Abstract
Various cnidarians have adapted their life style to interstitial habitats of marine sediments. Recently, for the first time a hydroid was reported from the interstitial brine channel system of Arctic fast ice. Due to its derived features, the new genus and species Sympagohydra tuuli was introduced. Here we describe findings of S. tuuli in sea ice at several sites within the central Arctic Ocean. In our view the results of this study do not allow assignment of Sympagohydra to any known family and we, therefore, suggest the introduction of a new family Sympagohydridae which is placed within the hydrozoan subclass Hydroidolina, order Anthomedusae, suborder Capitata. A first detailed histological analysis of S. tuuli is presented. In vivo analysis of locomotion and reproduction revealed a remarkable convergent evolution in S. tuuli and distant meiobenthic relatives. Shared traits are a flagellated epidermis enabling the animals to glide within small interstices by means of flagellar beating as well as an internalised embryogenesis. In S. tuuli gametogenesis occurs in the absence of gonophores inbetween gastro- and epidermis clearly separated from the epidermis. Budding was observed as the vegetative mode of reproduction. Documentation of feeding behaviour identified copepod nauplii and rotifers as prey items and demonstrates a high trophical position of the hydroids within the sympagic food web. Occurrence of reproducing individuals and pronounced tolerances towards changing temperatures and salinities indicate S.tuuli as a truly sympagic species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Life in interstitial habitats necessitates adaptations in size and locomotion and has shaped organisms with sometimes entirely new types of organisation. The limitations in the interstitial space of marine sands or comparable sediments results in trends towards vermiformity (worm-like appearance) and many aberrant interstitial species show larval features (Swedmark 1964). Members of almost all classes of invertebrates can be found in the marine interstitial environments (Giere 1993). Studies on these meiofaunal species, multicellular animals in the size range of about 20 μm–2 mm, contributed essentially to the understanding of evolution and species relationships.
In the twenties of last century, Adolf Remane in Kiel started to focus on the interstitial habitat of marine sediments and enlarged the understanding of a previously neglected biotope. His studies resulted in the discovery of the meiofaunal cnidarian Halammohydra sp. from marine sands (Remane 1927). In the following years, several other small sized cnidarian species with an interstitial life style were described (e.g. Schulz 1950; Swedmark and Teissier 1958; Salvini-Plawen 1966; Thomas et al. 1995) showing varying solutions to the restrictions of the environment. In meiofaunal cnidarians modes of locomotion within the interstitium range from caterpillar-like movement observable in Psammohydra nanna (Schulz 1950), to creeping by use of tentacles like in Cryptohydra thieli (Thomas et al. 1995), to gliding by means of beating flagella in Halammohydra sp. or Otohydra vagans (Remane 1927; Swedmark and Teissier 1958).
The brine channels in sea ice are a very special interstitial habitat, which develops when seawater freezes. The exclusion of ions from the developing ice crystal matrix leads to the formation of brine which concentrates within the ice in small pockets and interconnected channels, in diameter from micro- to millimeters (Weissenberger et al. 1992). Within the brine channels a diverse meiofaunal community is established. Characteristic elements of the Arctic sympagic (ice associated) meiofauna are copepods, plathelminthes, nematodes and rotifers (Schnack-Schiel 2003). Trophic relationships within the sympagic community are largely unknown, but sympagic meiofauna is mainly assumed to feed on sympagic algae (Grainger and Hsiao 1990; Arrigo and Thomas 2004, and references therein; Gradinger et al. 2005). Throughout the year sympagic species are confronted with low temperatures and correspondingly high salinities in winter and moderate temperature and brackish conditions during the summer melting period, temperatures ranging from 0°C to at least −22°C and salinities from close to 0 up to 220 (Schünemann and Werner 2005).
Recently, for the first time a hydroid was reported in fast ice from samples near Barrow, Alaska (Bluhm et al. 2007). Based on a combination of derived characters the new genus and species S. tuuli was introduced (Piraino et al. 2008). Due to the interstitial mode of living and because of the presence of an extensible epidermal foot projection, Sympagohydra was placed within the Protohydridae (Piraino et al. 2008). S. tuuli was found in the lowermost part of coastal fast ice and showed a size range from 0.2 up to 1.1 mm. Although no analysis of gut contents had been conducted yet, a trophic relationship with juvenile stages of copepods and polychaetes was suggested based on results of a factor analysis (Bluhm et al. 2007).
