Abstract
The breeding performance and population trends of Adélie penguins (Pygoscelis adeliae) was studied at Esperanza/Hope Bay, Antarctic Peninsula, by comparing an area with low levels of human disturbance (LLD) and an area with high levels of human disturbance (HLD), close to an Argentine research station. From 1995/1996 to 2004/2005 (except for 1999/2000 and 2003/2004), the following population parameters were measured in both areas: (1) the number of breeding pairs, (2) the number of chicks in creches and (3) the number of chicks produced by breeding pairs. Counts were made for 26 breeding groups situated in the LLD area and 63 breeding groups located in the HLD area. The number of chicks per breeding pair was obtaobtained by following 100 marked nests in each area. All parameters were measured as described in the CCAMLR Monitoring Program protocols. The magnitude and direction (increasing or decreasing) of the changes in breeding population size and the number of chicks creched were similar in both areas. Overall, the number of breeding pairs decreased from 4,744 to 2,968 (37.4%) in the LLD area, and from 8,744 to 5,378 (38.6%) in the HLD area. The number of chicks fledged increased from 3,808 to 4,065 (6.7%) in the LLD area, and decreased from 6,991 to 6,712 (4%) in the HLD area. Breeding success (chicks fledged per marked nest) did not differ significantly between areas for most of the seasons compared. In 1996/1997, breeding success was significantly higher in the HLD area. Our data suggest that environmental influences currently exert greater effects than human disturbance on the penguin population at Esperanza Bay.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nesting colonial waterbirds are potentially vulnerable to human intrusion (e.g. Carney and Sydeman 1999; Nisbet 2000). Adélie penguins (Pygoscelis adeliae) live and breed around the entire Antarctic continent (Woehler 1993), where they reproduce during the austral summer on ice-free areas. In Antarctica, ice-free areas near the coast are rather rare. These areas are also used as sites for research stations and regularly visited by tourists, which implies that penguins and humans often occur together. In the past years, human activity in Antarctica has been increasing, mainly due to logistic operations and tourism (Enzenbacher 1992, 1994; Patterson et al. 2003), and this has led to concern about the effects of human disturbance on penguin populations. Although agonistic behaviour is expected as a response to human presence, it is rather difficult to ascertain the potential long-term effects of human disturbance on penguin populations. Some studies have confirmed the effects of human visitation and disturbance on penguin physiology and behaviour (Culik et al. 1990; Wilson et al. 1989, 1990, 1991; Giese 1998; Giese and Riddle 1999), breeding performance (Woehler et al. 1994; Giese 1996) and population trends (Woehler et al. 1994) while others have not (Fraser and Patterson 1997; Patterson and Fraser 1998; Cobley and Shears 1999; Cobley et al. 2000; Micol and Jouventin 2001).
The Adélie penguin colony at Esperanza/Hope Bay, Antarctic Peninsula, is one of the largest in Antarctica, with 120,000 breeding pairs (Myrcha et al. 1987). At this site, there are some Adélie breeding groups close to an Argentine research station (Esperanza Station) and thus exposed to high levels of human disturbance from commercial tourism and the station personnel who often approach the nesting areas. Additionally, the operation of ships and helicopters to re-supply the station further increases the level of disturbance throughout the breeding season. Other areas of the colony are far from the base and therefore mostly undisturbed. As human–penguin interaction is likely to increase, it is particularly important to obtain information on the potential effects of human disturbance.
The aim of this study was to determine if the effects of high levels of human disturbance on nesting Adélie penguins could be detected in selected reproductive parameters (clutch size, hatching success and overall reproductive success) using data collected over a 10-year period. We also used count data to investigate potential long-term effects on penguin populations by comparing breeding population sizes between disturbed and undisturbed areas.
