Abstract
Global warming has led to a strong deterioration of the Arctic sea ice cover. Ice thickness, age and coverage have been strongly declining in recent years. Brine channels that form in sea ice when seawater freezes represent a unique habitat for bacteria, algae, proto- and small metazoans. We hypothesized that the loss of multi-year ice and the more prevalent formation of first-year ice even in central regions of the Arctic will lead to changes in the Arctic sea ice meiofauna community composition. We therefore analysed the sea ice meiofauna community composition of three different ice types sampled in summer and autumn 2007. Young, thin ice of few cm thickness was typified by taxa of pelagic origin or with good swimming abilities (ciliates, pelagic foraminifera, rotifers and platyhelminthes). Harpacticoid copepods and nematodes with poor swimming abilities were prevalent in older, thicker (>0.5 m) first- and multi-year ice. Brash ice—which was likely a mix of older broken ice, slush and pancake ice—was characterized by a high abundance of platyhelminthes and rotifers. An experimental analysis of colonization efficiencies of artificial thin ice also revealed that species with poor swimming ability are less successful to colonize newly forming thin ice. We conclude that observed and predicted changes in the ice formation regime will likely result in changes in the composition of Arctic sea ice communities. We predict negative effects particularly for species with low dispersal capacities like harpacticoid copepods and endemic nematodes, as these are less successful in colonizing newly forming thin ice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Global warming has resulted in a strong decrease in Arctic summer sea ice extent in the last decades (Stroeve et al. 2007, 2011; Serreze and Stroeve 2015), and climate models predict a further decrease in Arctic summer sea ice extent and sea ice volume and an almost ice-free Arctic Ocean (<103 km3 sea ice in summer) in the 2050s (Melia et al. 2015). The amount of summer open-water areas in the central Arctic increased significantly with the strong decline in sea ice extent in the recent past and coincides with a gradual loss of multi-year ice and a decrease in average sea ice thickness (Maslanik et al. 2007; Haas et al. 2008; Kwok et al. 2009; Barber et al. 2009; Kwok and Cunningham 2015; Serreze and Stroeve 2015). A very pronounced loss of summer sea ice in the central Arctic Ocean occurred in 2007, followed by a slight recovery in 2009–2011. A new record minimum was reached in 2012, and further very low extents were observed in 2015 and 2016 (Serreze and Stroeve 2015; Vizcarra 2016). Ongoing global warming will likely lead to a further destabilization of the Arctic sea ice cover and to further increases in open-water areas in summer (e.g. Barnhart et al. 2016). Increased open-water areas in summer will naturally coincide with increased new ice formation in autumn, especially in Russian shelf areas like the Laptev Sea, but also over the deep waters of the central Arctic. How such changes in sea ice dynamics impact the Arctic sea ice meiofauna diversity is not well understood.
Sea ice is pervaded by small brine channels, which form when seawater freezes, as salts in solution are not incorporated into the crystal matrix, but accumulate as brine between forming ice crystals (Weeks and Ackley 1986). These brine channels form a habitat for a unique community of bacteria, algae, proto- and small metazoans (Horner et al. 1992). Metazoans found within Arctic sea ice comprise species of evolutionarily benthic origin like harpacticoid copepods (Carey and Montagna 1982; Kern and Carey 1983; Grainger 1991; Carey 1992), nematodes (Tchesunov 1986; Tchesunov and Riemann 1995; Riemann and Sime-Ngando 1997; Blome and Riemann 1999), platyhelminthes (Janssen and Gradinger 1999; Friedrich and Hendelberg 2001), cnidarians (Bluhm et al. 2007; Siebert et al. 2009) and polychaete larvae (Carey and Montagna 1982; Grainger et al. 1985; Carey 1992), as well as species of pelagic origin like rotifers (Chengalath 1985; Friedrich and De Smet 2000) and calanoid copepods (Kramer and Kiko 2011). Several nematodes are endemic to Arctic sea ice (Riemann and Sime-Ngando 1997).
We hypothesize that these sea ice meiofauna organisms have different dispersal capacities and different capabilities to colonize newly formed ice. Further loss of multi-year ice and increased open-water areas in summer could have a negative impact on low-dispersal species and benefit species with high dispersal capacities. We tested our hypothesis using two approaches. On the one hand, we sampled the bottom layer of thick first-year and multi-year sea ice, newly formed thin ice and brash ice—which was likely a mix of older broken ice, slush and pancake ice—at different locations in the central Arctic. In case of similar long-range dispersal capacities and colonization efficiencies of all sea ice meiofauna taxa, we would expect similar meiofauna communities in all three ice types. Furthermore, we sampled the sub-ice water layer underneath the thin and the brash ice in order to characterize exchange processes between the water and the ice. On the other hand, we conducted an experimental analysis of colonization success of one pelagic and three sea ice meiofauna taxa.