During expedition ARK-XXII/2 with RV “Polarstern” to the central Arctic Ocean and adjacent regions close to Svalbard, Franz-Josef Land and Sewernaja Semlja we studied various aspects of sympagic meiofaunal biology and ecology. S. tuuli was found at several sites. We present observations on cnidome composition, locomotion, modes of reproduction and feeding behaviour as well as a detailed histological analysis. Phylogenetic implications of these findings and convergent adaptations within Cnidaria due to an interstitial life style are discussed.
Methods
Sampling
Sympagic hydroids were collected at six stations during expedition ARK-XXII/2 (28 July–07 Oct 2007, Table 1, Fig. 1) of RV “Polarstern” to the central Arctic Ocean. Level ice was sampled using an engine-powered KOVACS ice corer (internal diameter 9 cm) and bottom ice was collected from blocks sawed from level ice using an ice saw. Large-volume ice samples were taken along the cruise track at various locations (Table 1) by picking up ice pieces directly from the water as described by Kiko et al. (2008). At several stations sub-ice water underneath newly formed sea ice was sampled using a hand-held plankton net (mesh size 20 μm). In order to avoid osmotic and temperature stress for the animals, ice samples were slowly melted in a surplus of 0.2 μm filtered seawater (FSW) at 4°C (Garrison and Buck 1986). Samples were then either transferred to 0°C or directly concentrated over a 20 μm gauze at 0°C. Animals were sorted from the samples at 0°C and kept in 0.2 μm filtered seawater at T = 0°C (±3°C) and S = 33 (±2).
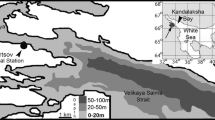
Sampling sites of sympagic hydroids on “Polarstern” cruise ARK-XXII/2 in the central Arctic Ocean and adjacent regions in August–October 2007. White areas are ice covered (ice chart from 15 September 2007, Spreen et al. 2008)
In vivo observation and cnidome analysis
In vivo observations on board were made and documented using a stereomicroscope Leica WILD MZ 12.5 and camera Olympus C-4040ZOOM with stereomicroscope adaptor Olympus C3040-ADU. In vivo observations at Kiel University were made and documented using stereomicroscope Olympus SZX16 and Olympus DP71 digital camera. Feeding behaviour was documented after addition of copepod nauplii and rotifers to the culture medium. Cnidome analysis was conducted after mechanical disruption of nonfixed or fixed tissue and on fixed whole mounts.
Preparation of whole mounts for light microscopy and type conservation
Hydroids were relaxed in 2% urethane prior to fixation in 4% paraformaldehyde. Animals were transferred to 70% ethanol and subsequently to 90% ethanol/glycerol. After ethanol had evaporated animals were put on slides and enclosed using paraffin. Alternatively animals were stained in Grenacher Borax-Carmine (Fluka), differentiated in acidic ethanol, dehydrated in an alcohol series and, after transfer to xylene, embedded using Eukitt resin.
Transmission and scanning electron microscopy
Hydroids were relaxed in 2% urethane prior to fixation in 3.5% glutaraldehyde in 0.05 mol l−1 sodium cacodylate buffer, pH 7.4, for 18 h at 4°C. After washing with 0.075 mol l−1 sodium cacodylate buffer for 30 min, animals were postfixed with 1% OsO4 in 0.075 mol l−1 sodium cacodylate buffer for 2 h at 4°C.
After additional washing with 0.075 mol l−1 sodium cacodylate buffer for 30 min, the tissue was dehydrated in EtOH and embedded in Agar 100 resin (Agar Scientific, Ltd., Essex) for thin sectioning. Semithin sections were stained according to Richardson et al. (1960) with a solution containing 0.5% Methylene Blue, 0.5% borax and 0.5% Azur II in ddH2O at 60°C for 1–2 min and analyzed on a Zeiss Axioscope fluorescence microscope with an Axiocam (Zeiss) digital camera. Ultrathin sections were contrasted with 2.5% uranylacetate for 5 min and lead citrate solution (prepared freshly from lead acetate and sodium citrate) for 2 min (Reynolds 1963) and analyzed using a transmission electron microscope EM 208 S (Philips).
