Abstract
The Antarctic Peninsula is one of the areas where the climate is changing at the fastest pace, having several effects on the populations of pygoscelid penguins. Few studies have analysed the variation in immune parameters of antarctic birds in a geographical context; thus, analyses of geographical differences in the immune components of wild pygoscelid penguins are still scarce. Leukocyte counts in birds provide information on their immunity and physiological stress. The objective of this study was to analyse the leukocyte counts in penguins of the genus Pygoscelis (gentoo, Adélie and chinstrap penguins), covering sites along the South Shetland Islands and some islands on the west coast of the Antarctic Peninsula. Our results revealed differences in the number of heterophils and eosinophils and in the heterophil/lymphocyte ratio in the northeastern populations of gentoo and Adélie penguins as compared to the rest of the colonies studied. The results contribute to better understanding of the variations in physiological parameters of penguins related to a geographical context.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The western coast of the Antarctic Peninsula is one of the areas in the world with the fastest warming trends (Vaughan et al. 2003; Meredith and King 2005; Turner et al. 2009; Ainley and Tin 2012). In some regions such as Faraday/Vernadsky research station (65°15′S, 64°16′W), the average temperature increased by 0.53 °C per decade from 1956 to 2006, whereas in the South Orkney Islands, the average temperature increased by 0.20 °C per decade in a 100-year record (Turner et al. 2009). One of the main effects of this temperature increase has been reported to be a reduction in mean annual sea ice extent (Fan et al. 2014). Such changes have affected wildlife as well, with a decrease in krill density (Atkinson et al. 2004, Flores et al. 2012) and changes in the populations of krill-dependent predators, including krill-dependent penguins (Trivelpiece et al. 2011). For instance, in the South Shetlands, chinstrap penguin populations have declined 60 % on average (Barbosa et al. 2012). In Stranger Point, 25 de Mayo/King George Island, the Adélie penguin populations decreased by 62 % between 1996 and 2006, while the gentoo breeding population size increased by 68 % (Carlini et al. 2009).
Global changes in climate and human movements affect the health of wildlife and their ecosystems, with shifts in the distribution and abundance of parasites and pathogens (Daszak et al. 2000; Harvell et al. 2002). It has been hypothesised that these shifts can lead to an increase in the distribution area of parasites and pathogens towards the poles (Sutherst 2001). It is therefore expected that the environmental changes affecting the Antarctic Peninsula could be reflected in the distribution and/or abundance of parasites and pathogens present in this region. Unfortunately, this cannot be currently tested because of the lack of detailed information about the geographical variation of these organisms in Antarctica (Barbosa and Palacios 2009). However, it is possible to study such variability in the hosts by using information of their immune system, as it is one the most important defence mechanisms against parasites and pathogens (Zuk and Stoehr 2002).
Penguins are easy to study and are hosts of ectoparasites such as ticks, fleas and biting lice, endoparasites such as helminths and several diseases produced by both viruses and bacteria (see Barbosa and Palacios 2009 and Grimaldi et al. 2014 for a review). Regarding tick infestation, it has been seen that it can vary in different locations along a geographical gradient from northeast to southwest in the Antarctic Peninsula (Barbosa et al. 2011), consistent with expectations under a climate change scenario. Regarding immunity, studies on the variation in the immune system of Antarctic pygoscelid penguins have reported a geographical pattern in their humoral immune response, showing that immunoglobulin levels increase towards the northwest from sites in the Antarctic Peninsula to sites in the South Shetland Islands (Barbosa et al. 2007a). However, although some studies have been carried out in particular situations or specific locations, no other components of the immune system, such as the cellular immunity, have been studied in pygoscelid penguins (see D’Amico et al. 2014a, b; Vanstreels et al. 2014; Vleck et al. 2000; Zinsmeister and VanDer Heyden 1987). Considering that all the components of the immune system could differ in their response (Adamo 2004), it is necessary to have as much information as possible about different immunological parameters, including the cellular immunity.
