Abstract
Global warming, and its consequences, constitute one of the main stressors for organisms worldwide, affecting different factors such as the geographic distribution and the abundance of parasites, which in turn can affect the immune system of their hosts, and vice versa. Therefore, it is important to have baseline information on immune parameters of organisms in order to make future comparisons within this changing ecological context. Here, we report on the leukocyte counts of the Antarctic pack ice seals, the crabeater (Lobodon carcinophaga), Weddell (Leptonychotes weddellii) and leopard (Hydrurga leptonyx) seals, sampled off the western Antarctic Peninsula. We captured and sampled seals in the pack ice off the Danco Coast, Antarctica in the austral summers, January to March, of 2015 and 2016. The leukocyte counts, along with the counts of each different leukocyte (e.g., basophil, neutrophil, eosinophil, lymphocyte and monocyte), were made from blood smears viewed under the light microscope. As a potential stress indicator, we examined whether seals with lice, so presumably under greater physiological stress, had changes in leukocyte counts, including higher ratios of neutrophil-to-lymphocytes (N/L ratio). Leukocyte counts were different among the seal species. While crabeater and Weddell seals had higher neutrophil counts, followed by lymphocyte counts, leopard seals had the reverse pattern. Basophil, eosinophil, and lymphocyte counts were higher in the leopard seal, while the N/L ratio, as well as the neutrophil counts, were higher for the crabeater seal. We show, for the Weddell seal, that the animals with lice were more likely to have higher N/L ratios. This suggests that future research into the potential of the N/L index as a stress indicator, that incorporates additional stress parameters including cortisol concentrations, oxidative damage, as well as other measures of immune function, is warranted for the pack ice seals. Our results are a first step towards establishing leukocyte count baselines for the Antarctic pack ice seals off the western Antarctic Peninsula.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The study of immune function has acquired greater importance for understanding the life histories of organisms (Norris and Evans 2000), not only because it is the primary defence against pathogens in animals (Zuk and Stoehr 2002), but also because animals adjust their investment in the immune system depending on energetic resources available (Norris and Evans 2000). Different factors and environmental conditions can cause an animal to reallocate resources to other systems, and so away from the immune system (Davis et al. 2008); for example, when there are changes in body condition (Castellini et al. 1993; Brock et al. 2013), geography (Cammen et al. 2011), breeding status (Deerenberg et al. 1997), or the presence of pathogens, parasites (Mos et al. 2006), or contaminants (de Swart et al. 1996).
Cellular immunity, although only one component of an organisms’ immune system, is the first line of immune defence. Cellular immunity is an easily monitored immune parameter, assessed by the number of white blood cells, also called leukocytes (Roitt et al. 2001). Leukocytes can be divided in to neutrophils, eosinophils, basophils, and monocytes. Leukocytes function through phagocytosis (i.e., engulf and ingest) pathogens (Roitt et al. 2001) but the neutrophils are the primary phagocytic leukocytes. Neutrophils are of the most abundant circulating leukocyte and play a fundamental role in the innate immune response (Nathan 2006). Eosinophils are involved in the defence against parasites, particularly gastrointestinal parasites, and allergic reactions. Basophils are associated with the defence against parasitic infections and allergens (Yamanishi et al. 2017). Monocytes are the largest of the leukocytes and play the role of phagocytosis of foreign organisms (Roitt et al. 2001).
Lymphocytes belong to the acquired immunity and tend to be pathogen-specific (Roitt et al. 2001). Lymphocytes are involved in a variety of immunological functions such as the production of immunoglobulins and modulation of immune defence (Campbell 1995). Although leukocytes make up the first line of the immune defence against infections or diseases in vertebrates, they are also produced in response to several stress-induced conditions (Davis et al. 2008). In fact, it has been proposed that a change in leukocyte profiles, specifically the ratio of neutrophils-to-lymphocytes (N/L), could be used as an index to identify that an individual is experiencing greater physiological stress (Davis et al. 2008). This is because during periods of stress, such as poor feeding conditions, severe climate change or other novel situations, an individual can reallocate resources to the immune system leading to increased numbers of neutrophils relative to the number of lymphocytes (e.g., higher N/L ratio). Therefore, N/L ratios may be an early-warning signal to identify vertebrate populations that are under stress.
Currently, global warming and its consequences for environmental change constitute one of the main stressors for organisms worldwide. These environmental changes may affect the geographical distribution, abundance, and virulence of parasites and diseases, which in turn can have an effect on the immune system of organisms (Harvell et al. 2002). Therefore, it is important to have baseline information on immune parameters of organisms in order to make future comparisons in this changing ecological context.
