the Crozet Shag Phalacrocorax [atriceps] melanogenis at Marion Island, 1994/95-2002/03. Afr J Mar Sci 25:537–547. https://doi.org/10.2989/18142320309504043
Crawford RJM, Dyer BM, Upfold L, Makhado AB (2014) Congruent, decreasing trends of gentoo penguins and Crozet shags at sub-Antarctic Marion Island suggest food limitation through common environmental forcing. Afr J Mar Sci 36:225–231. https://doi.org/10.2989/1814232X.2014.926293
Creuwels JCS, Stark JS, Woehler EJ, van Franeker JA, Ribic CA (2005) Monitoring of a southern giant petrel Macronectes giganteus population on the Frazier Islands, Wilkes Land, Antarctica. Polar Biol 28:483–493. https://doi.org/10.1007/s00300-004-0663-8
Croxall JP, Prince PA (1980) Food, feeding ecology and ecological segregation of seabirds at South Georgia. Biol J Linn Soc 14:103–131. https://doi.org/10.1111/j.1095-8312.1980.tb00101.x
Croxall JP, Prince PA, Hunter I, McInnes SJ, Copestake PG (1984) The seabirds of the Antarctic Peninsula, islands of the Scotia Sea, and Antarctic continent between 80W and 20W: their status and conservation. International Council for Bird Preservation Technical Publication No.2:637–666
Dunn M (2020) Counts of South Georgia shag Leucocarbo [atriceps] georgianus nests at Signy Island, South Georgia, from 1947–2020. UK Polar Data Centre BAS, Natural Environment Research Council, UK Research & Innovation. Cambridge. Version:1.0. https://doi.org/10.5285/CA60BFEC-9070-4967-920E-0C42554AD649
Dunn MJ, Adlard S, Taylor AP, Wood AG, Trathan PN, Ratcliffe N (2021) Un-crewed aerial vehicle population survey of three sympatrically breeding seabird species at Signy Island South Orkney Islands. J Polar Biol. https://doi.org/10.1007/s00300-021-02831-6
Dunn MJ, Forcada J, Jackson JA, Waluda CM, Nichol N, Trathan PN (2019) A long-term study of gentoo penguin (Pygoscelis papua) population trends at a major Antarctic tourist site, Goudier Island, Port Lockroy. Biodivers Conserv 28:37–53. https://doi.org/10.1007/s10531-018-1635-6
Dunn MJ, Jackson JA, Adlard S, Lynnes AS, Briggs DR, Fox D, Waluda CM (2016) Population size and decadal trends of three penguin species nesting at Signy Island South Orkney Islands. PLoS ONE 11:e0164025. https://doi.org/10.1371/journal.pone.0164025
Dunn MJ, Jackson JA, Adlard S, Phillips RA (2015) Population size and trends of southern giant petrels (Macronectes giganteus) nesting at Signy Island, South Orkney Islands. Polar Biol. https://doi.org/10.1007/s00300-015-1855-0
Forcada J, Trathan PN (2009) Penguin responses to climate change in the Southern Ocean. Global Change Biol 15:1618–1630
Forcada J, Trathan PN, Reid K, Murphy EJ, Croxall JP (2006) Contrasting population changes in sympatric penguin species in association with climate warming. Global Change Biol 12:411–423
Green K, Woehler EJ, Copley PB, Jones RT (1998) A review of the population status of the Heard Island cormorant Phalacrocorax nivalis. Mar Ornithol 26:69–74
Harris CM et al. (2015) Important bird areas in Antarctica 2015. BirdLife International and Environmental Research & Assessment Ltd. Cambridge. pp 313. http://www.era.gs/resources/iba/Important_Bird_Areas_in_Antarctica_2015_v5.pdf
Hinke JT, Salwicka K, Trivelpiece SG, Watters GM, Trivelpiece WZ (2007) Divergent responses of Pygoscelis penguins reveal a common environmental driver. Oecologia 153:845–855
IUCN (2021) The IUCN Red List of Threatened Species. Version 2020–2023 https://www.iucnredlist.org. Accessed 12 March 2021