Materials and methods
Sampling region and ice conditions
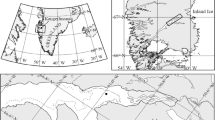
The present study was undertaken as part of the expedition ARK XXII/2 of R/V Polarstern (28 July–7 October 2007) to the central Arctic Ocean during the boreal summer–autumn transition in 2007. Ice from three larger categories was sampled: old thick ice (9 first-year, 1 second-year and 1 third-year ice floe; ice thickness 101–262 cm; information on ice age based on delta-18O data determined at the same station but at a different location on the same floe, personal communication Tobias Roeske), young thin ice (dark and light nilas; ice thickness 2–5 cm) and brash ice (a mix of older broken ice, slush and some pancake ice; ice thickness ~8 cm; Fig. 1). Fifteen samples of young thin ice were obtained during 8 station occupations, and 2 samples of brash ice were obtained during 1 station occupation. Between samplings during one station occupation, RV Polarstern was moved several hundred metres to provide a true replication. Samples of old thick ice were taken within the closed pack ice zone of the central Arctic Ocean (Fig. 1a). The ice concentration along the cruise track in this part of the study area was between 8/10 and 10/10. The modal ice thickness (obtained from airborne electromagnetic induction sounding) was 0.9 m without secondary modes; the mean ice thickness was 1.2 m (Haas et al. 2008). The open-water fraction based on these airborne surveys was about 2%. From 18 August on, new ice (dark or light nilas) occurred occasionally on melt ponds, narrow breaks or leads. According to daily AMSR-E ASI sea ice concentration distribution maps (not shown), the minimum ice extent in the study area northeast of Severnaja Semlaya was observed on 13 September 2007. Continued advection of mild air inhibited substantial new ice growth for another 2 days; before on 16 September 2007, the ice cover in the study region started to increase. Samples from thin ice (dark and light nilas) were taken from 17 to 19 September in an area covered with 90% new thin ice and approximately 10% older ice floes (ship-based observations; Fig. 1b, c). This area of mainly new thin ice extended in a belt from the thick pack ice to a band of brash and pancake ice, which separated the thin ice cover from the open water. The belt of thin ice had a width of 150–200 km. The area in the sampling region covered by thin ice was approximately 40,000 km2; that of the entire thin ice region along the thick ice tongue was about 75,000 km2 on 17 September. The latter area increased within 1 day by 10,000 km2 to about 85,000 km2 on 18 September. Please see online resource 1 for a detailed description of the algorithm to derive thin ice thickness and a comparison to in situ measurements. Brash ice samples were taken from within the band of brash and pancake ice. This brash ice tongue marked in Fig. 1b) was likely a relict of the sea ice cover of the last winter mixed with some newly formed pancake and slush ice. Water depths at all sampling stations was >1000 m, except for sampling near Franz-Josef-Land (327 m) and Severnaja Zemlya (726 m). According to the routine meteorological observations aboard the R/V Polarstern, air temperatures remained above −10 °C during the entire expedition. While passing through the thin ice covered region, air temperatures were −3 to −4 °C with only light winds. No precipitation was observed during this time.
Sampling region and sampling positions. a Cruise track, ASI algorithm ice concentration as derived from Advanced Microwave Scanning Radiometer (AMSR-E) 89 GHz data for 6 September 2007 and sampling positions for bottom ice samples (BI), indicated by circles. The first and last bottom ice sampling stations are indicated with sample ID (BI-MonthMonthDayDay). The boxed area is shown in b. Ice concentration legend only apply to a. b NOAA-17 Advanced Very High-Resolution Radiometer channel 1 reflectance image of the young ice sampling region for 17 September 2007, 5:42 UTC. Sampling positions are indicated through rectangles. The northernmost rectangle indicates TI-1, and the southernmost Br-1 and Br-2 as well as W-9 and W-10. Areas covered by old thick ice or brash and pancake ice are indicated. c Picture of the typical appearance of the areas covered by new ice, lower border of the picture approximately 10 m. d Picture of the typical appearance of the area covered by brash ice with the sampling cage in operation. e Sample retrieval through an opening in the bottom of the cage
Sampling of sea ice and sub-ice water
Thick level sea ice was sampled along the cruise track every 3–10 days from 2 August to 16 September 2007 (11 stations in total). During each station, four bottom ice sections (lowermost 5 cm) of thick level ice were sampled within a 1 m2 area using an engine-powered KOVACS ice corer with an internal diameter of 9 cm. The respective samples are abbreviated BI-mmdd—BI as short form for bottom ice, mmdd being the sampling date (month and day). Sampling was restricted to the lowermost 5 cm, as previous research had revealed that in summer, more than 90% of sea ice meiofauna within a vertical section through an ice floe is found in the lowermost 2–6.5 cm (Gradinger et al. 1999). The median sample volume was 0.29 l melted ice. Thickness of snow and deteriorated ice was measured at five randomly chosen locations around each coring site using a ruler stick. Deteriorated ice is here defined as ice that has lost its coherent structure due to brine drainage and melting, but is distinguishable from snow due to its large ice grains.
Thin and brash ice samples were obtained on ten stations from 17 to 19 September 2007 (abbreviation for thin ice samples: TI-numerical a/b). For this, a cage was craned right above the ice surface and samples were retrieved through an opening in the floor (Fig. 1d, e). Pieces of thin ice were cut out of the ice sheet in close vicinity to each other with a saw. A large opening was thereafter cut into the thin ice to lower a 20-µm plankton net with an opening diameter of 25 cm. Ice shavings were carefully removed from the opening, and exclusion of ice shavings and crystals was checked visually during sample retrieval. To deploy the plankton net in brash ice, all ice pieces were pushed away or removed to yield a large opening for the net. The special sampling setting (hovering above the ice in a craned cage) did not allow for more sophisticated methods of sub-ice fauna sampling (e.g. the use of an under-ice pump). The employed net samples—besides sea ice meiofauna potentially present in the sub-ice water layer—were mostly micro-zooplankton and meso-zooplankton in the same size range as sea ice meiofauna. Two tows from 5.5 m depth to the surface were performed, and the samples were fixed with borax-buffered formaldehyde for later zooplankton counts. Water samples for the determination of salinity and Chl-a concentration were taken directly from the surface through the same opening (abbreviation for Water samples: W-Numerical). Entire chunks of brash ice were sampled with large beakers (abbreviation for brash ice samples: Br-numerical a/b).
Sample processing and analysis
One bottom ice section or two thin or brash ice samples per station were melted directly in the dark at 4 °C within 24–36 h for the determination of bulk salinity (with a WTW microprocessor conductivity meter LF 196) and chlorophyll-a (Chl-a) and phaeopigment (Phaeo) concentration (as described previously, Kiko et al. 2008). In short, melted ice samples were filtered on Whatman GF/F filters, extracted in 90% acetone, homogenized and analysed fluorometrically with a Turner Designs 10-AU digital fluorometer. Calibration of the fluorometer was conducted using a dilution series of spinach Chl-a measured with a photometer. Ice samples for meiofauna analyses (three bottom ice sections, two ice cuts or two ice blocks per station) were melted in the dark at 4 °C in a surplus of 0.2-µm-filtered seawater (FSW; 200 ml per 1-cm core length) in order to reduce salinity stress for the organisms (Garrison and Buck 1986). Within 24 h after complete melting of the ice, the samples were concentrated over a 20-µm gauze.