The western Antarctic Peninsula (wAP) has been significantly affected by climatic changes illustrated by a greater frequency of strong winds that, since the late 1990s, have resulted in cyclonic conditions north of the Peninsula (Turner et al. 2016). These changes have amplified the warming of the sea surface of the wAP region over the last 60–100 years (Turner et al. 2016) which has resulted in the loss of sea ice habitat (Stammerjohn et al. 2008), critical habitat for the Antarctic fauna as well as important fishing grounds (Forcada et al. 2012; Meade et al. 2015).
Pinnipeds, as top predators and sea-ice dependent species, have been particularly impacted (Siniff et al. 2008). There are four Antarctic pinnipeds that are directly associated with the sea ice, and consequently, vulnerable to the warming in this region (Siniff et al. 2008; Forcada et al. 2012; Meade et al. 2015): the crabeater seal (Lobodon carcinophaga); Weddell seal (Leptonychotes wedellii); leopard seal (Hydrurga leptonyx); and the Ross seal (Ommatophoca rossii). The loss of sea ice reduces the suitable habitat for breeding, resting and/or protection from predators, for the pack-ice seals (Siniff et al. 2008; Costa et al. 2010; Forcada et al. 2012; Rogers et al. 2013; Meade et al. 2015), increasing also the distance to foraging grounds (Burns et al. 2004; Southwell et al. 2005, 2012; Forcada et al. 2012). The magnitude of impact upon each of the pack ice seal species is likely to be different (Siniff et al. 2008). For instance, the species that depend on sea ice for most, or at least critical periods of their life history, and/or those that are krill-specialist, should be more affected than species with fewer habitat requirements and/or a wider prey spectrum (Siniff et al. 2008).
It has been proposed that the crabeater seal would be the species most impacted by climate change-related alterations. Crabeater seals are ice-dependent and their diet is almost exclusively composed of Antarctic krill. Weddell seals are also an ice-obligated species, but its diet includes fishes and squids (Siniff et al. 2008; Daneri et al. 2012, 2018). Finally, leopard seal might be less directly influenced by these changes since they have the ability to adapt and use different ice floe types and that they exploit a wider range of prey, from krill, cephalopods, fish, seabirds, seals and fur seals (Siniff and Stone 1985; Rogers and Bryden 1995; Hall-Aspland and Rogers 2004; Krause et al. 2015).
The wAP pack ice seals have the highest density populations within the circumpolar Antarctic sea ice (Forcada et al. 2012; Southwell et al. 2012; Rogers et al. 2013). Not only is the wAP region experiencing great environmental changes, this area also supports an important fishery (Forcada et al. 2012). Fishing has the potential to cause additional stress on the prey base of the wAP pack ice seals (Forcada et al. 2012). Thus, the need to monitor the health of the wAP seal populations would be valuable. Different parameters have been examined, including monitoring serum proteins (Gray et al. 2005), as well as haematological parameters (Gray et al. 2009; Yochem et al. 2009), such as the leukocyte counts (Davis et al. 2008; Gray et al. 2009; Yochem et al. 2009). As leukocytes offer protection against a variety of stressors they could be useful as a proxy to monitor physiological stress (Maceda-Veiga et al. 2015).
We report here on the leukocyte counts of three sympatric Antarctic pinnipeds, the Weddell, leopard and crabeater seal. We examine whether seal sex, body condition, moult state or lice loads influenced basophil, neutrophil, eosinophil, lymphocyte, and monocyte counts.
Materials and methods
Study area
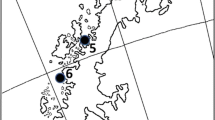
The study area, the sea ice off the Danco Coast, western Antarctic Peninsula, extended from Cape Herschell to Spring Point (Fig. 1) and included the Antarctic Specially Protected Area (ASPA) No. 134 “Cierva Point” (64°09′23″S, 60°57′17″W). The study was conducted over two austral summers, from January to March of 2015 and 2016. Seals were accessed on ice floes in the pack ice off the coast, whereas Weddell seals were also captured on beaches.