Leukocyte counts (i.e. the proportion of the different types of circulating leukocytes) is one of the parameters most commonly assessed to find information on the cellular immune response and the health and physiological stress of animal populations (Apanius 1998; Davis et al. 2008). Leukocytes make up an important component of the immune system as the primary line of defence against pathogens (Roitt et al. 2001). Therefore, their concentrations are often of particular importance and the interest in obtaining data on leukocyte numbers is rapidly growing among animal ecologists (Davis et al. 2008). The leukocyte types known as granulocytes (heterophils, eosinophils and basophils) and monocytes, associated with the innate immune system, act as an initial non-specific protection mechanism of wide range during the early stages of infestation. The specific protection is driven by the acquired immunity (lymphocytes), which provides the vertebrate immune system with the ability to recognise and remember specific pathogens (Roitt et al. 2001). Different pathogens and environmental stimuli can lead to specific patterns of leukocyte proliferation and activation (Campbell 1995). The ratio between heterophils/lymphocytes (H/L) has been described as a measurement of stress in birds (Davis et al. 2008). The H/L ratio is a reliable indicator because it is very sensitive to different stressors such as injuries, severe heat, disease, extreme exercise, food deprivation, contamination and/or exposure to novel social situations (Vleck et al. 2000; Davis et al. 2008; Müller et al. 2011; Banbura et al. 2013).
The aim of this study was to analyse the variation in cellular immunity by means of determining the leukocyte counts of different populations of three Antarctic pygoscelid penguins along the South Shetland Islands and some islands on the west coast of the Antarctic Peninsula. The Antarctic Peninsula shows great environmental variability in relation to climate change (e.g. Ducklow et al. 2007; Martinson et al. 2008; Montes-Hugo et al. 2009). We have previously found that along the Antarctic Peninsula, penguin populations differ in different physiological parameters such as immunoglobulin levels (Barbosa et al. 2007a), heat shock proteins (Barbosa et al. 2007b) and the expression of coloured traits (Barbosa et al. 2012) and that the abundance of some parasites such as ticks (Barbosa et al. 2011), the presence of pollutants (Jerez et al. 2011) and the genotoxic damage to penguins (De Mas et al. 2015) show geographical variability. Considering such variability, we expected to find geographical variation in cellular immunity, such as increases of leukocytes in northeastern sites where penguins showed an abundance of parasites (Barbosa et al. 2011; Diaz et al. 2013) or higher H/L values in places where penguins are more exposed to pollutants (Jerez et al. 2011) or human activities (Barbosa et al. 2013). We also wanted to establish a baseline for future comparisons in the context of climate change.
Materials and methods
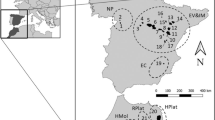
Breeding colonies of chinstrap, gentoo and Adélie penguins located in the South Shetland Islands (Barton Point and Stranger Point, 25 de Mayo/King George Island; Hannah Point, Livingston Island and Vapour Col, Deception Island) and in islands located on the west coast of the Antarctic Peninsula (George Point, Ronge Island; Yalour Island and Avian Island) (Table 1; Fig. 1) were visited during December 2009 and January 2010. Data from Adélie, gentoo and chinstrap penguins from 25 de Mayo/King George Island were taken from D’Amico et al. (2014a) and used as the northeasternmost comparative breeding colonies in this study. Adults, without sex distinction, were randomly captured on the beach using a long handled net in order to minimise disturbance in the breeding colonies (Barbosa et al. 2007a, b). The penguins were sampled when the chicks were in guard phase to avoid the likely effects of variation related to the breeding period. The penguins sampled showed no external signs of illness or injuries.