For scanning electron microscopy analyses animals were fixed and postfixed as described above, subsequently critical point dried (BAL-TEC CPD 030) and afterwards sputtered with gold using a sputter coater SCD 050 (BAL-TEC). Samples were examined using scanning microscope DSM 940 (Zeiss).
Conserved material
Specimens 1–2: 84°06.19′N, 109°57.87′E, NOs. ZMK C.1501–1502 (borax carmin stained whole mount). Specimens 3–7: 84°06.19′N, 109°57.87′E, NOs. ZMK C.1503–1506 (glycerol embedded whole mounts). Specimens 8–12: 84°06.19′N, 109°57.87′E, NOs. ZMK C.1507–1510 (Richardson stained semi thin sections). Material is stored at the Zoological Museum of Kiel University, Germany.
Isolation of COI/16S/18S/28S partial DNA sequence information
For the isolation of partial ribosomal DNA sequence information three live animals from station 5 were put in 15 μl Millipore water and boiled for 8 min in the presence of Chelex 100 chelating resins (Sigma). After centrifugation for 15 min at 11,000g, supernatant containing genomic template was used in PCR reactions using primer combinations LSU/28S-F GCTAAGCTTTGACGAGTAGG, LSU/28S-R CTGCCACAAGCCAGTTATC; 18S-F GATCCTGCCAGTAGTCATATG, 18S-R GAGTCAAATTAAGCCGCAGG (Hemmrich et al. 2007). Partial 16S was amplified using primer SHA ACGGAATGAACTCAAATCATGT and SHB TCGACTGTTTACCAAAAACATA (Cunningham and Buss, 1993). Partial cytochrome c oxidase subunit I (COI) was amplified using primer LCO-1490 GGTCAACAAATCATAAAGATATTGG and HCO-2198 TAAACTTCAGGGTGACCAAAAAATCA (Folmer et al. 1994). In the latter two cases DNA template was isolated from the organic phase of a standard TRIZOL RNA extraction from 100 animals (pooled from station 3 and 4). The organic phase was isolated and an equal volume back extraction buffer [4 mol l−1 guanidine thiocyanate, 50 mmol l−1 sodium citrate, 1 mol l−1 Tris (free base)] was added. After mixing and centrifugation at 12,000g for 30 min at 20°C, the aqueous phase was transferred to a new tube. An equal volume of isopropanol was added and the DNA was precipitated by centrifugation at 12,000g for 15 min at 4°C.
Resulting PCR fragments were cloned into pGEMT vector (Promega, Madison, Wisconsin) and transformed into electrocompetent DH10B Escherichia coli cells (Invitrogen, Karlsruhe, Germany). For each gene four plasmids were sequenced using a LI-COR 4300 DNA Analyzer plate sequencer (LICOR Biosciences, Lincoln, Nebraska). Sequences (COI/16S/18S/28S) have been submitted to GenBank (Accession NOs. FJ554626, FJ554625, FJ554628, FJ554627).
Phylogenetic analysis
Phylogenetic analyses were based on a concatenated, Gblocked DNA alignment of partial 16S, 18S and 28S ribosomal sequence information from a previous study (Cartwright et al. 2008, TreeBase accession No. S2066). The alignment (5,046 characters) was complemented with sequence information available for S. tuuli (2,889 bp). As the original alignment had been optimised by deletion of alignment ambiguities respective nucleotides were removed from sequences of S. tuuli. Analogously to Cartwright et al. (2008), the resulting alignment was analysed using the phylogenetic inference packages PAUP* 4.0 (Swofford 1998) and Garli0.96 beta (Zwickl 2006). Maximum parsimony (MP) heuristic analyses (PAUP* 4.0) were performed using 500 random addition sequences, TBR branch swapping and 100 bootstrap replicates with 10 random addition sequences per replicate. Maximum likelihood (ML) searches (Garli0.96) were performed under the GTR + I + G model. The respective rates were estimated using the Akaike information criterion of Model Test (Posada and Crandall 1998). 100 bootstrap replicates (two search replications) were run. Analyses were repeated four times and delivered consistent results. The data matrix and the ML consensus tree can be found in the supplementary material (S2).
