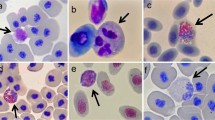
Abstract
Assessing numbers of leukocytes in salamanders and other amphibians can be useful metrics for understanding health or stress levels of individuals in a population. In this chapter we describe the procedures for obtaining blood samples from amphibians, preparing blood films for microscopy, counting, and identifying cells. We also provide reference values for amphibian leukocytes for use in interpreting leukocyte data. From our assessment of the published and unpublished literature, “non-stressed” salamanders would have a leukocyte profile where 60–70% of cells are lymphocytes, 17–30% are neutrophils, 1–4% are eosinophils, 4–12% are basophils, and 2–6% are monocytes. In Ambystoma spp., the eosinophil abundance can be notably higher (30% of all white blood cells), for reasons unknown. Finally, the neutrophil–lymphocyte ratio of most non-stressed salamanders tends to be between 0.3 and 0.4 (sometimes less), while the ratios of stressed salamanders tend to be over 1.0.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Forson D, Storfer A (2006) Atrazine increases ranavirus susceptibility in the tiger salamander, Ambystoma tigrinum. Ecol Appl 16(6):2325–2332
Gervasi SS, Foufopoulos J (2008) Costs of plasticity: responses to desiccation decrease post-metamorphic immune function in a pond-breeding amphibian. Funct Ecol 22(1):100–108
Cabagna MC et al (2005) Hematological parameters of health status in the common toad Bufo arenarum in agroecosystems of Santa Fe Province, Argintina. Appl Herpetol 2:373–380
Raffel TR et al (2006) Negative effects of changing temperature on amphibian immunity under field conditions. Funct Ecol 20:819–828
Rutherford PL, McRuer DL, Forbes MR (2005) Condition and immune traits of frogs from Ontario baitshops: risks of practice not ameliorated by sale of healthy frogs. Herpetol Rev 36(2):129–133
Shutler D, Smith TG, Robinson SR (2009) Relationships between leukocytes and hepatozon spp. in green frogs, Rana clamitans. J Wildl Dis 45(1):67–72
Davis AK, Durso AM (2009) Leukocyte differentials of northern cricket frogs (Acris c. crepitans) with a compilation of published values from other amphibians. Herpetologica 65(3):260–267
Davis AK, Golladay C (2019) A survey of leukocyte profiles of red-backed salamanders from Mountain Lake, Virginia, and associations with host parasite types. Comp Clin Pathol 28:1743–1750
Barriga-Vallejo C et al (2015) Assessing population health of the Toluca axolotl Ambystoma rivulare (Taylor, 1940) from Mexico, using leukocyte profiles. Herpetol Conserv Biol 10(2):592–601
Kim HJ, Jung YJ (2020) The emerging role of eosinophils as multifunctional leukocytes in health and disease. Immune Netw 20(3):14
Carnevale S et al (2020) The complexity of neutrophils in health and disease: focus on cancer. Semin Immunol 48:13
Demir A et al (2020) Ratio of monocytes to lymphocytes in peripheral blood in children diagnosed with active tuberculosis. J Pediatric Res 7(2):97–101
Jain NC (1986) Schalm’s veterinary hematology, vol 1221. Lea & Febiger, Philadelphia
Thrall MA (2004) Hematology of amphibians. In: Thrall MA, Baker DC, Lassen ED (eds) Veterinary hematology and clinical chemistry: text and clinical case presentations. Lippincott Williams & Wilkins, Philadelphia, PA
Thrall MA et al (2006) Veterinary hematology and clinical chemistry. Blackwell Publishing, Ames, IA, p 518
Kiesecker JM (2002) Synergism between trematode infection and pesticide exposure: a link to amphibian deformities in nature? Proc Natl Acad Sci 99(15):9900–9904
Rohr JR et al (2008) Agrochemicals increase trematode infections in a declining amphibian species. Nature 455(7217):1235–1U50
Shutler D et al (2015) Nematode parasites and leukocyte profiles of Northern Leopard Frogs, Rana pipiens: location, location, location. Can J Zool 93(1):41–49
Koprivnikar J et al (2019) Endocrine and immune responses of larval amphibians to trematode exposure. Parasitol Res 118(1):275–288
Ussing AP, Rosenkilde P (1995) Effect of induced metamorphosis on the immune system of the axolotl, Ambystoma mexicanum. Gen Comp Endocrinol 97(3):308–319
Davis AK (2009) Metamorphosis-related changes in leukocyte profiles of larval bullfrogs (Rana catesbeiana). Comp Clin Pathol 18(2):181–186
Davis AK et al (2009) New findings from an old pathogen: intraerythrocytic bacteria (Family Anaplasmatacea) in red-backed salamanders Plethodon cinereus. Ecohealth 6(2):219–228
Marcogliese DJ et al (2009) Combined effects of agricultural activity and parasites on biomarkers in the bullfrog, Rana catasbeiana. Aquat Toxicol 91(2):126–134
Davis AK, Maney DL, Maerz JC (2008) The use of leukocyte profiles to measure stress in vertebrates: a review for ecologists. Funct Ecol 22:760–772