Samples of thin and brash ice as well as sub-ice water for faunistic analyses were fixed with borax-buffered formaldehyde (2% in FSW) for later analyses; samples of thick ice were analysed directly onboard at 0 °C. In total, 33 bottom ice sections, 15 thin ice samples, 4 brash ice samples and 8 water samples were taken. Meiofauna was identified and counted using a stereomicroscope (Leica WILD MZ 12.5 and Leica MZ 16 F, 20–100× magnification, transmitted and impinging light). Samples were not split prior to analysis. A detailed compilation of all data stemming from the environmental sampling can be found in online resource 2.
Enrichment indices accounting for abundance changes due to brine loss were calculated according to Gradinger and Ikävalko (1998) as I S = (X I/S I) * (S W/X W), where X I is the ice parameter value, S I the bulk salinity of the ice, S W the water salinity, and X W the water parameter value. An index value of 1 indicates no change in the parameter relative to changes in ice salinity, an index value >1 indicates enrichment, and an index value <1 indicates depletion.
It needs to be kept in mind that differences in sampling techniques and sample volumes have impact on the size distribution and diversity of the communities observed. This holds particularly true for the comparison of water and ice samples, as for the former considerably larger volumes were sampled. This could—as more specimens are caught and analysed—lead to a higher diversity estimate. Nevertheless, the relative contributions of the dominant taxa, which are the focus of our analysis, are not expected to change with different sampling volumes.
Statistical comparisons of faunal communities
Nonparametric two-tailed Mann–Whitney U tests were performed to test for differences in the population means between (1) all possible combinations of the three ice types sampled and (2) each of the three ice types against the underlying water in terms of bulk abundances of different taxonomic groups of metazoans, protozoans and eggs. Missing cases were excluded test-by-test. If several samples were taken, these were included in the tests as individual samples.
To test for differences in community structure between the different sample types, a one-way analysis of similarities (ANOSIM; max 999 permutations) was applied pairwise to the abundance data. This nonparametric permutation test contrasts differences between sample types with differences between stations of the same sample type to test the null hypothesis that the similarity between the stations of the same sample type is greater than or equal to the similarity within the sample type. In order to visualize and further investigate grouping patterns of the samples with respect to community structure, a hierarchical agglomerative clustering (group-average linkage) was performed and significance of clustering was tested with a similarity profile test (SIMPROF; 1000 permutations, 999 simulated profiles). Furthermore, non-metric multi-dimensional scaling (MDS) to two dimensions was performed (starting configuration from MDS to three dimensions, method of steepest descent, Kruskal fit scheme 1, stop criterion: improvement in stress value <0.01, 25 restarts) to visualize the similarity of sample types. Meiofauna taxa discriminating and typifying the different sample types were identified by the similarity percentages method (SIMPER). Eggs and the samples W-3, W9 and TI-1 were not included in the ANOSIM, MDS and SIMPER analyses. In cases where two or more samples were taken, arithmetic means of these were used for analyses.
All multivariate analyses normally included metazoans, protozoans and eggs and were based on the Bray–Curtis similarities or dissimilarities calculated from fourth-root-transformed abundance data. The significance level for all statistic tests on biological parameters was 5% (p value <0.05). Univariate and multivariate analyses were performed with the software packages SPSS (2001) and PRIMER version 6 (Clarke and Gorley 2006), respectively.
Experimental investigation of the colonization of new ice
The ability of different sympagic and pelagic taxa to colonize newly forming and formed sea ice was tested in two parallel experiments. Abundant representatives of the sea ice meiofauna (Halectinosoma spp., rotifers, red acoel platyhelminthes) and the sub-ice community (Oithona sp.) were chosen for the experiment. Nematodes and Tisbe spp. were not available in sufficient numbers. Experiments were performed in a temperature-controlled room (set at −2 °C) in the dark. Five hundred-millilitre beakers were filled with 350-ml-filtered seawater diluted with Millipore® water to an initial salinity of 20 g/kg. A relatively mild freezing temperature was chosen to avoid complete freezing of the experimental beakers, whereas a low initial salinity was chosen to reach a water salinity close to 35 g/kg after ice formation. As the air temperature was below the freezing point of the seawater, ice formation took place at the water surface in all beakers. Ice growth proceeded for about 2 days, after which only little further growth occurred and about 170 ml of water remained unfrozen. Ice temperature and bulk salinity of the newly formed ice were measured on three ice samples grown and incubated in parallel to the experiment proper under the same conditions as described. Brine volume for these three ice samples was calculated according to Frankenstein and Garner (1967).
Ice formed during the colonization experiment had a thickness of 3.2 ± 1 cm (average ± SD, n = 20). Ice temperature was −1.3 ± 0.3 °C (n = 20), water temperature −1.6 ± 0.3 °C (n = 24), and water salinity 32.5 ± 3.5 g kg−1 (n = 24). Mean bulk salinity of artificial ice, grown in parallel to the experiment proper under the same conditions, was 10.0 ± 0.3 g kg−1 (n = 3). Mean brine volume fraction was 33 ± 5% (n = 3).
In the first experiment, animals were added to the beakers prior to ice formation, whereas in the second experiment, animals were added to the water fraction at day 2, i.e. when the ice had already formed. In both experiments, red acoel platyhelminthes (n = 14), Halectinosoma sp. (n = 21), rotifers (n = 60) and Oithona sp. (n = 25) were used, with three replicates each. The experiments were incubated for 6 days, after which ice temperature and thickness as well as water temperature, salinity and volume were measured. After measurement of the abiotic parameters, the ice was separated from the water fraction and melted in a surplus of filtered seawater. Dead and alive animals from the water fraction were counted immediately. Dead and alive animals from the ice were counted after melting in a surplus of 0.2-µm-filtered seawater and enrichment over a 20-µm gauze as described above. For each treatment, a colonization index C was calculated as the ratio of live animals in the ice to total live animals (ice and water) by the end of the experiment. Nonparametric U tests and Kruskal–Wallis tests were applied to test for differences in colonization efficiency both between the different experimental approaches (with and without ice present at the start) and between taxa.