Salinity and temperature tolerance
Test animals (n = 10 each) were transferred stepwise with 24 h acclimation time in each transit media (ΔS = 5 with temperatures adjusted to the respective freezing point at high salinities) from filtered seawater FSW (S = 33) to salinities of S = 5 and S = 80 and temperatures of 0°C and −4.6°C, respectively (tested salinity/temperature (°C) combinations: 5/0.0; 7.5/0.0; 10/−0.6; 15/-0.9; 20/-1.1; 25/−1.4; 30/-1.7; 40/-2.2; 45/-2.5; 50/-2.8; 55/-3.1; 60/-3.5; 65/-3.7; 70/-4.0; 75/-4.4; 80/-4.6). Control animals (n = 10) were held at 0°C in FSW (S = 33). Medium in the control was exchanged in parallel to that of the treatments to mimic handling stress. Water temperatures were adjusted with a thermobath. Filtered seawater was adjusted to the respective salinities by diluting with MilliQ® water or by adding artificial sea salt (Tropic Marin®). Salinities were measured with a WTW microprocessor conductivity meter LF 196 (accuracy: S = ±0.2) or for salinities above 70 using a hand-held refractometer (accuracy: S = ±1.0). Media were adjusted to the respective temperatures, before the animals were transferred. Flagellar movement was taken as the sign of vitality and checked after each incubation step. When the animals did not move anymore, the experiment was stopped, the animals were transferred stepwise back to FSW (S = 33) and vitality was checked again.
Results
Distribution
Hydroids (355 in total) were found in ice samples at six different sites in the central Arctic Ocean in the time from August to October (Fig. 1, Table 1). Four hydroids (two individuals each) were collected from samples of the lowermost 10 cm of an ice core or blocks of sea ice (Stations 1, 4). 350 animals were collected from samples of sea ice directly taken from the water (Stations 2, 3, 5). A single hydroid was found in sub-ice water from underneath newly formed sea ice (Stations 6). Water depth at the sampling sites varied from 692 to 4,450 m.
General morphology and histology
Hydroids showed a vermiform to ovoid body shape (Fig. 2a–c). Body length in live animals varied from 193 to 1,040 μm and body width from 64 to 355 μm (n = 25). A row of three to four filiform tentacles surrounds a central tubular hypostome terminating in a simple mouth-opening (Fig. 2a, b). The tentacles are highly extensible and can reach up to twice the body length in relaxed state (Fig. 2a, and also below in Fig. 6g). In vivo observations demonstrate a proboscis-like organisation of the hypostome which is extensible and can be bent to all sides (supplementary video V1). While tentacles are highly contractile and react immediately upon mechanical disturbance, the body column does not perform fast contractions upon the same treatment. The aboral part varies in size and shape between individuals but always has an epidermis clearly separated from the gastrodermis. Inbetween these two epithelial layers reside highly vacuolated cells associated with the gastrodermis. More basally an acellular mesoglea can be observed (Fig. 2b).