Blood samples were collected from the metatarsal vein with a 1-ml syringe within 5 min of capture to minimise capture and handling stress (Davis 2005). Thin blood smears were prepared with a drop of fresh blood, air dried, fixed with absolute ethanol for 3 min and stained with Tinción 15 (Biopur S.R.L., Rosario, Argentina). The smears were examined with a light microscope scanning monolayer fields with similar densities of erythrocytes for all individuals (Campbell 1995). The proportion of each leukocyte type was obtained from a sample of 100 leukocytes in 1000x (oil immersion) classified into basophils (B), heterophils (H), eosinophils (E), lymphocytes (L) and monocytes (M) (Campbell 1995). The relative leukocyte counts (RLC) per 10,000 erythrocytes were estimated by counting the number of all erythrocytes in one microscopic visual field and multiplying it with the number of the microscopic visual fields that were scanned until reaching 100 leukocytes, following Lobato et al. (2005). The H/L ratio was calculated from the leukocyte counts. For each of the three species, ten smears were randomly selected to analyse the repeatability. Repeatability was calculated for RLC, H, E, L and M following Lessells and Boag (1987). Basophils were not included because of the many zeros found in the matrix. Repeatability was high for the three penguin species (Table 2; P < 0.001).
Leukocyte counts and H/L ratios were statistically described and compared between the penguin populations studied using the nonparametric Kruskal-Wallis H test and nonparametric multiple comparisons (STATISTICA version 7.0) because of the lack of normality and homoscedasticity of the data (Sokal and Rohlf 1995). To maintain an experiment-wise error rate of 0.05, we used a Bonferroni adjustment (Rice 1989) of α = 0.007 for n = 7 parameters compared.
Results
A total of 227 penguins were sampled in the seven sites. In most of the sites, L was the most abundant leukocyte type followed by H for the three species, except for the population of gentoo penguins at Stranger Point (25 de Mayo/King George Island), which showed an inverse pattern (Table 3). Gentoo and Adélie penguin populations showed statistical differences among the sites sampled, while chinstrap penguins showed no statistical differences among populations.
For gentoo penguins, RLC (H 2,67 = 15.7, P < 0.007), H (H 2,67 = 15.7, P < 0.007), E (H 2,67 = 15.7, P < 0.007) and H/L (H 2,67 = 15.7, P < 0.007) showed significant differences, with the highest values at Stranger Point (multiple comparisons, P < 0.007) and lower values in the other two more southwestern locations, Hannah Point and Ronge Island (multiple comparisons, P < 0.007) (Table 3 and Fig. 2).
Graphs show the mean (points) and standard error (whiskers) of the relative leukocyte counts (a) and heterophil-lymphocyte ratios (b) of the three penguin species in each site sampled. Sites are shown in a northeast-southwest order from the left to the right. BACH chinstrap penguins from Barton, STGE/STAD gentoo and Adélie penguins from Stranger Point, HAGE/HACH gentoo and chinstrap penguins from Hannah Point, DECH chinstrap penguins from Deception Island, ROGE/ROCH gentoo and chinstrap penguins from Ronge Island, YAAD Adélie penguins from Yalour Island and AVAD Adélie penguins from Avian Island
For Adélie penguins, RLC values were higher for the population sampled at Avian Island (H 2,73 = 10.6, multiple comparisons, P < 0.007) (Table 3, Fig. 2). Values at Stranger Point and Yalour Island were statistically similar (multiple comparisons, P < 0.007). L values were also higher at Avian Island and similar in the other two sites (H 2,73 = 27.8, multiple comparisons, P < 0.007). H was statistically similar in Stranger Point and Avian Island and lower at Yalour Island (H 2,73 = 25.6, multiple momparisons, P < 0.007, Table 3). E values were highest at Stranger Point (H 2,73 = 16.1, multiple comparisons, P < 0.007, Table 3). Finally, the H/L ratio was highest at Stranger Point (H 2,73 = 29.9, multiple comparisons, P < 0.007, Table 3, Fig. 2).