Results
Characteristics of the different sample types
Bulk salinity was low and quite uniform in the bottom ice sections of thick ice, whereas it was higher and more variable in thin ice and brash ice (Table 1). Pigments (Chl-a and Phaeo) were enriched in thin and slightly enriched in brash ice in comparison with the underlying water column (Tables 1, 2). Significantly higher pigment concentrations (U test) were found in the bottom ice sections of thick ice compared to thin ice, brash ice and water. Protozoans and rotifers were found in significantly higher numbers (U test) in thin ice and brash ice compared to the underlying water. Platyhelminth abundance was also slightly but not significantly higher (Fig. 2). Abundance of protozoans was found to be highest in bottom sections of thick ice. In bottom sections of thick ice, protozoans were dominant, which was also the case for the water column, while in thin ice rotifers, and in brash ice rotifers and platyhelminthes were dominating. A detailed account of environmental and biological measurements, including abundance of all meiofauna taxa, is given in online resource 2.
Composition of zooplankton and meiofauna in the respective sample types. The relative proportion in terms of median abundance of all metazoans or all meta- and protozoans is depicted in the pie charts, and the median bulk abundance in Ind. l−1 for each group is indicated by the number associated with the respective pie chart piece. Values to indicate the median abundance of metazoa are associated with the left, and of protozoa with the right pie charts
Composition of thick ice meiofauna
Thick ice had the most heterogeneous meiofauna and the highest number of taxa of the ice types examined. The meiofauna was usually dominated by ciliates which accounted for 59–97% (median 83%) of total abundance (Fig. 2; online resource 2). The most abundant metazoan components of the meiofauna were rotifers (range 1–19%; median 6%), Halectinosoma sp. (range 0–31%; median 0%), platyhelminthes (range 0–17%; median 0%) and Tisbe sp. (range 0–5%; median 0%). Nematodes were abundant at one station (8% relative meiofauna abundance at that station). Only few nauplii were found, mainly on the last four stations, which coincides with the observation that egg-bearing harpacticoids (Halectinosoma sp.) were mainly found at those stations. Median egg abundance before 20 August was 19.4 eggs. l−1 (range 0–86.4 eggs. l−1), whereafter considerably more eggs were found, with a median abundance of 582.8 eggs l−1 (range 34.3–1614.7 eggs. l−1).
Composition of the thin ice meiofauna
The thin ice meiofauna was usually dominated by metazoans, with a median proportion of 87% (range: 0–97%) of the total meiofauna abundance (Fig. 2; online resource 2). In particular, the thin ice meiofauna was strongly dominated by rotifers, with a median proportion of 68% (range 0–91%) and maximum abundance of 187.8 Ind. l−1. The only other abundant taxa were ciliates (range 6–100%; median 12%) and platyhelminthes (range 0–26%; median 4%). Nauplii were very scarce (range 0–1%; median 0%), and harpacticoid copepods and nematodes were missing in all thin ice samples, except for one nematode found at station TI-8b.
Composition of brash ice meiofauna
The brash ice meiofauna was always dominated by metazoans, with a median proportion of 73% (range: 68–77%) of the total meiofauna abundance (Fig. 2; online resource 2). Rotifers and platyhelminthes were most abundant with median shares of 40% (range 34–47%) and 31% (range 29–34%), respectively. Ciliates and foraminifera (mainly the pelagic Neogloboquadrina pachyderma) were less abundant with respective shares of 13% (range 12–15%) and 14% (range 11–16%) of the meiofauna. Median total abundance of foraminifera in brash ice was 5.1 Ind. l−1 (range: 4.0–6.2 Ind. l−1), which was higher (not significant, U test) than within thin ice (median 0 Ind. l−1; range 0–2.3 Ind. l−1) and significantly higher (U test) than in bottom ice sections of thick ice (median 0 Ind. l−1; range 0–6.7 Ind. l−1). Harpacticoid copepods and nematodes were missing in all brash ice samples. Few nauplii were found.
Composition of the sub-ice fauna
The sub-ice fauna was always dominated by ciliates accounting for 46–93% (median 75%) of the total abundance. Other protozoan components were foraminifera ranging from 0 to 7% (median 2%) and radiolaria ranging from 0 to <1% (median <1%) of the total zooplankton (Fig. 2; online resource 2). Metazoans made up 6–52% (median 22%). Amongst metazoans nauplii, Oithona sp. and rotifers were most abundant, accounting for 44–67% (median 52%), 18–41% (median 25%) and 5–24% (median 7%) of the zooplankton, respectively. Harpacticoids were not present, but single nematodes were found at two thin ice sampling stations and one brash ice sampling station. Two individuals of the cnidarian Sympagohydra tuuli were found: one at station W-6 and one at W-7.
Enrichment indices
Although radiolarians were present in low, foraminifera and Oncaea sp. in medium and Oithona sp. in high numbers in the water column, none of the four taxa was enriched in thin ice (Table 2). Foraminifera were, nevertheless, strongly and Oncaea sp. slightly enriched in brash ice. Ciliates as well as platyhelminthes, rotifers and eggs were enriched in both ice types, with ciliates being only weakly enriched and the others being strongly enriched. Nauplii were found only in one out of two brash ice stations (070919-Br) and were slightly enriched in thin ice. Abundance of cnidarians, nematodes and harpacticoid copepods in the water column was too low to allow the calculation of a reasonable enrichment index.
Comparison of the different sample types
Total abundance of metazoans was not significantly different between thin, brash and bottom ice sections of thick ice, and metazoans were significantly more abundant in these ice types than in the water column (U test). Protozoans were significantly more abundant in bottom ice sections of thick ice than in thin and brash ice and again significantly more abundant in all three ice types than in the water column. Protozoan abundance did not differ significantly between brash and thin ice. Eggs were significantly more abundant in thin ice than in brash ice and bottom ice sections of thick ice and significantly more abundant in all three ice types than in the water column. Abundance of eggs was not significantly different between brash ice and bottom ice sections of thick ice.