Sample collection
A total of 65 seals (27 Weddell, 11 leopard and 27 crabeater seals) were sedated for sample collection (Table 1). The animals were chemically sedated using Tiletamine/Zolazepam 1:1 (1.3 mg/kg) following procedures outlined in Higgins et al. (2002). Twenty (20) ml of blood was collected from the extradural intra-vertebral vein in the lower lumbar region using an 18 G × 31/2″ spinal needle following the method described by Geraci and Smith (1975). The sex, cohort (juvenile or adult), moult state (moult complete or not) were determined by visual inspection. Standard length, measured as the straight-line from snout-to-tail, and axillary girth, measured around the seal behind the fore flippers, were measured to the nearest cm while the seal lay in ventral recumbency. Seals were weighed using digital scales while held suspended, in a sling, from a metal tripod. To estimate lice load, we used the standard technique developed for procedures where only a brief-time of manipulation was available (e.g., here constrained by sedation window), such that all lice were collected, using forceps to be later counted, from the hind flippers, along with a visual inspection of the entire body (Thompson et al. 1998).
Sample processing
Thin blood smears were prepared in the field at time of sampling. A drop of fresh blood, directly from the syringe, was placed and spread on the slide. It was air-dried and fixed for 3 min with 93% ethanol. Blood smears were stained in the laboratory within 5 h using Tinción 15 (Biopur S.R.L., Rosario, Argentina). Leukocyte counts were performed using the light microscope, where monolayer fields with similar densities of erythrocytes were scanned (~ 200 erythrocytes per field) (Campbell 1995; Lobato et al. 2005). To estimate relative leukocyte counts, per 10,000 erythrocytes, we followed the procedure described by Lobato et al. (2005), where we counted all erythrocytes in a single microscopic visual field and multiplied this by the number of the microscopic visual fields (~ 50) scanned, until 100 leukocytes had been reached. The proportion of each of the leukocytes was calculated from a subsample of 100 leukocytes in 1000 × (oil immersion) and classified into basophils, neutrophils, eosinophils, lymphocytes, and monocytes (Campbell 1995). The neutrophil-to-lymphocyte ratio (N/L) was calculated as the ratio of neutrophil to lymphocyte counts. To assess the repeatability of the leukocyte counts, eight smears were selected randomly for each of the three seal species. For each species three leukocyte counts were made by the same observer, for each of the eight blood smears. Repeatability values were calculated following Lessells and Boag (1987) for leukocyte, neutrophil, eosinophil, lymphocyte and monocyte counts, and values fell between 74 and 98%. Repeatability values were not calculated for the basophil counts because the data was zero-inflated, i.e., there were too many zeros in the matrix.
Statistical analysis
Data were statistically described and compared among the three species using the nonparametric Kruskal–Wallis H test and nonparametric multiple comparisons (Sokal and Rohlf 2012). We used Spearman rank correlations to test whether leukocyte counts were related to a seals’ body condition. Body condition was calculated as the residual of the regression between body mass (kg) and standard length (cm), where positive values indicate good body condition (see details in Arnould 1995; van den Hoff et al. 2006).
To establish whether leukocyte counts were influenced by various factors we performed a series of multiple linear regressions with backward stepwise selection to identify model of best fit. We retained factors significant at p < 0.05 and models at r2 > 0.1. For the Weddell and crabeater seals, the following factors were included: sex, age (juvenile/adult), moult (moulting/not moulting), body condition and presence of lice (present or absent). However, due to sample size constraints, only the factors body condition, moult and lice burden were included for the leopard seal (Table 1). All analyses were performed using STATISTICA version 7.0 and significance is reported using an alpha of 0.05.
Results
Neutrophils and lymphocytes were the most abundant leukocytes for all three species (Table 2). For Weddell seals, neutrophils, and then lymphocytes, were the most abundant leukocytes (Table 2). The presence of lice was a positive predictor of lymphocyte counts per 10,000 erythrocytes, such that Weddell seals with lice (Antarctophthirus carlinii) had higher lymphocyte counts (Table 3). There was no relationship between body condition and the presence of lice (Table 3). Neither the seals’ sex, age, body condition or stage of moult had an influence on leukocyte counts per 10,000 erythrocytes (Table 3).
For leopard seals, lymphocytes and then neutrophils were the most abundant leukocyte. The presence of lice was a positive predictor of neutrophil and basophil counts, seals with lice had higher basophil and neutrophil counts per 10,000 erythrocytes (Table 3). Leopard seal body condition was positively correlated with N/L ratios (Spearman rank correlation, r = 0.41, n = 11, p = 0.0050). Leopard seals had the highest basophil (Kruskal–Wallis test, H(2,65) = 9.8, p = 0.0210) and eosinophil (Kruskal–Wallis test, H(2,65) = 8.3, p = 0.0364) counts per 10,000 erythrocytes than the other seal species.