For chinstrap penguins, none of the parameters measured showed statistical differences among the populations (RLC counts: H 2,87 = 2.4, P > 0.007; B: H 2, 87 = 0.19, E: H 2,87 = 5.5, H: H 2,87 = 0.04, L: H 2,87 = 0.03; M: H 2,87 = 2.31, P > 0.007 and H/L: H 2,87 = 0.0008, P > 0.007) (Table 3). In the two sites where chinstrap and gentoo populations overlapped (i.e. Hannah Point and George Point), leukocyte counts showed no differences (Mann-Whitney U test, P > 0.007).
Discussion
To our knowledge, only one study has assessed the geographical variation in the immune parameters of the pygoscelid penguins living in Antarctica. This study showed that the populations of penguins living further northeast have higher immunoglobulin levels than the others (Barbosa et al. 2007a). Our results of leukocyte counts evidenced that RLC, L, H, E and H/L ratios were different among the different populations of gentoo and Adélie penguins sampled, which can be explained in a geographical context. In addition, we found no leukocyte differences for the populations of chinstrap penguins sampled.
Gentoo penguins showed the highest RLC in the northeastern site Stranger Point. In general, higher RLCs indicate higher exposure to parasites and pathogens (Roitt et al. 2001; Butler and McGraw 2010). As we expected, along with increased RLC values, the proportion of H and E was higher at the northeastern site Stranger Point. These phagocytic cells are the first line of defence of the innate immune response against gastrointestinal parasites incorporated through the diet (Shutler and Marcogliese 2011). Recently, it has been reported that gentoos at Stranger Point harbour gastrointestinal helminth parasites, showing high richness, abundance and intensities of these parasites (Diaz et al. 2013), probably due to their wider dietary spectrum. This would in turn explain the results found in the RLC counts. It is interesting to mention that a similar result was found by Barbosa et al. (2007a) in their study of immunoglobulins (IgY), where gentoo penguins showed the highest level of immunoglobulins at this site, which was also attributed to a higher impact of parasites or pathogens. The effect of the gastrointestinal infestation could also be causing the increased H/L ratio values in gentoo penguins in the northeast sites, since there is evidence that this ratio is influenced by diseases and infections, as well as by the stress hormones produced as a result of the infection (Davis et al. 2008). It has also been reported that anthropogenic activities have a strong impact on physiological parameters such as H/L ratios in penguins (Barbosa et al. 2013). Although the research and logistic activities tend to concentrate on relatively small areas or specific locations, the human activities developed in Stranger Point can also promote the increase in H/L at this site.
In contrast to that observed in gentoos, Adélie penguins displayed the highest RLC values at the southwesternmost site of Avian Island; also L was higher at this site. Since this is the first report of leukocytes at the site, the highest L values could be normal for the species and can then be used as baseline values. Adélie penguins, similarly to gentoos, displayed the highest granulocyte values (H and E) at Stranger Point. Although several stressors can increase these values (Apanius 1998), most of these stressors produce particularly increased H values, which in turn lead to increased H/L values, as described for gentoos. H/L ratios were the highest for the northeast population of Adélie penguins. In particular, Adélie penguins are ice-dependent penguins that need ice for foraging during the breeding period. Their diet is composed almost entirely of krill, a species that has shown a significant reduction as a result of the increase in the sea surface temperature due to the climate warming (Ainley et al. 2010). As a consequence, Adélie penguins at Stranger Point decreased by 62 % in a 10-year period (Carlini et al. 2009). Furthermore, human activities, as described for gentoos, can also add to these impacts, as this species is very sensitive to human disturbances (Carlini et al. 2009). These environmental stressors, as we expected, could be causing the increased values of H/L ratios, which are the highest for the northeast population of Adélie penguins.