The community structures of each thin ice, brash ice and bottom ice sections of thick ice on the one hand and water on the other hand were significantly different with regard to the abundances of the different taxa (pairwise ANOSIM). Furthermore, also thick ice on the one hand and both thin and brash ice on the other hand differed significantly in meiofauna composition, whereas thin ice and brash ice were not significantly different from each other (pairwise ANOSIM). This is also obvious from a multi-dimensional scaling (MDS) analysis, according to which particularly the water samples are distinctly separated from the ice samples (Fig. 3). Also the thick ice is clearly separated from the other two ice types. The water samples on the one hand and all ice samples on the other hand formed two statistically significant clusters with only 34% similarity between these clusters; the similarity between the likewise significant clusters of thick ice on the one hand and the two other ice types on the other hand was 51% (cluster analysis, SIMPROF).
A SIMPER analysis, based on average contributions of the taxa to overall similarities within sample types or dissimilarities between sample types (within-group similarity, sim, or between-group dissimilarity, dissim, and similarity or dissimilarity divided by standard deviation, sim/sd or dissim/sd), revealed that nauplii, rotifers, Oithona sp. and Oncaea sp. (sim > 9%, sim/sd > 7) typified samples from the water column. The typifying taxa for thin ice as well as for thick ice were rotifers and ciliates (sim > 17%, sim/sd > 3). (Identification of typifying taxa for brash ice was not possible by this method, which requires at least three samples.) Rotifers and platyhelminthes discriminated water from thin ice (dissim > 10%, dissim/sd > 1), while the taxa discriminating water from brash ice were rotifers, foraminifera and platyhelminthes (dissim > 9%, dissim/sd > 8). Thin ice and brash ice were discriminated from each other by ciliates and rotifers (dissim > 10%, dissim/sd > 2). Thick ice was discriminated from thin ice by ciliates, platyhelminthes and Halectinosoma spp. (dissim > 6%, dissim/sd > 1) and from brash ice by ciliates, foraminifera, platyhelminthes and Halectinosoma spp. (dissim > 6%, dissim/sd > 1).
Colonization efficiencies
Tests for differences between the two experiments (ice present when animals were added to the experimental beakers versus ice growing after the addition of animals) revealed no significant differences (U test) in colonization efficiency C for any of the taxa. Therefore, treatments of both experiments were regarded as replicates, and differences in C between the taxa were tested for with a Kruskal–Wallis test, which revealed significant global differences. Mann–Whitney U tests applied thereafter to each pair of taxa revealed that Halectinosoma sp. (median proportion C of organism found within the ice = 0.36) colonized the ice more efficiently than Oithona sp. (median proportion C = 0.00) (Fig. 4). Furthermore, platyhelminthes (median proportion C = 0.83) colonized the ice more efficiently than rotifers (median proportion C = 0.07), and again rotifers colonized it more efficiently than Oithona sp. There were no significant differences between the colonization efficiencies neither of platyhelminthes and Halectinosoma sp., nor between rotifers and Halectinosoma sp. A detailed account of the animals found in the ice and water fraction after the experiment is given in online resource 3.
Discussion
Our study confirms the hypothesis that interspecies differences in dispersal strategies and colonization efficiencies exist for sea ice meiofauna organisms and result in different capabilities to establish and maintain populations within sea ice. Observed differences in sea ice meiofauna communities in the different ice types sampled are likely the result of different long-range dispersal, colonization, survival and reproduction strategies.
Long-range dispersal of sea ice meiofauna
Our comparison of the different ice types revealed significant differences between young and older ice, with the latter harbouring a community that also contains taxa with a benthic lifestyle (harpacticoid copepods, nematodes and platyhelminthes). It is mostly assumed that these species colonize sea ice in shallow water areas of the Eurasian and Alaskan shelves mainly when the sea ice forms (e.g. Kern and Carey 1983; Gradinger et al. 2009). Pack ice motion, especially the transpolar drift, can then result in the long-range dispersal of these organisms throughout the Arctic. Another possibility suggested recently is the colonization of younger ice from older persisting floes (Kramer et al. 2011). The thick ice observed during our study was mainly first-year ice (Haas et al. 2008), probably originating from the Eurasian shelf, but nearby Svalbard and Franz-Josef Land, also second-year ice and third-year ice were found (Haas et al. 2008) and colonization in some areas might thus have occurred from second-year ice. Dispersal within the sympagic realm can take place within the ice and to a larger extent via the sub-ice layer (Kiko et al. 2008). Horizontal dispersal of single organisms within the ice is probably restricted to a few metres. Friedrich and Hendelberg (2001) observed that Arctic acoel platyhelminthes moved with an approximate speed of 14.4 m/d through the brine channel system. As this movement is probably not directed, dispersal distances per day will be considerably smaller and thus not sufficient to effectively colonize new habitats. The sub-ice layer is probably more important for the dispersal of sympagic species (Kiko et al. 2008). During the melt season, sympagic organisms (e.g. harpacticoid copepods) are found within a low-salinity sub-ice layer directly underneath the ice, whereas pelagic species mostly seem to avoid the low-salinity sub-ice layer (Werner 2006). The establishment of a halocline could restrain the dispersal of sympagic organisms vertically and result in almost exclusively horizontal transport in close vicinity to the ice underside. Such a mechanism could thus be effective on scales of at least tens to hundreds of metres. Whether it is effective on larger scales remains to be shown. During our expedition, a low-salinity sub-ice layer was no longer observed from 13 September 2007 onwards (data not shown) shortly prior to the thin ice sampling. This might partly explain why only species with a pelagic origin or good swimming ability were found in the thin ice samples.