Habitus of Sympagohydra tuuli. a Live animals showing varying body shape and differentially well developed aboral parts of the body column. t: tentacle, ga: gastrodermis, scale bar 200 μm. b Live animal in contracted state with gonadal cell clusters (g) and highly vacuolated cells (v) basally from the gastrodermis (ga). h Hypostome, t tentacle, ep epidermis, m acellular mesoglea, scale bar 100 μm. c Live animal with sparsely distributed nematocysts (nc) on the body column. Scale bar 100 μm
Histology revealed insights into the internal anatomy of the polyps (Figs. 3, 4, 5). The epidermis consists of a uniform epithelial layer along the whole body column and epidermal cells are characterised by a prominent nucleus (Figs. 2b, 3a, b). These nuclei give the epidermal surface a structured appearance. Longitudinal sections of various animals support the existence of an acellular mesoglea in the aboral part of the body column in some individuals (Fig. 3b). Analysing the gastrodermis revealed that the lining of the hypostome is regionally specialised (Fig. 3a). Within the upper part of the hypostome the gastrodermis is composed of secretory cells of two types—a granular one, containing numerous, small, regular granula, and a spumous one with irregular vesicle morphology (Figs. 3a, 4a, b). The gastrodermis of the lower hypostome is composed of highly vacuolated cells (Figs. 3a, 4c). The nuclei of these cells are orientated towards the lumen of the hypostome. Generally gastrodermal epithelial cells are ciliated (Fig. 4a). Within the body column the gastrodermis contains gland cells with zymogenic vesicles (Fig. 3a, 4d) and epithelial cells packed with phagocytic vacuoles (Fig. 3a). The gastrodermis of the tentacles shows a chordal organisation (Fig. 3f) and is separated from the gastrodermis of the body column by a supporting lamella.
Internal anatomy of Sympagohydra tuuli. a Longitudinal section of whole animal. c, d and e indicate levels of cross sections from different animals as shown in Fig. 3c, d and e, respectively. st: stenotele, sc: secretory cell region, to: tentacle onset, dn developing nematocysts, g gonadal cell cluster, gc gastric cavity, ep epidermis, z zymogenic gland cell, scale bar 50 μm. b Longitudinal section of an individual showing the basal part with highly vacuolated cells (v) and an acellular mesoglea (m). Scale bar 50 μm. c Cross section of the hypostome shortly below the secretory cell region with a stenotele (st) residing within the gastrodermis. t Tentacle, scale bar 50 μm. d Cross section shortly above the tentacle formation zone with stenoteles (st) residing within the gastrodermis and gonad (g) within the inter-tentacle region. n Nematocysts of the tentacle, scale bar 50 μm. e Cross section below the tentacle formation zone showing two major gonadal cell clusters (g) and developing nematocysts (dn). The epidermis (ep) is separated by a thin mesolamella (m). gc Gastric cavity, scale bar 50 μm. f Cross section of a tentacle showing chordal organization of the gastrodermis. Scale bar 50 μm
Ultrastructure of Sympagohydra tuuli. a Cross section of the apical part of the hypostome showing oral stenoteles (st), ciliated gastrodermal cells and a region with secretory cells of two types—spumous secretory cells (ssc) and granular secretory cells (gsc). Mo Mouth opening. b Enlargement of a showing different vesicle morphologies. gr granular vesicle, sp: spumous vesicle. c Cross section of the hypostome shortly below the secretory cell region showing vacuolated gastrodermal cells and well defined longitudinal (L) and radial (R) myoid processes. ep epidermis, ga gastrodermis, m mesoglea. d Zymogenic gland cell of the gastrodermis. e Gonadal cell cluster (g) consisting of homogenous appearing cells. The gonad resides in between gastrodermis and epidermis, which is separated by the mesolamella. z zymogenic gland cell of the gastrodermis
Cnidome composition of Sympagohydra tuuli. a Light microscopic image of a tentacle and the apical part of the hypostome with stenoteles around the mouth opening and a single nematocyst type within the tentacle. n Nematocyst, st stenotele, mo mouth opening, scale bar 50 μm. b Ultrastructure of an oral stenotele within the epidermis. c Stenotele (non-fixed) of the oral region (left) and schematic drawing. Scale bar 10 μm. d Nematocyst of the tentacle (non-fixed) with thread coiled in two large loops (left) and a small one (middle) and schematic drawing. Scale bar 10 μm. e Ultrastructure of a nematocyst of the tentacle bearing a cnidocil (cn). f Nematogenic region with developing nematoblasts in different developmental stages. un undifferentiated nematoblast, en early nematoblast with nascent capsule, ln late nematoblast containing capsule with internalised shaft. g Ultrastructure: separation of nematogenic region from the epidermis (ep) by the mesolamella (m). ln capsule of late nematoblast
Well-developed myoid processes in both epidermal layers can be found in the hypostome (Fig. 4c) and the tentacles. This corresponds to the flexibility of both structures.