For chinstrap penguins, neither the RLC counts, types of leukocytes nor H/L ratios showed statistical differences among the populations sampled. The chinstrap penguin populations in the Antarctic Peninsula are decreasing (Barbosa et al. 2012, Lynch et al. 2012), which probably means that the same driver is operating at a regional level on this species. Therefore, the lack of differences found in our study could be explained by the fact that the degree of potential stressors affecting cellular immunity may be similar in the populations sampled (i.e. gastrointestinal parasites). In chinstrap penguins breeding at Ardley Island, South Shetlands (62°13′S, 58°55′W), Zinsmeister and VanDerHeyden (1987) reported higher values of H and lower values of the rest of the leukocyte types than those found by us in the present study for all the breeding colonies sampled. However, this discrepancy could be due to the very small sample size used in their study (only two chinstrap penguins). Vanstreels et al. (2014) compared two close populations on 25 de Mayo/King George Island and found no differences between leukocyte types. These authors found higher H and H/L ratio values than those found by us for chinstraps and found lower values of eosinophils, basophils and monocytes. However, we cannot conclude whether such differences are related to geographical differences because the sampling time was later than in our case or to the different year of sampling.
Chinstrap populations overlapped with gentoo ones in two of the locations sampled (Hannah Point and George Point). However, we found no differences in leukocyte counts for either species at these two sites.
Few studies have analysed the variation in immune parameters in a geographical context in Antarctica. Barbosa et al. (2007a) reported for the first time a geographical variation in immunoglobulin levels to assess humoral acquired immunity in pygoscelid penguins. Results of that study showed a clear geographical pattern, with higher immunoglobulin levels in the northeastern locations in the three pygoscelid species studied. Here, we found the same pattern in gentoo penguins but the opposite in Adélie penguins and no geographical differences in chinstrap penguins. Published results about associations of the different components of the immune system are contradictory. Some studies have found positive associations between humoral and cellular immunity (e.g. Morales et al. 2004), while others have found negative associations (e.g. Johnsen and Zuk 1999). Our results agree with both possibilities depending on the species and suggest that the relationships between humoral and cellular immunity in pygoscelid penguins could be species-specific.
The present study, which incorporates the variation of leukocyte counts in the same species of penguins in several sites in Antarctica, increases the knowledge of how immune parameters could vary in a geographical context. However, the number of populations sampled (three or four populations of each species) could be considered low to generalise the conclusions and more populations should be sampled at the same time. However, logistic constraints make this scenario very difficult to achieve.
Finally, it is possible that the sample timing (see date of sampling in Table 1) could have influenced our results. However, most of the sampling (five locations) was carried out within 5 days and it is unlikely that this timing affected our results. In 25 de Mayo/King George Island, sampling was carried out 10 days before the next sampling for gentoos, 22 days before the next sampling for chinstraps and 38 days before the next sampling for Adélies. In this case, the results could have been influenced by the sample collection time. However, as breeding time is different in the different populations (i.e. Adélies in King George Island breed before Adélies in Avian Island), all the individuals were sampled during the chick-rearing period in spite of the time differences. Thus, a sampling time effect could be ruled out. Nevertheless, to avoid any concern, simultaneous sample collection at the different locations should be considered in future studies.