For crabeater seals, neutrophils, and then lymphocytes, were the most abundant leukocytes (Table 2). All crabeater leukocyte counts were influenced by the year sampled (Table 3). However, monocytes were influenced by both year sampled and negatively by the seals’ body condition such that seals with lower body condition had higher monocyte counts per 10,000 erythrocytes (Table 3). The body condition of crabeater seals was positively correlated with basophil counts per 10,000 erythrocytes (Spearman rank correlation, r = 0.55, n = 23, p = 0.0047) and negatively with the presence of lice. The seals sex, or state of moult had no influence on leukocyte counts per 10,000 erythrocytes (Table 3). Of the three species, the crabeater seal had the highest neutrophil counts per 10,000 erythrocytes (Kruskal–Wallis test, H(2,65) = 9.7, p = 0.0390) and the highest N/L ratios (Kruskal–Wallis test, H(2,65) = 7.6, p = 0.0027).
Discussion
The use of haematological parameters, specifically leukocyte profiles, as an alternate method for physiological stress assessment has proven a useful tool in conservation physiology studies (Davis et al. 2008; D’Amico et al. 2016a, b). We report here on the leukocyte counts for three Antarctic pack ice seals: the Weddell seal, leopard seal and crabeater seal, during the austral summer. We examined specifically whether the presence of lice was a potential stressor and influenced haematological parameters.
Weddell seal
We found that Weddell seals with lice had higher lymphocyte counts, as well as higher neutrophil-to-lymphocyte ratios. There was no relationship between the presence of lice and the number of eosinophils or basophils, leukocytes that are typically involved in parasite defence, particularly the eosinophils (Yamanishi et al. 2017). However, as here the factor ‘lice’ was considered as binary, either present or absent, rather than as the number of lice, the ‘lice load’, we may have limited our ability to determine if the lice burden impacted eosinophil or basophil counts. However, the finding that, for the Weddell seal, there was a link between the presence of lice and higher neutrophil-to-lymphocyte ratios is encouraging. An increase in the ratio of neutrophils-to-lymphocytes may be an indirect stress measure as it can be proportional to glucocorticoid release (Davis et al. 2008). Stress induces a reduction in the number of lymphocytes circulating in the blood stream; this is because high glucocorticoid levels affect a redistribution of lymphocytes from the blood to the tissues where they are needed (Dhabhar 2002). Conversely, increased glucocorticoids stimulate neutrophil redistribution from bone marrow into the blood stream, resulting in an increase in the neutrophil count (Bishop et al. 1968). The population of Weddell seals on the western Antarctic Peninsula that were studied here (~ 64°09′S) are within the northerly range for this species. We found that they had higher neutrophil counts, followed by lymphocyte counts, a pattern shown in southern Weddell seals at McMurdo Sound (~ 77°50′S) (Yochem et al. 2009). Higher proportions of neutrophils followed by lymphocytes are also reported for captive northern phocid seals, the harp seal (Pagophilus groenlandicus), harbour seal (Phoca vitulina), and ringed seal (Pusa hispida) (Engelhardt 1979).