Materials and methods
Study area
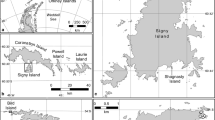
The present study was carried out at Esperanza/Hope Bay, Antarctic Peninsula (63°24′S, 57°01′W) during ten austral summers from 1995/1996 to 2004/2005 (except for 1999/2000 and 2003/2004 due to the late arrival of the research group at the sampling area). The study was conducted in two different areas following definitions given by Acero et al. (1991). These authors identified an area with low levels of human disturbance (LLD) and another with high levels of human disturbance (HLD), the latter being close to the Argentine Antarctic Station (Fig. 1). Both areas hold breeding groups of Adélie penguins (here defined as a group of penguins breeding as a geographically continuous unit located within one larger area) exposed to very different levels of human disturbance.
While penguins breeding in the LLD area are almost free from human disturbance, with the exception of scientific research, penguins breeding in the HLD area are affected by disturbances associated with the supply of the base and frequent visits by tourist ships and station personnel.
During the study period the supply of the base during December usually involved the use of helicopters (Sea King or Super Puma) making continuous flights every 10 min for approximately 48 h with the exception of a few hours where there were no flights. During these flights it was reported that some penguins occupying nests with eggs/chicks left their nests returning some seconds latter (Acero et al. 1991). The authors also observed that it was the first flight which had the most important effect on the breeding groups with the noise of the helicopters being the main cause of disturbance. However, it was also noted that there was no skua predation at Esperanza Bay which differs with that reported for other colonies (Acero et al. 1991).
Tourist ships arriving at Esperanza Station usually visit the facilities of the base and the breeding groups in close proximity to them which are located in the HLD area. The mean number of tourists who visited Esperanza Station during the study period per season was 1,273 (IAATO, http://www.iaato.org/tourism_stats.html). During the 10 years of the study, Esperanza Station was ranked among the 30 most popular places visited in Antarctica when sorted by the number of tourists that landed (IAATO). Finally we were not able to quantify the level of disturbance generated by the personnel of the base. While the smallest distance between a station facility and the nearest breeding group in the HLD is 39 m with the farthest being 199 m, the distances from the breeding groups controlled in the LLD area are between 0.7 and 1.3 km. It is therefore expected that the level of potential disturbance in both areas will differ significantly.
Size of the breeding groups
The study was carried out on 26 breeding groups in the LLD area and 63 breeding groups in the HLD area. The size of the breeding groups in the LLD area was more homogeneous than in the HLD area. Mean size at the LLD area was 182 ± 108 (mean ± SD) breeding pairs (range 80–509), while in the HLD area it was 139 ± 173 (mean ± SD) breeding pairs (range 9–1157), 1 week after peak egg laying in 1995/1996. The difference in the size of the groups selected was due to the fact that while all the breeding groups were counted in the clearly defined HLD area (see Fig. 1), the groups at the LLD area were chosen for control purposes and are only a part of the whole population of this area.
Breeding population size and chicks creched
In 26 breeding groups of the LLD area and in 63 breeding groups of the HLD area, penguins occupying nests with eggs and chicks creched were counted on two occasions during the season as follows: 1 week after peak egg-laying (14–20 November) and when at least two-thirds of the chicks were in crèches (2–12 January).
Three separate counts were made for each breeding group according to standard CCAMLR Ecosystem Monitoring Program Methods (CCAMLR 2003) and the average values were recorded.
Chicks produced per breeding pair
When the first egg of Adélie penguins was observed in the colony, 200 nests were marked (one hundred in each area). Nests were marked following a transect across ten breeding groups in each area and followed every 5 days until chicks hatched and every 2 days until chicks entered a creche. At each sampling date the numbers of abandoned nests, eggs/chicks and chicks in creches were registered according to CCAMLR Ecosystem Monitoring Program Methods (CCAMLR 2003).
The measures of reproductive success used were as follows: the number of eggs laid per breeding pair, the number of chicks hatched per egg laid and the number of chicks surviving to creche per egg laid.
Statistical analyses
Comparative analyses of the breeding success of Adélie penguins between low and high levels of disturbance groups were done using chi-square tests after Yates’ correction.