References
Adamo S (2004) How should behavioural ecologists interpret measurements of immunity. Anim Behav 68:1443–1449
Ainley D, Tin T (2012) Antarctica. In: Hilty JA, Chester CC, Cross MS (eds) Climate and conservation. Island Press, Washington, pp 267–277
Ainley D, Russell J, Jenouvrier S, Woehler E, Lyver O’B, Fraser WR, Kooyman GL (2010) Antarctic penguin response to habitat change as Earth’s troposphere reaches 2 °C above preindustrial levels. Ecol Monogr 80:49–66
Apanius V (1998) Stress and immune defense. Adv Stud Behav 27:133–153
Atkinson A, Siegel V, Pakhomov E, Rothery P (2004) Longterm decline in krill stock and increase in salps within the Southern Ocean. Nature 432:100–103
Banbura J, Skwarska J, Banbura M, Gladalski M, Holysz M et al (2013) Spatial and temporal variation in heterophil-to-lymphocyte ratios of nestling passerine birds: comparison of blue tits and great tits. PLoS ONE. doi:10.1371/journal.pone.0074226
Barbosa A, Palacios MJ (2009) Health of Antarctic birds: a review of their parasites, pathogens and diseases. Polar Biol 32:1095–1115. doi:10.1007/s00300-009-0640-3
Barbosa A, Merino S, Benzal J, Martinez J, Garcia-Fraile S (2007a) Geographic variation in immunoglobulin levels in pygoscelid penguins. Polar Biol 30:219–225. doi:10.1007/s00300-006-0175-9
Barbosa A, Merino S, Benzal J, Martinez J, Garcia-Fraile S (2007b) Population variability in heat shock protein among three Antarctic penguin species. Polar Biol 32:1239–1244. doi:10.1007/s00300-007-0284-0
Barbosa A, Benzal J, Vidal V, D’Amico V, Coria N, Diaz J, Motas M, Palacios Motas MJ, Cuervo J, Ortiz J (2011) Seabird ticks (Ixodes uriae) distribution along the Antarctic Peninsula. Polar Biol 34:1621–1624. doi:10.1007/s00300-011-1000-7
Barbosa A, Benzal J, De León A, Moreno J (2012) Population decline of chinstrap penguins (Pygoscelis antarctica) on Deception Island, South Shetlands, Antarctica. Polar Biol 35:1453–1457. doi:10.1007/s00300-012-1196-1
Barbosa A, Eva De Mas E, Benzal J, Diaz JI, Motas M, Jerez S, Pertierra L, Benayas J, Justel A, Lauzurica P, Garcia-Peña FJ, Serrano T (2013) Pollution and physiological variability in gentoo penguins at two rookeries with different levels of human visitation. Antarct Sci. doi:10.1017/S0954102012000739
Butler MW, McGraw KJ (2010) Relationships between dietary carotenoids, body tissue carotenoids, parasite burden, and health state in wild mallard (Anas platyrhynchos) ducklings. Arch Biochem Biophys 504:154–160
Campbell TW (1995) Avian hematology and cytology. Iowa State University Press, Ames
Carlini R, Coria NR, Santos MM, Negrete J, Juares MA, Daneri GA (2009) Responses of Pygoscelis adeliae and P. papua populations to environmental changes at Isla 25 de Mayo (King George Island). Polar Biol 32:1427–1433. doi:10.1007/s00300-009-0637-y
D’Amico VL, Bertellotti M, Diaz JI, Coria N, Vidal V, Barbosa A (2014a) Leucocyte levels in some Antarctic and non-Antarctic penguins. Ardeola 61:145–152. doi:10.13157/arla.61.1.2014.145
D’Amico VL, Coria N, Palacios MG, Barbosa A, Bertellotti M (2014b) Physiological differences between two overlapped breeding Antarctic penguins in a global change perspective. Polar Biol. doi:10.1007/s00300-014-1604-9
Daszak P, Cunningham AA, Hyatt AD (2000) Emerging infectious diseases of wildlife: threats to biodiversity and human health. Science 287:443–449
Davis AK (2005) Effects of handling time and repeated sampling on avian white blood cell counts. J Field Ornithol 76:334–338
Davis AK, Maney DL, Maerz JC (2008) The use of leukocyte profiles to measure stress in vertebrates: a review for ecologists. Funct Ecol 22:760–777. doi:10.1111/j.1365-2435.2008.01467.x