Colonization of new sea ice habitats
Sea ice meiofauna species found in the sub-ice layer need to re-colonize the sea ice. We observed signs of both, passive entrainment—the incorporation of sea ice meiofauna organisms into newly forming ice due to physical mechanisms—and active colonization or habitat choice. A slight enrichment of foraminifera in newly formed thin ice was likely the result of passive entrainment. Spindler and Dieckmann (1986) already proposed earlier that high abundances of foraminifera—small, sticky protozoans with no means of active movement—are the result of passive enrichment during frazil ice formation. Active choice of habitats has been suggested to account for differences in community composition of the mobile components of the sub-ice zooplankton and sea ice meiofauna (Werner 2006; Kiko et al. 2008; Gradinger et al. 2010). The results of our colonization experiment partly mirror the results from environmental sampling. Oithona sp. was not found in any of the ice types sampled and was able to avoid incorporation into newly forming artificial thin ice. Enrichment indices for platyhelminthes and rotifers are high for thin ice. For platyhelminthes, this goes in hand with our colonization experiments, during which we observed a very high affinity of platyhelminthes to growing and already present sea ice. The particular enrichment of platyhelminthes observed in bottom sections of Arctic pressure ridges in summer has likewise been suggested to be related to a high sea ice affinity (Gradinger et al. 2010). For rotifers, the environmental results are contrary to our experimental results, as we found a low colonization efficiency in the experiment. As we performed our experiment in the dark, we omitted light as a potentially decisive clue for sea ice colonization. Several rotifers show a positive phototactic response (Clement 1993 and references therein), including sea ice rotifers (S. Siebert, personal communication). In nature, the ice cover is the brightest object in the habitat and rotifers will probably direct their movement to this light source. Studies on Arctic polychaete larvae (Gradinger et al. 2009) and the Antarctic calanoid copepod Paralabidocera antarctica (Tanimura et al. 2002) have shown that these also show positive phototactic responses. Light thus seems to be a major cue for the colonization of sea ice for at least some sympagic meiofauna taxa.
The harpacticoid copepod Halectinosoma sp. showed a considerable affinity to artificial sea ice, but was not found in environmental thin ice samples, nor in the sub-ice water directly underneath. Our experimental design did not test for differences in swimming ability, as the distance between the beaker bottom and the ice underside was only about 8 cm. Therefore, colonization of the artificial ice in our experiment was relatively easy even for taxa with poor swimming ability. We suggest that poor swimming ability was the reason for the absence of harpacticoids in our environmental thin ice samples, as source habitats such as level thick ice floes were several kilometres away. In comparison, thin ice that developed on saline meltwater ponds on level thick ice was colonized by harpacticoid copepods and nematodes (Kramer and Kiko 2011). Similarly, in coastal areas the sea floor and the ice cover are in close proximity, which makes the colonization of sea ice through species with a (partly) benthic lifestyle or poor swimming ability (P. antarctica nauplii, polychaete larvae, harpacticoids and nematodes; Carey and Montagna 1982; Carey 1992; Tanimura et al. 2002; Gradinger et al. 2009) easier. The fact that some of these species like polychaete larvae are only found in coastal sea ice supports the hypothesis of differential dispersal and colonization capacity as a major factor in shaping sea ice communities.
Succession and differential survival
Whereas differences in colonization capacity are one likely explanation for the differences in community structure found for the different ice types, also different suitability of sea ice in terms of abiotic factors, food availability and predation pressure for the incorporated and colonizing organisms is probably partly responsible for the different community structures found. In our MDS analysis, thin ice samples grouped in between thick ice and water, and we found significant differences between thick ice, thin ice and water. This could be interpreted as a change from a community dominated by pelagic organisms (thin ice) to one dominated by organisms with a sympago-benthic lifestyle (thick ice). This is likely a result of a slower colonization of new ice by sympago-benthic organisms, which, nevertheless, are better adapted to a life within the brine channel network due to their evolutionary origin and resulting adaptations to an interstitial lifestyle (Krembs et al. 2000). These adaptations enable them to use the sea ice habitat as a refuge from predators and as a feeding ground with ample food supply. This seems to be especially valid for ciliates, which were much more abundant in thick ice than in thin ice in our data set. Variable, sometimes extreme salinities and temperatures within the brine channel system (Schünemann and Werner 2005), furthermore, select for sympago-benthic organisms, as these are often more salinity tolerant and temperature tolerant than pelagic organisms (Grainger and Mohammed 1990; Gradinger and Schnack-Schiel 1998; Kiko 2010).
Reproduction and seasonality
Sea ice is a very dynamic habitat; large areas are lost every year during the melt season or through export to lower latitudes, where the ice finally melts. Maintenance of viable populations in sea ice seems only possible if successful dispersal strategies are coupled with a reproduction of the respective species, thereby accounting for losses due to displacement into unsuitable habitats. Very high egg abundances were found in thick ice and also in thin ice and brash ice during our late summer/early autumn expedition. Furthermore, in samples taken for life observations, more egg-carrying harpacticoids were found towards the end of the summer season. This goes in hand with the observation of Schünemann and Werner (2005) that in summer, copepodids, platyhelminthes, nematodes and rotifers are found in sea ice, whereas these are nearly absent in winter, when nauplii dominate. We suggest that many meiofauna species reproduce in late summer/early autumn, when the food availability and quality within the ice is still good (as indicated through high Chl-a concentrations in all ice types observed during our study), the habitat becomes more stable (no melting of the ice) and the sea ice habitat extends due to new ice formation.
Deterioration of old ice—who is last?
We interpret the brash ice sampled as being mainly remnants of deteriorated older ice, possibly with a fraction of newly formed ice. The observed meiofauna species composition therefore likely is representative for the last stage of an ice floe and is influenced by the flushing with seawater as indicated by the high bulk salinity of the ice. If we assume that the meiofauna composition of the original ice cover was similar to that observed in our first- and multi-year ice samples, consisting of mainly harpacticoids, nematodes, platyhelminthes and rotifers, we can conclude that flushing is detrimental to nematodes and harpacticoids, likely due to their low swimming ability. Platyhelminthes seem to be able to remain within or in close contact with the ice also under these conditions, likely due to their high affinity to sea ice mentioned above. Nevertheless, it seems that they are also flushed out of the ice, as their enrichment index is lower for brash ice than for thin ice. This is also the case for rotifers. Both taxa are worm like and likely rather negatively affected by flushing in comparison with foraminifers. High abundance and enrichment of foraminifera in the brash ice could be a result of scavenging when the ice is flushed with sea water. Scavenging of particles was observed during formation of brash and pancake ice under turbulent conditions (Ackley 1982; Garrison et al. 1989). Again, differential affinity to the ice and differential scavenging properties are able to partly explain the community composition of the ice sampled.