Results
Breeding population size
Both areas showed very similar trends in their breeding population sizes (Table 1; Fig. 2). At the LLD area, the breeding population decreased from 4,744 to 2,968 pairs in 26 breeding groups, which represented a decrease of 37.4% over the 10-year study period. In four of the seven seasons (1996/1997, 1997/1998, 1998/1999 and 2000/2001), the numbers of breeding pairs were higher than in the 1995/1996 season (Table 1; Fig. 2).
Trends in breeding population size (nests with eggs) of Adélie penguins breeding at low levels of human disturbance (LLD) area (n = 26) and at high levels of human disturbance (HLD) area (n = 63) expressed as a percentage of nesting pairs present during the 1995/1996 season. 1995 refers to the 1995/1996 season and so forth. Data are given for the 1995/1996–2004/2005 period at Esperanza Bay, Antarctic Peninsula
The breeding population in the HLD area decreased from 8,753 to 5,378 pairs in 63 breeding groups, which represented a decrease of 38.6% (Table 1; Fig. 2). The numbers of breeding pairs were higher than during 1995/1996 for two of the six seasons (1996/1997 and 2000/2001) in the HLD area (breeding pairs were not counted during the 1998/1999 season). Although, to avoid biases in our results, comparisons between areas were made on the basis of the original groups marked during 1995/1996, four new breeding groups were identified in the HLD area in 2000/2001 which totalled less than 100 breeding pairs in that and the following seasons. If these new breeding groups were considered, the decrease in the breeding population from 1995/1996 to 2004/2005 in the HLD area would represent 37.7% instead of the 38.6% reported.
Chicks in creches
The total number of chicks in creches showed a similar variation in both of the areas studied (Fig. 3a). The number of chicks creched was higher in the seven seasons in relation to the number recorded in 1995/1996 in the LLD area. The highest number of chicks was counted in 2000/2001 (7,568 chicks, representing an increase of 98%) and the lowest in 2004 (4,065 chicks, representing an increase of 6.7%). In the HLD area the number of chicks in creches was higher in five of the seven seasons in relation to the counts carried out in 1995/1996. The highest number was found in 1998/1999 (13,008 chicks, representing an increase of 86%) while the lowest number was recorded in 2001/2002 (6,558 chicks, representing a decrease of 6%). The inter-annual increases or decreases in the numbers of chicks counted were similar at both sites (Fig. 3b).
a Number of chicks counted at the low levels of human disturbance (LLD) and high levels of human disturbance (HLD) areas. b Percent change in the total number of chicks creched at Esperanza Bay at the LLD and HLD areas. The magnitude and direction of changes (increasing or decreasing) is calculated relative to the preceding year’s censuses. If more than a year elapsed between censuses, the resultant change was divided by the number of years since the previous count to give an average annual change over the intervening period. c Chicks creched per nest marked in LLD (n = 100) and HLD areas (n = 100). 1995 refers to the 1995/1996 season and so forth. Data are given for the 1995/1996–2004/2005 period at Esperanza Bay, Antarctic Peninsula
Chicks produced per breeding pairs
Information on breeding success is summarised in Table 2 and Fig. 3c. The proportion of breeding pairs laying 0, 1 or 2 eggs differed between areas for the 1995/1996 season (χ 2 = 6.13, P < 0.05). There were no significant differences in the proportion of breeding pairs laying 0, 1 or 2 eggs between the nests marked in the HLD and LLD areas for any of the other seasons compared (chi-square, P > 0.05 for every season).
The proportion of chicks hatched per egg laid was significantly higher in the HLD area than in the LLD area during 1996/1997 (χ2 = 4.77, P < 0.05) and 1997/1998 (χ 2 = 5.24, P < 0.05) but did not differ in the other seasons compared (chi-square, P > 0.05 for 1995/1996, 1998/1999, 2000/2001, 2001/2002, 2002/2003 and 2004/2005).