De Mas E, Benzal J, Merino S, Valera F, Palacios MJ, Cuervo JJ, Barbosa A (2015) Erythrocytic abnormalities in three Antarctic penguin species along the Antarctic Peninsula: biomonitoring of genomic damage. Polar Biol. doi:10.1007/s00300-015-1667-2
Diaz JI, Fusaro B, Longarzo L, Coria NR, Vidal V, Jerez S, Ortiz J, Barbosa A (2013) Gastrointestinal helminths of Gentoo penguins (Pygoscelis papua) from Stranger point, 25 de Mayo/King George Island, Antarctica. Parasitol Res. doi:10.1007/s00436-013-3341-3
Ducklow HW, Baker K, Martinson DG, Quetin LB, Ross RM, Smith RC, Stammerjohn SE, Vernet M, Fraser WR (2007) Marine pelagic ecosystems: the West Antarctic Peninsula. Philos Trans R Soc Lond B 362:67–94
Fan T, Deser C, Schneider DP (2014) Recent Antarctic sea ice trends in the context of the Southern Ocean surface climate variation since 1950. Geophys Res Lett 41:2419–2426. doi:10.1002/2014GL059239
Flores H, Atkinson A, Kawagushi S, Krafft B, Milinevsky G, Nicol S, Reiss C, Tarling GA, Werner R, Bravo Rebolledo E, Cirelli V, Cuzin-Roudy J, Fielding S, Groeneveld J, Haraldsson M, Lombana A, Marschoff E, Meyer B, Pakhomov EA, Rombolá E, Schmidt K, Siegel V, Teschke M, Tonkes H, Toullec J, Trathan P, Tremblay N, Van de Putte A, van Franeker JA, Werner T (2012) Impact of climate change on Antarctic krill. Mar Ecol Prog Ser 458:1–19. doi:10.3354/meps09831
Grimaldi WW, Seddon PJ, Lyver PO’B, Shinichi Nakagawa S, Tompkins DM (2014) Infectious diseases of Antarctic penguins: current status and future threats. Polar Biol. doi:10.1007/s00300-014-1632-5
Harvell CD, Mitchell CE, Ward JR, Altizar S, Dobson AP, Ostfeld RS, Samuel MD (2002) Climate warming and disease risks for terrestrial and marine biota. Science 296:2158–2162
Jerez S, Motas M, Palacios MJ, Valera F, Cuervo JJ, Barbosa A (2011) Concentration of trace elements in feathers of three Antarctic penguins: geographical and interspecific differences. Environ Pollut 159:2412–2419. doi:10.1016/j.envpol.2011.06.036
Johnsen TS, Zuk M (1999) Parasites and tradeoffs in the immune responses of female red jungle fowl. Oikos 86:487–492
Lessells CM, Boag PT (1987) Unrepeatable repeatabilities: a common mistake. Auk 104:116–121
Lobato E, Moreno J, Merino S, Sanz JJ, Arriero E (2005) Haematological variables are good predictors of recruitment in nestling pied flycatchers (Ficedula hypoleuca). Ecoscience 12:27–34
Lynch HJ, Naveen R, Trathan PN, Fagan WF (2012) Spatially integrated assessment reveals widespread changes in penguin populations on the Antarctic Peninsula. Ecology 93:1367–1377. doi:10.1890/11-1588.1
Martinson DG, Stammerjohn SE, Iannuzi RA, Smith RC, Vernet M (2008) Western Antarctic Peninsula physical oceanography and spatio-temporal variability. Deep Sea Res II 55:1964–1987. doi:10.1016/j.dsr2.2008.04.038
Meredith MP, King JC (2005) Rapid ocean climate change at the WAP. Geophys Res Lett 32:L19604. doi:10.1029/2005GL024042
Montes-Hugo M, Doney SC, Ducklow HW, Fraser W, Martinson D, Stammerjohn SE, Schofield O (2009) Recent changes in phytoplankton communities associated with rapid regional climate change along the Western Antarctic Peninsula. Science 323:1470–1473
Morales J, Moreno J, Merino S, Tomás G, Martínez J, Garamszegi LZ (2004) Associations between immune parameters, parasitism, and stress in breeding pled flycatcher (Ficedula hypoleuca) females. Can J Zool 82:1484–1492. doi:10.1139/Z04-132
Müller C, Jenni-Eiermann S, Jenni L (2011) Heterophils/lymphocytes-ratio and circulating corticosterone do not indicate the same stress imposed on Eurasian kestrel nestlings. Funct Ecol 25:566–576. doi:10.1111/j.1365-2435.2010.01816.x