Leopard seal
The leopard seals with lice had higher proportions of basophils, but there was no relationship with eosinophils. Although basophils have been shown to be involved in parasite defence, as mentioned, it is the eosinophils that are usually the most important leukocyte in the host-defence against parasites (Yamanishi et al. 2017). Our study was conducted on animals off the northern wAP, and represents the northern Antarctic range for the leopard seal (64°09′S). An earlier study examined haematological parameters of a more southerly population (Davis Sea, 68°34′S), as well as vagrant animals, considered to be clinically ‘sick’, sampled well north of the Antarctic pack ice (33°51′S) off the Australian coastline (Gray et al. 2005). Haematological parameters can vary between populations, however, as the earlier study used different methodology we cannot compare leukocyte counts directly. The wAP leopard seals had lower proportions of neutrophils (mean = 43.3%). As neutrophil numbers increase with stress (Bishop et al. 1968), it was unsurprising that the wAP seals had lower neutrophil proportions than the ‘sick’ seals (mean = 69%; SD = 55–83%; Gray et al. 2009); however, it was interesting that they also had lower proportions than the southern population (mean = 59.2% SD = 55.6–63.0%; Gray et al. 2009). An increase in stress is linked with lower lymphocyte counts (Bishop et al. 1968) so it was unsurprising that the wAP leopard seals had higher proportions of lymphocytes (mean = 48.3%) compared to the ‘sick’ vagrant leopard seals (mean = 20%; SD = 9.5–31.9%; Gray et al. 2009), but they also had higher proportions than the southern population (mean = 16.6%; SD = 11.3–21.9%; Gray et al. 2009). Overall, the haematological parameters of the wAP leopard seals, the lower proportions of neutrophils, higher proportions of lymphocytes and therefore a lower neutrophil-to-lymphocyte ratio, may suggest that the wAP pack ice environment is more favourable for leopard seals than the Davis Sea. In fact, the pack ice off the wAP supports the highest densities of leopard seals recorded (Forcada et al. 2012), much higher than in the Davis Sea (Rogers and Bryden 1997; Southwell et al. 2008; Rogers et al. 2013). The wAP leopard seals had similar proportions of basophils (mean = 1.9%) to the southern population (mean = 2.12%; SD = 1.42–3.16%; Gray et al. 2009) although the ‘sick’ leopard seals had extremely low proportions of basophils (mean = 0.05%; SD = 0–0.27%; Gray et al. 2009). Yet the wAP leopard seals had lower proportions of eosinophils (mean = 4.2%) than the southern seals (mean = 16.5%; SD = 13.9–19.6%; Gray et al. 2009) which were within a similar range to the ‘sick’ seals (mean = 1.2%; SD = 0–4.6%; Gray et al. 2009).
Crabeater seal
Crabeater seals with lice were in poorer body condition; however, we found no relationship between the presence of lice and eosinophils or basophils, leukocytes typically associated with parasitic infections. The neutrophil-to-lymphocyte ratios of the crabeater seals were higher than the other seal species. All leukocyte counts were influenced by the year that the samples had been collected; however, this may be an artefact of unbalanced sampling as there were 21 samples in 2015 and only five in 2016.
Neutrophil-to-lymphocyte ratios to monitor wildlife health
Monitoring neutrophil-to-lymphocyte ratios could be a valuable approach to monitor wildlife health of populations in remote and/or difficult sites to access, such as in our study here in the Antarctic, where access to the animals and sampling procedures are limited by logistic constraints and climatic conditions. Lice burden may be a potential trait to examine physiological stress on wild pack ice seals. Pinnipeds are typically infested with the sucking lice of the family Echinophthiriidae. Although sucking lice are usually considered harmless, they can cause skin damage inducing the production of phagocytic cells (Allen 1994). Thompson et al. (1998) reported that although there was no difference between the leukocyte counts of lice-parasitized and nonparasitized common seals (Phoca vitulina), they had found a relationship between the haematological parameters of lice-parasitized seals and body condition. Animals in poorer condition had reduced haemoglobin concentration, haematocrit, as well as lower numbers of erythrocytes, suggesting that lice infestation indirectly influenced diving ability (Thompson et al. 1998). Although infestations of the suckling louse, Antarctophthirus microchir, had little direct influence on the haematological parameters of Australian sea lion, Neophoca cinerea, pups, these animals had high endemic hookworm, Uncinaria sanguinis, infections (Marcus et al. 2015). In further studies, increasing the number of seals that had high lice burdens, and quantifying the number of lice would permit us to elucidate possible further links between haematological parameters and seal health. In addition, incorporating stress parameters (e.g., such as cortisol levels, oxidative damage, as well as other measures of immune function), alongside with haematological parameters would be warranted.
Seal sex and moult stage
The absence of significant differences in the haematological parameters with seal sex and moult stage was not unexpected, in fact this supports previous findings reported for pinnipeds, including studies on more southern populations of the leopard seal (Gray et al. 2009) and Weddell seal (Yochem et al. 2009), as well as other phocid seal species: the harp seal (Vallyathan et al. 1969); gray seal, Halichoerus grypus (Hall 1998); and the southern elephant seal, Mirounga leonina (Lane et al. 1972) and northern elephant seal, Mirounga angustirostris (Goldstein et al. 1998).
We report on the haematology parameters of predominantly adult populations of three sympatric Antarctic pack ice seals, the Weddell, leopard and crabeater seal. These results represent circulating leukocyte values (Davis et al. 2008); leukocytes can be stored in the body to be released, or redistributed in response to stressors or infectious agents (Davis et al. 2008). We had access to limited haematological parameters; however, in further research it would ideal to include broader parameters, such as, packed cell volume (PCV), erythrocyte count, haemoglobin concentration, haematocrit, red cell distribution width (RDW), mean platelet volume (MPV) and platelet distribution width (PDW). Here we explore approaches to monitor the health of these ice-dependent seals at the northern range of their distribution, the western Antarctic Peninsula. This region is undergoing unprecedented environmental change; it is vulnerable to ongoing warming, as well as increased fishery and tourism pressure.