Summary, conclusions and outlook
The general composition of the Arctic sea ice meiofauna of thick ice > 0.5 m observed during this study is consistent with previous studies (e.g. Gradinger et al. 1999, 2005, 2010). Mainly harpacticoids, nematodes, platyhelminthes and rotifers were found also in 2007. Nevertheless, there are indications that taxa with a low dispersal capacity—which often are dominant and characteristic components of the sea ice meiofauna—are less successful in very young ice (this study) and seasonal sea ice (Kramer et al. 2011). We therefore conclude that concomitant with a decline of multi-year ice, changes in the community composition and possibly also an overall reduction in diversity and abundance already has taken place in the Arctic and will continue in case of further global warming. This conclusion is supported by the observation that thin ice (dark and light nilas) over deep waters is colonized by only few representatives of the Arctic sea ice meiofauna. The area successively covered by thin ice in our sampling region was with 85,000 km2 (the size of, for example, Austria, Europe or South Carolina, USA) extremely large. The increasing likeliness of formation of such extensive new ice covers in a warmer climate will negatively affect the overall diversity of the Arctic sea ice meiofauna as these areas are difficult to colonize.
References
Ackley SF (1982) Ice scavenging and nucleation: two mechanisms for incorporation of algae into newly-formed sea ice. EOS 63:54–55
Barber DG, Galley R, Asplin MG, DeAbreu R, Warner K-A, Pucko M, Gupta M, Prinsenberg S, Julien S (2009) Perennial pack ice in the southern Beaufort Sea was not as it appeared in the summer of 2009. Geophys Res Lett. doi:10.1029/2009GL041434
Barnhart KR, Miller CR, Overeem I, Kay JE (2016) Mapping the future expansion of Arctic open water. Nat Clim Change 6:280–285. doi:10.1038/nclimate2848
Blome D, Riemann F (1999) Antarctic sea ice nematodes, with description of Geomonhystera glaciei sp. nov. (Monhysteridae). Mitt Hamb Zool Mus Inst 96:15–20
Bluhm BA, Gradinger R, Piraino S (2007) First record of sympagic hydroids (Hydrozoa, Cnidaria) in Arctic coastal fast ice. Polar Biol 30:1557–1563
Carey AG Jr (1992) The ice fauna in the shallow southwestern Beaufort Sea, Arctic Ocean. J Mar Syst 3:225–236
Carey AG Jr, Montagna PA (1982) Arctic sea ice faunal assemblage: first approach to description and source of the underice meiofauna. Mar Ecol Prog Ser 8:1–8
Chengalath R (1985) The Rotifera of the Canadian Arctic sea ice, with description of a new species. Can J Zool 63:2212–2218
Clarke KR, Gorley RN (2006) Primer v6: user manual/tutorial. PRIMER-E Ltd, Plymouth, p 190
Clement P (1993) The phylogeny of rotifers: molecular, ultrastructural and behavioural data. Hydrobiologia 255(256):527–544
Frankenstein G, Garner R (1967) Equations for determining the brine volume of sea ice from −0.5 °C to −22.9 °C. J Glaciol 6:943–944
Friedrich C, De Smet WH (2000) The rotifer fauna of Arctic sea ice from the Barents Sea, Laptev Sea and Greenland Sea. Hydrobiologia 432:73–89
Friedrich C, Hendelberg J (2001) On the ecology of Acoela living in the Arctic Sea ice. Belg J Zool 131(Supplement 1):213–216
Garrison DL, Buck KR (1986) Organism losses during ice melting: a serious bias in sea ice community studies. Polar Biol 6:237–239
Garrison DL, Close AR, Reimnitz E (1989) Algae concentrated by frazil ice: evidence from laboratory experiments and field measurements. Antarct Sci 1:313–316
Gradinger RR, Ikävalko J (1998) Organism incorporation into newly forming Arctic sea ice in the Greenland Sea. J Plankton Res 20:871–886
Gradinger RR, Schnack-Schiel SB (1998) Potential effect of ice formation on Antarctic pelagic copepods: salinity induced mortality of Calanus propinquus and Metridia gerlachei in comparison to sympagic acoel turbellarians. Polar Biol 20:139–142
Gradinger RR, Friedrich C, Spindler M (1999) Abundance, biomass and composition of the sea ice biota of the Greenland Sea pack ice. Deep-Sea Res Pt II 46:1457–1472
Gradinger RR, Meiners K, Plumley G, Zhang Q, Bluhm BA (2005) Abundance and composition of the sea-ice meiofauna in off-shore pack ice of the Beaufort Gyre in summer 2002 and 2003. Polar Biol 28:171–181
Gradinger RR, Kaufmann MR, Bluhm BA (2009) Pivotal role of sea ice sediments in the seasonal development of near-shore Arctic fast ice biota. Mar Ecol Prog Ser 394:49–63
Gradinger RR, Bluhm B, Iken K (2010) Arctic sea-ice ridges—safe heavens for sea-ice fauna during periods of extreme ice melt? Deep-Sea Res Pt II 57:86–95
Grainger EH (1991) Exploitation of Arctic sea ice by epibenthic copepods. Mar Ecol Prog Ser 77:119–124
Grainger EH, Mohammed AA (1990) High salinity tolerance in sea ice copepods. Ophelia 31:177–185
Grainger EH, Mohammed AA, Lovrity JE (1985) The sea ice fauna of Frobisher Bay, Arctic Canada. Arctic 38:23–30
Haas C, Pfaffling A, Hendricks S, Rabenstein L, Etienne J-L, Rigor I (2008) Reduced ice thickness in Arctic Transpolar Drift favors rapid ice retreat. Geophys Res Lett. doi:10.1029/2008GL034457
Horner R, Ackley SF, Dieckmann GS, Gulliksen B, Hoshiai T, Legendre L, Melnikov IA, Reeburgh WS, Spindler M, Sullivan CW (1992) Ecology of sea ice biota. 1. Habitat, terminology, and methodology. Polar Biol 12:417–427
Janssen HH, Gradinger R (1999) Turbellaria (Archoophora: Acoela) from Antarctic sea ice endofauna: examination of their micromorphology. Polar Biol 21:410–416