Overall reproductive success (the number of chicks creched per egg laid) was significantly lower in the LLD area than in the HLD area during 1996/1997 (χ 2 = 8.32, P < 0.05) but did not differ in any of the other seasons compared (chi-square, P > 0.05 for 1995/1996, 1997/1998, 1998/1999, 2000/2001, 2001/2002, 2002/2003 and 2004/2005).
Discussion
Breeding population size
One of the possible adverse effects of human disturbance on the breeding colonies is its potential effect on breeding population size. It is known that late during the breeding season, prebreeders are present and prospecting areas on the periphery of the colonies for future reproduction (Sladen 1958). The experience gained by these young prebreeding birds during the reoccupation phase in a given year influences their subsequent choice of breeding site (Ainley et al. 1983). Moreover, it has been shown in many birds species that previous success at a breeding site is linked to the probability of their returning to that breeding site (Greenwood and Harvey 1982). Both the prebreeders’ and/or the breeders’ subsequent choice of a breeding location could be influenced by human disturbance by dissuading prebreeders or by affecting the breeding success and consequently the future choice of breeding site by breeding birds. As a result, breeding population trends could differ in areas with different levels of human activity because of the differences in the recruitment rate to the population and/or changes in the distribution of breeding pairs. Woehler et al. (1991) reported that the Adélie penguin population at Shirley Island shifted from the eastern end of the Island, near Casey Station, to the western end and related the shift observed to differences in the levels of human disturbance. Moreover, the disturbance associated with human visitors was suggested to be responsible for the lack of an increase in the breeding population at Shirley Island near Casey Station (Woehler et al. 1994). On the other hand, there was no evidence that a high frequency of tourist visits to Gentoo penguin (Pygoscelis papua) colonies at Port Lockroy for over a decade had adverse effects on their population trends (Cobley and Shears 1999; Cobley et al. 2000). Moreover, Micol and Jouventin (2001) did not find evidence for adverse effects on the population trends of Adélie penguins at Terre Adélie attributable to human impact.
To determine population trends in penguins, a long-term study (decadal and longer) is necessary, since their populations exhibit important inter-annual fluctuations, which also implies that spot censuses could give misleading impressions of the actual status of the population (Croxall et al. 1988). Over the 10-year period of our study the breeding population size of Adélie penguins at Esperanza/Hope Bay exhibited inter-annual fluctuations between 7.1 and 29.0% in the LLD area and between 2.8 and 23.8% in the HLD area (Table 1). The population has now decreased by a very similar proportion at both sites (37.4 and 38.6% at the LLD and HLD areas, respectively). There were also similar overall breeding population trends through the study period in both areas (Fig. 2), all of which suggests that the changes observed occurred independently of human disturbance and that these trends were mainly driven by environmental factors probably acting on the availability of food resources during winter. In the same period of our study, another Adélie breeding rookery located in the Antarctic Peninsula region showed a constant decline in its breeding population trend (Santos et al. 2004). This population is located on Isla 25 de Mayo (King George Island) within the Antarctic Specially Protected Area No. 132 and can be visited only under permit. This breeding population has therefore been free of human disturbance and decreased by 69% during the 1995–2004 period (A.R. Carlini and N.R. Coria, unpublished data). Even though the two colonies located in the Antarctic Peninsula region were subjected to different local environmental conditions, the data collected seem to reinforce the impression that the population trends observed in both areas at Esperanza Bay are mainly determined by environmental factors rather than by the effects of human disturbance. Our results are similar to those of Fraser and Patterson (1997), who showed that the breeding populations of Adélie penguins declined in both tourist visited and unvisited colonies in a study carried out over a 20-year period at Litchfield and Tongerson Islands, Antarctic Peninsula.