References
Allen JR (1994) Host resistance to ectoparasites. Rev Sci Technol 13:1287–1303
Arnould JPY (1995) Indices of body condition and body composition in females Antarctic fur seals (Arctocephalus gazella). Mar Mammal Sci 11:301–313
Bishop CR, Athens JW, Boggs DR, Warner HR, Cartwright GE, Wintrobe MM (1968) Leukokinetic studies: XIII. A non-steady-state kinetic evaluation of the mechanism of cortisone-induced granulocytosis. J Clin Invest 47:249–260
Brock PM, Hall AJ, Goodman SJ, Cruz M, Acevedo-Whitehouse K (2013) Immune activity, body condition and human-associated environmental impacts in a wild marine mammal. PLoS ONE 8(6):e67132
Burns JM, Costa DP, Fedak MA, Hindell MA, Bradshaw CJ, Gales NJ, et al (2004) Winter habitat use and foraging behavior of crabeater seals along the Western Antarctic Peninsula. Deep Sea Res Part 2 Top Stud Oceanogr 51: 2279–2303
Cammen K, Hoffman JI, Knapp LA, Harwood J, Amos W (2011) Geographic variation of the major histocompatibility complex in Eastern Atlantic grey seals (Halichoerus grypus). Mol Ecol 20:740–752
Campbell TW (1995) Avian hematology and cytology. Iowa State University Press, Ames
Castellini MA, Loughlin TR, Williams TM (1993) Blood chemistries and body condition of Steller sea lion pups at Marmot Island, Alaska. Mar Mamm Sci 9:202–208
Costa DP, Hückstädt LA, Crocker DE, McDonald BI, Goebel ME, Fedak MA (2010) Approaches to studying climatic change and its role on the habitat selection of Antarctic pinnipeds. Integr Comp Biol 50:1018–1030
D’Amico VL, Bertellotti M, Benzal J, Coria N, Vidal V, Díaz JI, Barbosa A (2016a) a) Leukocyte counts in different populations of Antarctic Pygoscelid penguins along the Antarctic Peninsula. Polar Biol 39:199–206
D’Amico VL, Coria N, Palacios MG, Barbosa A, Bertellotti M (2016b) b) Physiological differences between two overlapped breeding Antarctic penguins in a global change perspective. Polar Biol 39:57–64
Daneri GA, Carlini AR, Negri A, Allcock AL, Corbalán A (2012) Predation on cephalopods by Weddell seals, Leptonychotes weddellii, at Hope Bay, Antarctic Peninsula. Polar Biol 35:585–592
Daneri GA, Negri A, Coria NR, Negrete J, Libertelli MM, Corbalán A (2018) Fish prey of Weddell seals, Leptonychotes weddellii, at Hope Bay, Antarctic Peninsula, during the late summer. Polar Biol 41:1027–1031
Davis AK, Maney DL, Maerz JC (2008) The use of leukocyte profiles to measure stress in vertebrates: a review for ecologists. Funct Ecol 22:760–777
De Swart RL, Ross PS, Vos JG, Osterhaus AD (1996) Impaired immunity in harbour seals (Phoca vitulina) exposed to bioaccumulated environmental contaminants: review of a long-term feeding study. Environ Health Perspect 104:823–828
Deerenberg C, Arpanius V, Daan S, Bos N (1997) Reproductive effort decreases antibody responsiveness. Proc Royal Soc B 264:1021–1029
Dhabhar FS (2002) A hassle a day may keep the doctor away: stress and the augmentation of immune function. Integr Comp Biol 42:556–564
Engelhardt FR (1979) Haematology and plasma chemistry of captive pinnipeds and cetaceans. Aquat Mamm 7:11–20
Forcada J, Trathan PN, Boveng PL, Boyd IL, Burns JM, Costa DP, Fedak M, Rogers T, Southwell CJ (2012) Responses of Antarctic pack-ice seals to environmental change and increasing krill fishing. Biol Conserv 149:40–50
Geraci JR, Smith TG (1975) Functional hematology of ringed seals (Phoca hispida) in the Canadian Arctic. J Fish Board Can 32:2559–2564
Goldstein T, Johnson SP, Werner LJ, Nolan S, Hilliard BA (1998) Causes of erroneous white blood cell counts and differentials in clinically healthy young northern elephant seals (Mirounga angustirostris). J Zoo Wildl Med 1:408–412
Gray R, Canfield P, Rogers T (2005) Investigation of serum proteins in the leopard seal, Hydrurga leptonyx, in Prydz Bay, Eastern Antarctica and the coast of NSW, Australia. Comp Biochem Physiol B 142:67–78