Jenouvrier S, Barbraud C, Weimerskirch H (2003) Effects of climate variability on the temporal population dynamics of southern fulmars. Ecology 72:576–587. https://doi.org/10.1046/j.1365-2656.2003.00727.x
Kennedy M, Spencer HG (2014) Classification of the cormorants of the world. Mol Phylogenet Evol 79:249–257. https://doi.org/10.1016/j.ympev.2014.06.020
Lynch HJ, LaRue MA (2014) First global census of the Adélie penguin. Auk 131:457–466
Lynch HJ, Naveen R, Fagan WF (2008) Censuses of penguin, blue eyed shag Phalacrocorax atriceps and southern giant petrel Macronectes giganteus populations on the Antarctic Peninsula, 2001–2007. Mar Ornithol 36:83–97
Lynch HJ, Naveen R, Trathan PN, Fagan WF (2012) Spatially integrated assessment reveals widespread changes in penguin populations on the Antarctic Peninsula. Ecology 93:1367–1377
Lynch HJ, White R, Naveen R, Black A, Meixler MS, Fagan WF (2016) In stark constrast to widespread declines along the Scotia Arc, a survey of the South Sandwich Islands finds a robust seabird community. Polar Biol 39:1615–1625. https://doi.org/10.1007/s00300-015-1886-6
Marchant S, Higgins PJ (1990) Australian, New Zealand and Antarctic birds. Vol 1. Ratites to ducks, vol 1. Oxford University Press, Melbourne
Massom RA et al (2006) Extreme anomolous atmospheric circulation in the west Antarctic peninsula region in austral spring and summer 2001/2002, AND Its profound impact on sea ice and biota. J Climate 19:3544–3571. https://doi.org/10.1175/JCLI3805.1
Micol T, Jouventin P (2001) Long-term population trends in seven Antarctic seabirds at Pointe Geologie (Terre Adelie): human impact compared with environmental change. Polar Biol 24:175–185. https://doi.org/10.1007/s003000000193
Muggeo VMR (2003) Estimating regression models with unknown break-points. Stat Med 22:3055–3071. https://doi.org/10.1002/sim.1545
Muggeo VMR (2016) Testing with a nuisance parameter present only under the alternative: a score-based approach with application to segmented modelling. J Stat Comput Sim 86:3059–3067. https://doi.org/10.1080/00949655.2016.1149855
Orta J (1992) Family Phalacrocoracidae (Cormorants). In: del Hoyo J, Elliot A, Sargatal J (eds) Handbook of the birds of the world, vol 1. Lynx Editions, Barcelona, pp 326–353
Pfeifer C, Rummler MC, Mustafa O (2021) Assessing colonies of Antarctic shags by unamnned aerial vehicle (UAV) at South Shetland Islands, Antarctica. Antarct Sci. https://doi.org/10.1017/S0954102020000644
Phillips RA, Silk JRD, Massey A, Hughes KA (2019) Surveys reveal increasing and globally important populations of south polar skuas and Antarctic shags in Ryder Bay (Antarctic Peninsula). Polar Biol 42:423–432. https://doi.org/10.1007/s00300-018-2432-0
Prince PA, Croxall JP (1983) Birds of South Georgia: new records and re-evaluations of status. Br Antarctic Surv Bull 59:15–27
Rootes DM (1988) The status of birds at Signy Island, South Orkney Islands. Br Antarctic Surv Bull 80:87–119
Ryan PG, Cooper J, Dyer BM, Underhill LG, Crawford RJM, Bester MN (2003) Counts of surface-nesting seabirds breeding at Prince Edward Island, summer 2001/02. Afr J Mar Sci 25:441–451. https://doi.org/10.2989/18142320309504033
Schrimpf M, Naveen R, Lynch HJ (2018) Population status of the Antarctic shag Phalacrocorax (atriceps) bransfieldensis. Antarct Sci 30:1–9. https://doi.org/10.1017/S0954102017000530
Shaw P (1984) Factors affecting the breeding performance of the Antarctic blue-eyed shag (Phalacrocorax atriceps bransfieldensis). PhD, Durham University. http://etheses.dur.ac.uk/7469/.