Chicks in creches
It has been suggested that the size of the breeding groups could affect the breeding success (Oelke 1975). The lower breeding success of smaller colonies in comparison with bigger ones is usually explained by the fact that smaller breeding groups have a higher proportion of peripheral nests, which are occupied by younger and less experienced birds (Spurr 1973). The size of the breeding groups used to count total chicks in creches in both areas was different in our study (see Methods). Breeding groups in the HLD area were on average smaller than in the LLD area and included 13 small breeding groups (less than 30 breeding pairs). Thus if there had been a bias in the total number of chicks counted owing to different group sizes, a decrease in the overall breeding performance measured in the HLD area would have been expected. The general pattern as well as the inter-annual variation in the total number of chicks in creches counted in both areas was very similar (Fig. 3a, b). The total number of chicks varied enormously during the study period but it followed a very similar trend in both areas, which suggests that environmental influences on the penguin population at Esperanza Bay currently exert greater effects than human disturbance.
Chicks produced by breeding pairs
The breeding success (chicks produced per breeding pairs) of Adélie penguins was also very similar at both sites (Table 2; Fig. 3c) and is in accordance with the results reported in other studies. We measured breeding success by checking nests during breeding, which itself causes some disturbance. Giese (1996) found at Vestfold Hills that nest checking for scientific purposes causes a significant reduction in hatching success and chick survival in smaller colonies (less than 44 nests) when compared with control colonies, but that the difference was not significant in larger colonies (mean 70 nests). Since in our study the same protocol was applied to both sites every year, possible reductions in breeding success caused by nest checking are expected to have been similar at the LLD and HLD sites and the results therefore comparable. Additionally, the breeding groups used for marking nests have more than 70 nests, which according to the findings by Giese (1996) minimises the impact of nest checking. A possible effect of human disturbance on breeding performance is the lowering of penguins’ breeding success by increasing the chances of predators to procure food. At Vestfold Hills, predation by south polar skuas (Catharacta maccormicki) was the most likely cause of egg loss in breeding groups exposed to human disturbance (Giese 1996). At Esperanza Bay, there were less than ten breeding pairs of brown skuas (C. lonnbergi) occupying territories throughout the study period, and additionally 100 nonbreeding birds were present (N.R. Coria unpublished data). Thus, considering an estimated colony size of 120,000 breeding pairs, there were around 1,000 penguin pairs per skua, which means that predator pressure was much lower than at Vestfold Hills, where a ratio of 107 penguin pairs per skua was calculated (Giese 1996). Although this fact, per se, does not explain the lack of differences in breeding success between disturbed and undisturbed colonies in our study, it could help to understand that even though the overall response of penguins to human disturbance could be similar among different rockeries, it might not have any detectable effects on breeding success in the absence of additional factors, such as important levels of skua predation. Thus, the levels of human disturbance in the HLD area at Esperanza Bay are likely to have resulted, in most cases, only in an increased awareness, rarely affecting the birds by reducing their reproductive performance. Since Hope Bay has a long history of human presence, habituation and tolerance in penguins breeding at LLD area could also be a possible explanation of our results. Several studies have shown differences in birds response to human in areas of high and low human activity (Yorio and Boersma 1992; Nisbet 2000). Similarly to our study, no differences in breeding performance between disturbed and undisturbed colonies of Adélie penguins were found by Patterson and Fraser (1998) in the Antarctic Peninsula region, while Cobley and Shears (1999) reported that breeding performance of Gentoo penguins (P. papua) did not differ between disturbed and control colonies at Port Lockroy, one of the most visited tourist sites on the Antarctic Peninsula. Moreover, in a recent study carried out at Macquarie Island, Holmes et al. (2006) found no relationship between pedestrian activity and breeding success and reported a better breeding performance by Gentoo penguins in on-station areas in comparison to off-station breeding groups.