Cnidome
When analysing the cnidome of the hydroids, a group of stenoteles [fixed (n = 10): length 7.2–8.1 μm (mean 7.4 μm), width 4.9–6.3 μm (mean 5.5 μm)] could be observed around the hypostome (Figs. 3a, 4a, 5a–c). Light and electron microscopy revealed a single type of nematocysts within the tentacles (Fig. 5a, d, e) [fixed (n = 19): length 6.2–8.8 μm (mean 7.3 μm), width 2.8–4.8 μm (mean 3.3 μm), non-fixed (n = 54): length 6.5–10.6 μm (mean 8.9 μm), width 2.8–4.5 μm (mean 3.7 μm)]. In undischarged states the thread is coiled within the capsule in two larger loops and a smaller one (Fig. 5d). In discharged nematocysts, the length of the extruded isodiametric thread reaches 3.3–3.8 times the capsule length (Fig. 5a). This corresponds to the length of the coiled shaft in undischarged capsules. Capsules of the tentacle bear a cnidocil (Fig. 5e). Electron microscopy revealed the thread to be atrichous. Within the body column a third capsule type [fixed (n = 16): length 5.0–6.6 μm (mean 5.7 μm), width 2.6–3.8 μm (mean 3.4 μm)] could be identified (Fig. 2c) but no detailed insight into capsule morphology could be gained.
Using semithin sections and electron microscopy, developing nematoblasts could be documented at the height of the tentacle formation zone in between gastro- and epidermis (Figs. 3a, e, 5f). Figure 5f shows a cluster of differentiating nematoblasts in which three developmental stages can be distinguished. Undifferentiated cells are characterized by a large nucleus with a developed nucleolus. In early differentiating nematoblasts nascent capsules and a well-developed endoplasmatic reticulum can be identified. In later differentiation stages the internalised shaft is visible within the capsule. Cross-sectioning revealed stenoteles within the gastrodermis at various levels of the hypostome (Fig. 3c, d) indicating a translocation process towards the place of deployment at the apical region of the hypostome. Formation of nematocysts occurs apart from the epidermis which is clearly separated by the mesolamella (Fig. 5g).
Development and reproduction
Sexually mature animals (Figs. 2b, 3a) were found at sampling sites 2, 3, 5 and 6. In between gastro- and epidermis at the height of and below the tentacle formation zone reside up to four dense clusters of cells which we determined to be gonads (Figs. 2b, 3a, d, e, 4e). These clusters are predominantly located in the inter tentacle regions (Fig. 3d). Such packs of cells could be observed in an animal showing embryogenesis (Fig. 6a, b) and were interpreted as female gonads. The gametes are of gastrodermal origin as the epidermis is clearly separated by the mesolamella. In none of the histologically analysed animals (n = 14) male gonads characterised by developing sperm could be observed.
In vivo observation of internal embryogenesis and asexual reproduction via budding in Sympagohydra. a Polyps produce a single egg which develops internally. g gonadal cell cluster, scale bar 200 μm. b–f Developmental stages at 9,17, 24, 57 and 120 h after start of observation. From time point 70 h (not shown) the embryo was observed moving within the brood pouch. g gonad, scale is the same as in a. g Polyp bearing an advanced bud without distinct aboral part (arrow). Scale bar 100 μm
Embryogenesis could be documented in one case (Fig. 6). The parental polyp showed a single gonadal cell cluster (Fig. 6a, b). Hydroids produce an egg of around 100 μm diameter which develops internally in a brood chamber in between gastro- and epidermis close to the gastrodermis at a basal position (Fig. 6a). Cleavage of the egg is total (Fig. 6b). The first two cleavages generate four equal blastomeres (Fig. 6c). In the following blastomeres displace themselves and the embryo takes an indefinite form (Fig. 6d). Cell divisions were difficult to follow after 35 h. After around 70 h the embryo was observed moving within the brood chamber indicating the presence of flagellated ectoblastic cells. Movement of the embryo intensified in the next few hours. As evident from Fig. 6, body morphology of the parental polyp changed greatly over time (Fig. 6a–f). The parental polyp was lost after 120 h due to a freezing event in the dish. No signs of morphological differentiation of the larvae were detected to this time point.