Rice WR (1989) Analyzing tables of statistical tests. Evolution 43:223–225
Roitt I, Brostoff J, Male D (2001) Immunology. Mosby, London
Shutler D, Marcogliese DJ (2011) Leukocyte profiles of Northern Leopard Frogs, Lithobates pipiens, exposed to pesticides and hematozoa in agricultural wetlands. Copeia 2:301–307
Sokal RR, Rohlf FJ (1995) Biometry. W.H. Freeman, New York
Sutherst RW (2001) The vulnerability of animal and human health to parasites under global change. Int J Parasitol 31:933–948
Trivelpiece WZ, Hinke JT, Miller AK, Reiss CS, Trivelpiece SG, Watters GM (2011) Variability in krill biomass links harvesting and climate warming to penguin population changes in Antarctica. Proc Natl Acad Sci USA 108:7625–7628. doi:10.1073/pnas.1016560108
Turner J, Bindschadler RA, Convey P, Di Prisco G, Fahrbach E, Gutt J, Hodgson DA, Mayewski PA, Summerhayes CP (2009) Antarctic climate change and the environment. SCAR, Cambridge, ISBN: 978 0 948277 22 1
Vanstreels RET, Miranda FR, Ruoppolo V, de Almeida Reis AO, Schneider Costa E, de Lira Rodrigues, Pessôa A, Machado Torres JP, Teixeira Schmauder, da Cunha L, da Cruz Piuco R, Valiati VH, González-Acuña D, Labruna MB, Petry MV, Epiphanio S, Catão-Dias JL (2014) Investigation of blood parasites of pygoscelid penguins at the King George and Elephant Islands, South Shetlands Archipelago, Antarctica. Polar Biol 37:135–139. doi:10.1007/s00300-013-1401-x
Vaughan DG, Marshall GJ, Connolley WM et al (2003) Recent rapid regional climate warming on the Antarctic peninsula. Clim Change 60:243–274
Vleck CM, Vertalino N, Vleck D, Bucher T (2000) Stress, corticosterone, and heterophil to lymphocyte ratios in free-living Adélie penguins. Condor 102:392–400
Zinsmeister VAP, VanDerHeyden MJN (1987) Differential leucocyte counts from Pygoscelid penguins of Antarctica. J Wildl Dis 23:521–552
Zuk M, Stoehr AM (2002) Immune defense and host life history. Am Nat 160:9–22. doi:10.1086/342131
Acknowledgments
We appreciate the hospitality and logistic support of the Argentinean Antarctic Base “Carlini” and the Spanish Antarctic Base “Gabriel de Castilla”. We also thank the Spanish Polar Ship “Las Palmas”, which provided us with the logistic support and transport to the localities. We thank the logistic support provided by the Marine Technology Unit (CSIC) and Instituto Antártico Argentino (IAA). Permission to work in the study area and for penguin handling was given by the Spanish Polar Committee. We are very grateful to B. Fusaro and L. Longarzo for their help at “Carlini”. We thank Dr. Ralph Eric Thijl Vanstreels and the two anonymous reviewers for their insights, which have significantly improved our manuscript. Ma. Victoria Gonzalez Eusevi from Ingles Científico (www.inglescientifico.com) corrected the English language of the manuscript. This study was funded by the Spanish Ministry of Science and Innovation (Grant CGL2007-60369 and CTM2011-24427). VLD had a CONICET Postdoctoral fellowship and VV was supported by a Ph.D. Grant from the Spanish Council of Scientific Research (CSIC) and the European Social Fund (JAEPre08-01053). This study is a contribution to the International Polar Year project Birdhealth and to PINGUCLIM project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
D’Amico, V.L., Marcelo, B., Benzal, J. et al. Leukocyte counts in different populations of Antarctic Pygoscelid penguins along the Antarctic Peninsula. Polar Biol 39, 199–206 (2016). https://doi.org/10.1007/s00300-015-1771-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-015-1771-3