Rather unexpectedly, the proportion of chicks hatched per egg laid during 1996/1997 and 1997/1998 and the overall reproductive success (chicks creched per egg laid) during 1996/1997 were significantly higher in the HLD area (Table 2). Given the nearness of Esperanza Station, it could be reasonably expected that breeding success in this area would be lower if human disturbance had a negative effect on reproductive performance, or similar if the effects of human activity were negligible. Since an identical protocol was used over the study period, the proportion of peripheral and central nests marked was also similar in both areas throughout the study period. Other factors that are known to affect breeding success, such as age or previous breeding experience (Ainley et al. 1983), could not be directly assessed in our study but they are also expected to have been similar in both areas, since nests were randomly selected within each breeding group. The reason for the above results is unclear. However, since they are the reverse of what would be expected if human activity adversely affected the Adélie penguin population at the HLD area, the results reinforce the view that breeding performance was not affected by human activity. In conclusion, our data suggest that currently the potentially adverse effects of scientific research and tourism could have a minor effect as compared with the effects of changes in environmental variables at Esperanza Bay.
References
Acero JM, Agraz JL, Aguirre CA (1991) Análisis del Impacto Ambiental en Bahía Esperanza, Península Antártica. Instituto Antártico Argentino. Special Issue, pp 1–44
Ainley DG, Leresche RE, Sladen WJL (1983) Breeding ecology of the Adélie penguin. University of California Press, Los Angeles, 227 pp
CCAMLR (2003) Standard methods for monitoring parameters of predators species. CCAMLR Ecosystem Monitoring Program. CCAMLR, Hobart
Carney KM, Sydeman WJ (1999) A review of human disturbance effects on nesting colonial waterbirds. Waterbirds 22:68–79
Cobley ND, Shears JR (1999) Breeding performance of gentoo penguins (Pygoscelis papua) at a colony exposed to high levels of human disturbance. Polar Biol 21:355–360
Cobley ND, Shears JR, Downie RH (2000) The impact of tourists on Gentoo Penguins at Port Lockroy, Antarctic Peninsula. In: Davison W, Williams CH, Broady P (eds) Antarctic ecosystem: models for wider ecological understanding. Caxton Press, New Zealand, pp 319–323
Croxall JP, McCann TS, Prince PA, Rothery P (1988) Reproductive performance of seabirds and seals at South Georgia and Signy Island, South Orkney Islands, 1976–1987: implications for Southern Ocean monitoring studies. In: Sahrhage D (ed) Antarctic Ocean and resource variability. Springer, Berlin, pp 261–285
Culik B, Adelung D, Wolkes AJ (1990) The effect of disturbance on the heart rate and behaviour of Adélie penguins (Pygoscelis adeliae) during the breeding season. In: Kerry KR, Hempel G (eds) Antarctic Ecosystem ecological changes and conservation. Springer, Berlin, pp 177–182
Enzenbacher DJ (1992) Tourists in Antarctica: numbers and trends. Polar Rec 28(164):17–22
Enzenbacher DJ (1994) Antarctic tourism: an overview of 1992/93 season activities, recent developments and emerging issues. Polar Rec 30:105–116
Fraser WR, Patterson DL (1997) Human disturbance and long term changes in Adélie penguin populations: a natural experiment at Palmer Station, Antarctic Peninsula. In: Battaglia B, Valencia J, Walton DWH (eds) Antarctic communities: species structure and survival. Proceedings of the VI SCAR Biology Symposium. University Press, Cambridge, pp 445–452
Giese M (1996) Effects of human activity on Adélie penguin Pygoscelis adeliae breeding success. Biol Conserv 75:157–164
Giese M (1998) Guidelines for people approaching breeding groups of Adélie penguins (Pygoscelis adeliae). Polar Rec 34(191):287–292
Giese M, Riddle M (1999) Disturbance of emperor penguin Aptenodytes forsteri chicks by helicopters. Polar Biol 22:366–371