Kern JC, Carey AG Jr (1983) The faunal assemblage inhabiting seasonal sea ice in the nearshore Arctic Ocean with emphasis on copepods. Mar Ecol Prog Ser 10:159–167
Kiko R (2010) Acquisition of freeze protection in a sea-ice crustacean through horizontal gene transfer? Polar Biol 33:543–556
Kiko R, Michels J, Mizdalski E, Schnack-Schiel SB, Werner I (2008) Living conditions, abundance and composition of the metazoan fauna in surface and sub-ice layers in pack ice of the western Weddell Sea during late spring. Deep-Sea Res Pt II 55:1000–1014
Kramer M, Kiko R (2011) Brackish meltponds on Arctic sea ice—a new habitat for marine metazoans. Polar Biol 34:603–608
Kramer M, Swadling KM, Meiners K, Kiko R, Scheltz A, Nicolaus M, Werner I (2011) Antarctic sympagic meiofauna in winter: comparing diversity, abundance and biomass between the western Weddell Sea and the southern Indian Ocean. Deep-Sea Res Pt II 58:1062–1074
Krembs C, Gradinger R, Spindler M (2000) Implications of brine channel geometry and surface area for the interaction of sympagic organisms in Arctic sea ice. J Exp Mar Biol Ecol 243:55–80
Kwok R, Cunningham GF (2015) Variability of Arctic sea ice thickness and volume from CryoSat-2. Phil Trans R Soc A 373:20140517. doi:10.1098/rsta.2014.0157
Kwok R, Cunningham GF, Wensnahan M, Rigor I, Zwally HJ, Yi D (2009) Thinning and volume loss of the Arctic Ocean sea ice cover: 2003–2008. J Geophys Res. doi:10.1029/2009JC005312
Maslanik JA, Fowler C, Stroeve J, Drobot S, Zwally J, Yi D, Emery W (2007) A younger, thinner Arctic ice cover: increased potential for rapid, extensive sea ice loss. Geophys Res Lett. doi:10.1029/2007GL032043
Melia N, Haines K, Hawkins E (2015) Improved Arctic sea ice thickness projections using bias-corrected CMIP5 simulations. Cryosphere 9:2237–2251
Riemann F, Sime-Ngando T (1997) Note on sea-ice nematodes (Monhysteroidea) from Resolute Passage, Canadian High Arctic. Polar Biol 18:70–75
Schünemann H, Werner I (2005) Seasonal variations in distribution patterns of sympagic meiofauna in Arctic pack ice. Mar Biol 146:1091–1102
Serreze MC, Stroeve J (2015) Artic sea ice trends, variability and implications for seasonal ice forecasting. Philos T Roy Soc A 373:20140159. doi:10.1098/rsta.2014.0159
Siebert S, Anton-Erxleben F, Kiko R, Kramer M (2009) Sympagohydra tuuli (Cnidaria, Hydrozoa) – first report from sea ice of the central Arctic Ocean and insights into histology, reproduction and locomotion. Mar Biol 156:541–554
Spindler M, Dieckmann GS (1986) Distribution and abundance of the planktic foraminifer Neogloboquadrina pachyderma in sea ice of the Weddell Sea (Antarctica). Polar Biol 5:185–191
SPSS (2001) SPSS 11.0 brief guide. Upper Saddle River, New Jersey
Stroeve JC, Holland MM, Meier W, Scambos T, Serreze M (2007) Arctic sea ice decline: faster than forecast. Geophys Res Lett. doi:10.1029/2007GL029703
Stroeve JC, Serreze MC, Holland MM, Kay JE, Maslanik J, Barrett AP (2011) The Arctic’s rapidly shrinking sea ice cover: a research synthesis. Clim Change. doi:10.1007/s10584-011-0101-1
Tanimura A, Hoshiai T, Fukuchi M (2002) Change in habitat of the sympagic copepod Paralabidocera antarctica from fast ice to seawater. Polar Biol 25:667–671
Tchesunov A (1986) A new free-living nematode connected with Arctic sea ice. Zoologiceski J 65:1782–1787
Tchesunov AV, Riemann F (1995) Arctic sea ice nematodes (Monhysteroidea) with descriptions of Cryonema crassum gen. n., sp. n. and C. tenue sp. n. Nematologica 41:35–40
Vizcarra N (2016) Sluggish ice growth in the Artic. National Snow and Ice Data Center. http://nsidc.org/arcticseaicenews/2016/11/sluggish-ice-growth-in-the-arctic/. Accessed 9 Nov 2016
Weeks WF, Ackley SF (1986) The growth, structure, and properties of sea ice. In: Untersteiner N (ed) The geophysics of sea ice. NATO Adv Sci Inst Se 146, pp 9–164
Werner I (2006) Seasonal dynamics of sub-ice fauna below pack ice in the Arctic (Fram Strait). Deep-Sea Res Pt I 53:294–309
Acknowledgements
Thanks are first of all due to Iris Werner (Institute for Polar Ecology, Kiel, Germany) for her support during all phases of this study. We are grateful to the captain and the crew of R.V. Polarstern and the chief scientist U. Schauer (Alfred-Wegener-Institute, Bremerhaven, Germany) for constant support during ARK XXII/2. Thanks are also due to the reviewers who helped to improve the manuscript. The help of many colleagues, in particular A. Schneider (Institute for Polar Ecology, Kiel) and S. Siebert (University of California, Davis, USA), during the ice and laboratory work is gratefully acknowledged. Parts of this study were funded by a grant of the German Science Foundation (WE 2536/11–1,–2.). Most of the work presented here was organized with support and conducted at the former Institute for Polar Ecology, Kiel, Germany. Rainer Kiko, Henrike Mütze and Maike Kramer conducted their PhD theses at the Institute for Polar Ecology and would like to thank all colleagues for their support.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kiko, R., Kern, S., Kramer, M. et al. Colonization of newly forming Arctic sea ice by meiofauna: a case study for the future Arctic?. Polar Biol 40, 1277–1288 (2017). https://doi.org/10.1007/s00300-016-2052-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-016-2052-5