Wingfield JC, Romero LM (2001) Adrenocortical responses to stress and their modulation in free-living vertebrates. In: McEwen BS, Goodman HM (eds) Handbook of physiology, section 7: the endocrine system, Vol. IV: coping with the environment: neural and endocrine mechanisms. Oxford University Press, New York, pp 211–234
Dhabhar FS (2002) A hassle a day may keep the doctor away: stress and the augmentation of immune function. Integr Comp Biol 42(3):556–564
Dhabhar FS (2009) Enhancing versus suppressive effects of stress on immune function: implications for immunoprotection and immunopathology. Neuroimmunomodulation 16(5):300–317
Davis AK, Maney DL (2018) The use of glucocorticoid hormones or leucocyte profiles to measure stress in vertebrates: What's the difference? Methods Ecol Evol 9(6):1556–1568
Gormally BMG, Romero LM (2020) What are you actually measuring? A review of techniques that integrate the stress response on distinct time-scales. Funct Ecol 34(10):2030–2044
Reynolds AE, Pickard BL (1973) Normal blood values in some plethodontid salamanders. Herpetologica 29:184–188
Rivera M, Davis AK (2013) Evaluating a method for non-destructively obtaining small volumes of blood from gilled amphibians. Herpetol Rev 44(3):428–430
Homan RN et al (2003) Impact os varying habitat quality on the physiological stress of spotted salamanders (Ambystoma maculatum). Anim Conserv 6:11–18
Perpiñán D et al (2006) Comparison of three different techniques to produce blood smears from green iguanas, Iguana iguana. J Herpetol Med Surg 16:99–101
Wright KM (2001) Amphibian hematology. In: Wright KM, Whitaker BR (eds) Amphibian medicine and captive husbandry. Krieger Publishing Company, Malabar, FL, pp 129–146
Friedmann GB (1970) Differential white blood cell counts for the urodele Taricha granulosa. Can J Zool 48:271–274
Bennett MF, Alspaugh JK (1964) Some changes in the blood of frogs following administration of hydrocortisone. Virginia J Sci 15:76–79
Bennett MF et al (1972) Changes in the blood of newts, Notophthalmus viridescens, following administration of hydrocortisone. J Comp Physiol A 80:233–237
Bennett MF, Newell NC (1965) A further study of the effects of hydrocortisone on the blood of frogs. Virginia J Sci 16:128–130
Davis AK, Maerz JC (2008) Comparison of hematological stress indicators in recently captured and captive paedomorphic mole salamanders, Ambystoma talpoideum. Copeia 2008(3):613–617
Davis AK, Maerz JC (2010) Effects of exogenous corticosterone on circulating leukocytes of a salamander (Ambystoma talpoideum) with unusually abundant eosinophils. Int J Zool 2010:735937. https://doi.org/10.1155/2010/735937
DuRant SE et al (2015) Evidence of ectoparasite-induced endocrine disruption in an imperiled giant salamander, the eastern hellbender (Cryptobranchus alleganiensis). J Exp Biol 218:2297–2304
Davis AK (2009) The wildlife leukocytes website: the ecologis’s source for information about leukocytes of wildlife species. http://wildlifehematology.uga.edu
Coppo JA et al (2005) Blood and urine physiological values in captive bullfrog, Rana catesbeiana (Anura: Ranidae). Analecta Veterinaria 25(1):15–17
Cathers T et al (1997) Serum chemistry and hematology values for anesthetized American bullfrogs (Rana catesbeiana). J Zoo Wildl Med 28(2):171–174
Davis AK, Maerz JC (2011) Assessing stress levels of captive-reared amphibians with hematological data: implications for conservation initiatives. J Herpetol 45(1):40–44
de Assis VR et al (2015) Effects of acute restraint stress, prolonged captivity stress and transdermal corticosterone application on immunocompetence and plasma levels of corticosterone on the cururu toad (Rhinella icterica). PLoS One 10(4):21
Hadji-Azimi I, Coosemans V, Canicatti C (1987) Atlas of adult Xenopus laevis laevis hematology. Dev Comp Immunol 11:807–874
Voight GL (2000) Hematology techniques and concepts for veterinary technicians. Iowa State University Press, Ames, IA, p 139
Forzan MJ et al (2017) Clinical pathology of amphibians: a review. Vet Clin Pathol 46(1):11–33
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Science+Business Media, LLC, part of Springer Nature
About this protocol
Cite this protocol
Davis, A.K., Maerz, J.C. (2023). Assessing Leukocyte Profiles of Salamanders and Other Amphibians: A Herpetologists’ Guide. In: Seifert, A.W., Currie, J.D. (eds) Salamanders. Methods in Molecular Biology, vol 2562. Humana, New York, NY. https://doi.org/10.1007/978-1-0716-2659-7_29
Download citation
DOI: https://doi.org/10.1007/978-1-0716-2659-7_29
Published:
Publisher Name: Humana, New York, NY
Print ISBN: 978-1-0716-2658-0
Online ISBN: 978-1-0716-2659-7
eBook Packages: Springer Protocols