Greenwood PJ, Harvey PH (1982) The natal and breeding dispersal in birds and mammals. Anim Behav 28:1140–1162
Holmes ND, Giese M, Achurch H, Robinson S, Kriwoken LK (2006) Behaviour and breeding success of gentoo penguins Pygoscelis papua in areas of low and high human activity. Polar Biol 29:399–412
Micol T, Jouventin P (2001) Long-term population trends in seven Antarctic seabirds at Pointe Géologie. (Terre Adélie) Human impact compared with environmental change. Polar Biol 24:175–185
Myrcha A, Tatur A, Del Valle R (1987) Numbers of Adélie Penguins breeding at Hope Bay and Seymour Island rookeries (West Antarctica) in 1985. Pol Polar Res 8:411–422
Nisbet IC (2000) Disturbance, habituation, and management of waterbird colonies. Waterbirds 23:312–332
Oelke H (1975) Breeding behaviour and success in a colony of Adélie penguins Pygoscelis adeliae, at Cape Crozier, Antarctica. In: Stonehouse B (ed) The Biology of penguins. Macmillan Press, London, pp 363–395
Patterson DL, Fraser WR (1998) Long-term changes in Adélie penguin populations: human impact or environmental variability? NZ Nat Sci (supplement) 23:147
Patterson DL, Easter-Pilcher AL, Fraser WR (2003) The effects of human activity and environmental variability on long-term changes in Adélie Penguin populations at Palmer Station, Antarctica. In: Huiskes AHL, Gieskes WWC, Rozema J, Schorno RML, van der Vies SM and Wolff WJ (eds) Antarctic biology in a global context. Proceedings of VIIIth SCAR international biology symposium, Backhuys Publishers, Leiden, pp 301–307
Santos M, Carlini AR, Coria NR, Negrete J (2004) Decrease in a population of Adelia penguins at King George Island, Antarctica. In: Proceedings of the V international penguin conference. Ushuaia, Argentina. 5–10 Septiembre, p 70
Sladen JWL (1958) The Pygoscelid penguins. I Methods of study. II The Adèlie penguin. Falkland Islands dependencies survey. Scientific reports 17:97
Spurr EB (1973) Breeding of Adélie penguin (Pygoscelis adeliae) at Cape Bird. Ibis 117:324–338
Wilson RP, Coria NR, Spairani HJ, Culik BM (1989) Human-induced behaviour in Adélie penguins (Pygoscelis adeliae). Polar Biol 10:77–80
Wilson KL, Taylor RH, Barton KJ (1990) The impact of man on Adélie penguins at Cape Hallet, Antarctica. In: Kerry KR, Hempel G (eds) Antarctic ecosystems. Ecological change and conservation. Springer, Berlin, pp 183–190
Wilson RP, Culik BM, Danefield R, Adelung D (1991) People in Antarctica: how much do Adélies care? Polar Biol 11:363–370
Woehler EJ (1993) The distribution and abundance of Antarctic and Subantarctic penguins. SCAR, Cambridge
Woehler EJ, Slip DJ, Robertson LM, Fullagar PJ, Burton HR (1991) The distribution, abundance and status of Adélie penguins Pygoscelis adeliae at the Windmill Island, Wilkes Land, Antarctica. Mar Ornithol 19:1–19
Woehler EJ, Penney RL, Creet SM, Burton HR (1994) Impacts of human visitors on breeding success and long-term population trends in Adélie penguins at Casey, Antarctica. Polar Biol 14:269–274
Yorio P, Boersma PD (1992) The effects of human disturbance on Magellanic penguin Spheniscus magellanicus behaviour and breeding success. Bird Conserv Int 2:161–173
Acknowledgments
We would like to express our gratitude to M. Perez Cometo, D. Gaona, G. Blanco, C. MacDermock and D. Archuby for field assistance. We thank the members of Esperanza Station for their logistic support. We also thank Dr. EJ Woehler and two anonymous reviewers for helpful comments to improve the manuscript. This work was founded by the Dirección Nacional del Antártico.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Carlini, A.R., Coria, N.R., Santos, M.M. et al. Breeding success and population trends in Adélie penguins in areas with low and high levels of human disturbance. Polar Biol 30, 917–924 (2007). https://doi.org/10.1007/s00300-006-0251-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-006-0251-1