Shaw P (1986) Factors affecting breeding performance of Antarctic blue-eyed shags Phalacrocorax atriceps. Ornis Scand 17:141–150. https://doi.org/10.2307/3676863
Southwell C, McKinlay J, Emmerson L, Trebilco R, Newbery K (2010) Improving estimates of Adelie penguin breeding population size: developing factors to adjust one-off population counts for availability bias. CCAMLR Sci 17:229–241
Trathan PN et al (2015) Pollution, habitat loss, fishing, and climate change as critical threats to penguins. Conserv Biol 29:31–41. https://doi.org/10.1111/cobi.12349
Trathan PN, Ratcliffe N, Masden EA (2012) Ecological drivers of change at South Georgia: the krill surplus, or climate variability. Ecography 35:983–993. https://doi.org/10.1111/j.1600-0587.2012.07330.x
Trivelpiece ZW, Hinke JT, Miller AK, Reiss CS, Trivelpiece SG (2011) Variability in krill biomass links harvesting and climate warming to penguin population changes in Antarctica. Proc Natl Acad Sci USA 108:7625–7628
Van Franeker JA, Creuwels JCS, Van der Veer W, Cleland S, Robertson G (2001) Unexpected effects of climate change on the predation of Antarctic petrels. Antarct Sci 13:430–439
Wanless S, Harris MP, Morris JA (1992) Diving behaviour and diet of the blue-eyed shag at South Georgia. Polar Biol 12:713–719. https://doi.org/10.1007/BF00238872
Wanless S, Harris MP, Morris JA (1995) Factors affecting daily activity budgets of South Georgian shags during chick rearing at Bird Island, South Georgia. Condor 97:550–558
Woehler EJ, Blight L, Bullock I (2010) Ornithological observations at Eckener Point, Antarctic Peninsula. Polar Rec 46:279–281. https://doi.org/10.1017/S003224740999012X
Woehler EJ et al. (2001) A statistical assessment of the status and trends of Antarctic and Sub-Antarctic seabirds. Report on SCAR BBS Workshop on Southern Ocean seabird populations. SCAR. Cambridge. pp 43. https://www.scar.org/library/scar-publications/occasional-publications/3514-a-statistical-assessment-of-the-status-and-trends-of-antarctic-and-sub-antarctic-seabirds/file/
Woehler EJ, Croxall JP (1997) The status and trends of Antarctic and Sub-Antarctic seabirds. Mar Ornithol 25:43–66
Wood A (2020) Whole island counts of South Georgia Shag nests at Bird Island, South Georgia, from 1989–2020. UK Polar Data Centre NERC, UK Research & Innovation. Cambridge. https://doi.org/10.5285/FC18715A-07D6-4D03-B27C-124637338201
Acknowledgements
We thank all the staff of the British Antarctic Survey (BAS) Signy Island and Bird Island Research Stations who have contributed to and supported the South Georgia Shag monitoring programme. We are very grateful to Sarah Wanless and Mike Harris for kindly allowing us to publish their Bird Island data and Laura Gerrish for her mapping assistance. We also thank Richard Phillips and Norman Ratcliffe for their discussions, and the helpful advice and comments of Michael Schrimpf and two other anonymous reviewers.
Funding
This study is part of the Ecosystems component of the British Antarctic Survey Polar Science for Planet Earth Programme, funded by The Natural Environment Research Council.
Author information
Authors and Affiliations
Contributions
MJD conceived and designed the research. MJD, SA, ASL, DF and TIM contributed to the fieldwork. JAJ and MJD contributed to the data analyses. All authors contributed to and approved the written manuscript.
Corresponding author
Ethics declarations
Conflict of interest
None of the authors have conflicts of interest/competing interests.
Ethical approval
Our research was approved by the British Antarctic Survey Animal Welfare and Ethical Review Board, and permission was granted by the British Foreign and Commonwealth Office on behalf of HM Secretary of State, under Sections 12 and 13 of the Antarctic Act, 1994, 2013.
Consent to participate
Not applicable.
Consent for publication
All authors and the British Antarctic Survey Polar Science for Planet Earth Programme consent to publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dunn, M.J., Adlard, S., Lynnes, A.S. et al. Long-term population size and trends of South Georgia Shags (Leucocarbo [atriceps] georgianus) at Signy Island, South Orkney Islands and Bird Island, South Georgia. Polar Biol 45, 177–189 (2022). https://doi.org/10.1007/s00300-021-02978-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-021-02978-2