Gray RB, Rogers TL, Canfield PJ (2009) Health status of the Leopard-seal (Hydrurga leptonyx) in Prydz Bay, Eastern Antarctica. In: Kerry K, Riddle M (eds) Health of Antarctic wildlife: a challenge for science and policy. Springer, Berlin, pp 167–194. https://doi.org/10.1007/978-3-540-93923-8_10
Hall AJ (1998) Blood chemistry and hematology of gray seal (Halichoerus grypus) pups from birth to postweaning. J Zoo Wildl Med 1:401–407
Hall-Aspland SA, Rogers TL (2004) Summer diet of leopard seals, Hydrurga leptonyx, in Prydz Bay, Eastern Antarctica. Polar Biol 27:729–734
Harvell CD, Mitchell CE, Ward JR, Altizar S, Dobson AP, Ostfeld RS, Samuel MD (2002) Climate warming and disease risks for terrestrial and marine biota. Science 296:2158–2162
Higgins DP, Rogers TL, Irvine AD, Hall Aspland SA (2002) Use of midazolam/pethidine and tiletamine/zolazepam combinations for the chemical restraint of leopard seals (Hydrurga leptonyx). Mar Mamm Sci 18:483–499
Krause DJ, Goebel ME, Marshall GJ, Abernathy K (2015) Novel foraging strategies observed in a growing leopard seal (Hydrurga leptonyx) population at Livingston Island. Antarct Penins Anim Biotelemetry 3:24
Lane RA, Morris RJ, Sheedy JW (1972) A haematological study of the southern elephant seal, Mirounga leonina (Linn.). Comp Biochem Physiol A 42:841–850
Lessells CM, Boag PT (1987) Unrepeatable repeatabilities: a common mistake. Auk 104:116–121
Lobato E, Moreno J, Merino S, Sanz JJ, Arriero E (2005) Haematological variables are good predictors of recruitment in nestling pied flycatchers (Ficedula hypoleuca). Ecoscience 12:27–34
Maceda-Veiga A, Figuerola J, Martínez-Silvestre A, Viscor G, Ferrari N, Pacheco M (2015) Inside the Redbox: applications of haematology in wildlife monitoring and ecosystem health assessment. Sci Total Environ 514:322–332
Marcus AD, Higgins DP, Gray R (2015) Health assessment of free-ranging endangered Australian sea lion (Neophoca cinerea) pups: effect of haematophagous parasites on haematological parameters. Comp Biochem Physiol A 184:132–143
Meade J, Ciaglia MB, Slip DJ, Negrete J, Márquez MEI, Mennucci J, Rogers TL (2015) Spatial patterns in activity of leopard seals Hydrurga leptonyx in relation to sea ice. Mar Ecol Prog Ser 521:265–275
Mos L, Morsey B, Jeffries SJ, Yunker MB, Raverty S, Guise SD, Ross PS (2006) Chemical and biological pollution contribute to the immunological profiles of free ranging harbor seals. Environ Toxicol Chem 25:3110–3117
Nathan C (2006) Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol 6:173–182
Norris K, Evans MR (2000) Ecological immunology: life history trade-offs and immune defense in birds. Behav Ecol 11:19–26
Rogers T, Bryden MM (1995) Predation of Adélie penguins (Pygoscelis adeliae) by leopard seals (Hydrurga leptonyx) in Prydz Bay, Antarctica. Can J Zool 73:1001–1004
Rogers TL, Bryden MM (1997) Density and haul out behavior of leopard seals (Hydrurga leptonyx) in Prydz Bay, Antarctica. Mar Mamm Sci 13:293–302
Rogers T, Ciaglia MB, Klinck H, Southwell C (2013) Density can be misleading for low-density species: benefits of passive acoustic monitoring. PLoS ONE 8(1):e52542
Roitt I, Brostoff J, Male D (2001) Immunology. Mosby, London
Siniff DB, Stone S (1985) The role of the leopard seal in the tropho-dynamics of the Antarctic marine ecosystem. In: Siegfried WR, Condy PR, Laws RM (eds) Antarctic nutrient cycles and food webs. Springer, Berlin, pp 555–560
Siniff DB, Garrott RA, Rotella JJ, Fraser WR, Ainlley DG (2008) Projecting the effects of environmental change on Antarctic seals. Antarct Sci 20:425–435
Sokal RR, Rohlf FJ (2012) Biometry, 4th edn. WH Freeman and Company, New York
Southwell CJ, Kerry KR, Ensor PH (2005) Predicting the distribution of crabeater seals off east Antarctica during the breeding season. Mar Ecol Prog Ser 299:297–309
Southwell C, Paxton CG, Borchers D, Boveng P, Rogers T, William K (2008) Uncommon or cryptic? Challenges in estimating leopard seal abundance by conventional but state-of-the-art methods. Deep Sea Res Part 1 Oceanogr Res Pap 55:519–531
Southwell C, Bengston J, Bester M, Blix AS, Bornemann H, Boveng P, Cameron M, Forcada J, Laake J, Nordøy E, Plötz J, Rogers T, Southwell D, Steinhage D, Stewart BS, Trathan P (2012) review of data on abundance, trends in abundance, habitat use and diet of ice-breeding seals in the Southern Ocean. CCAMLR Sci 19:49–74
Stammerjohn SE, Martinson DG, Smith RC, Iannuzzi RA (2008) Sea ice in the western Antarctic Peninsula region: spatio-temporal variability from ecological and climate change perspectives. Deep Sea Res Part 2 Top Stud Oceanogr 55:2041–2058
Thompson PM, Corpe HM, Reid RJ (1998) Prevalence and intensity of the ectoparasite Echinophthirius horridus on harbour seals (Phoca vitulina): effects of host age and inter-annual variability in host food availability. Parasitol 117:393–403
Turner J, Lu H, White I, King JC, Phillips T, Hosking JC et al (2016) Absence of 21st century warming on Antarctic Peninsula consistent with natural variability. Nature 535:411–415
Vallyathan NV, George JT, Ronald K (1969) The harp seal, Pagophilus groenlandicus (Erxleben, 1777). V. Levels of haemoglobin, iron, certain metabolites and enzymes in the blood. Can J Zool 47:1193–1197
van den Hoff JO, Fraccaro R, Mitchell P, Field I, McMahon CL, Burton H, Blanchard W, Duignan P, Rogers T (2006) Estimating body mass and condition of leopard seals by allometrics. J Wildl Manag 69:1015–1023
Yamanishi Y, Miyake K, Iki M, Tsutsui H, Karasuyama H (2017) Recent advances in understanding basophil mediated Th2 immune responses. Immunol Rev 278:237–245
Yochem PK, Stewart BS, Gelatt TS, Siniff DB (2009) Health Assessment of Weddell Seals, Leptonychotes weddellii, in McMurdo Sound, Antarctica. In: Kerry K, Riddle M (eds) Health of Antarctic wildlife: a challenge for science and policy. Springer, Berlin, pp 123–138
Zuk M, Stoehr AM (2002) Immune defense and host life history. Am Nat 160:9–22
Acknowledgements
The study was financially and logistically supported by the Dirección Nacional del Antártico, Instituto Antártico Argentino. The permit for this work was granted by the Dirección Nacional del Antártico (Environmental Office). This research was funded by the Agencia de Promoción Científica y Tecnológica (PICT 2015-0082) and Lerner-Grey Fund for Marine Research. The authors express their support to the Argentinean scientific program, and thank the public politics during the period 2003–2015 that made possible this research. Field work was supported and funded by the Dirección Nacional del Antártico, Instituto Antártico Argentino PICTA 01–2010.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Ethical approval
The immobilisation and sampling of leopard, crabeater and Weddell seals within the Antarctic Specially Protected Area No. 134 were approved by the Dirección Nacional del Antártico, Program of Environmental Management and Tourism (PGAyT), Buenos, Aires, Argentina (Permit No. 8). Research procedures were reviewed and approved by the University of New South Wales’ Animal Care and Ethics Committee protocol numbers 08/103B and 11/112A to TR.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Leonardi, M.S., D’Amico, V.L., Márquez, M.E. et al. Leukocyte counts in three sympatric pack-ice seal species from the western Antarctic Peninsula. Polar Biol 42, 1801–1809 (2019). https://doi.org/10.1007/s00300-019-02551-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-019-02551-y