In four cases asexual reproduction via budding could be observed. Hydroids were found bearing almost mature buds which detached in the following. Bud formation took place shortly below the tentacle formation zone (Fig. 6g). In all observed cases attached young polyps were lacking a well-developed basal part (Fig. 6g).
Locomotion
Flagellated epidermal cells could be documented all over the body column up to the tentacle region (Fig. 7a) enabling a gliding mode of locomotion. Analysis of flagellated cells in greater detail revealed a prominent nucleus and a strong flagellar root with an accessory centriol (Fig. 7b, c). In these ectodermal cells of the lower body column myoid processes are not well developed. Apical ends of flagella show a shovel-like modification (Fig. 7d). In vivo observations allowed documentation of beating flagella (supplementary video V2). Depending on the beating mode animals move either with the oral or the aboral end ahead. The beating direction of flagella and consequently the direction of the gliding can be swiftly changed. During a directional change animals slow down and start over with increasing speed after a short detention (supplementary video V3). When rising in the water column—which always happened with the aboral end ahead—animals rotate around the longitudinal axis (supplementary video V4). Rising in the water column was frequently observed when temperatures in the culture medium were increased. Animals could be found gliding at the edge of the dish just below the surface of the medium.
Epidermal flagella in Sympagohydra tuuli used for locomotion. a Electron microscopic image showing flagellated epidermal cells all over the body column up to the tentacle region. t Tentacles, scale bar 20 μm. b Epidermal cell from the lower body column with prominent nucleus (n) and single flagellum. Scale bar 2 μm. c Enlargement from b flagellar root (r) and accessory centriol (as). Scale bar 1 μm. d Broadenings of flagella at distal ends (arrow)
Phylogenetic analysis
The phylogenetic relationship of S. tuuli was accessed using a concatenated alignment for 16S, 18S and 28S ribosomal sequence information which was based on an alignment of a previous study. The resulting trees under ML and MP (not shown) criterion corresponded to topologies presented in Cartwright et al. (2008) (Fig. 8, for full tree see supplement Fig. S1). Both ML and MP (not shown) analyses suggested a relationship of S. tuuli to species of a recently introduced filiferan clade Gonoproxima (Fig. 8).
Phylogenetic hypothesis using the maximum likelihood criterion on a concatenated dataset comprising partial 16S, 18S and 28S ribosomal sequence information of 112 hydrozoan taxa. For clarity reasons subtrees are compressed except for the clade Gonoproxima which houses Sympagohydra tuuli (for the full tree see Suppl. Fig. S1). Bootstrap values greater than 50 are indicated. Estimated parameters of the assumed model GTR + I + G were: A-C, 0.8877; A-G, 2.9294; A-T, 1.6218, C-G, 0.8694; C-T, 5.2844; G-T, 1.0000; assumed proportion of invariant sites: 0.5775, gamma shaped parameter 0.6086
Feeding behaviour
To document feeding behaviour, rotifers and copepod nauplii were offered to the hydroids and could be identified as prey items. Once they struck a tentacle or the hypostome, prey was captured and killed. In many cases swallowing of the prey item could be observed. The maximum prey size was only negligibly lower than the size of the hydroid (Fig. 9a–c). The reddish colour of the gastrodermis could be shown to result from pigment granules originating from prey as starvation lead to loss of colouration.
Salinity and temperature tolerance
As hydroids are exposed to a changing salinity and temperature regime throughout the year tolerances towards these abiotic factors were tested. After stepwise transfer to S = 75 and T = −4.4°C as well as to S = 10 and T = 0°C followed by a 24 h incubation period animals were still observed to move by means of flagellar beating. Movement of the animals was no longer observed after 24 h incubation at T = −4.6°C and S = 80 as well as at T = 0°C and S = 5. When slowly brought back to S = 33 animals resumed movement indicating that these salinities and temperatures are at least temporarily tolerated.
Discussion
Hydroids from Arctic pack ice of this study were determined as S. tuuli as they share the characteristic basal part of the body column and a whorl of solid filiform tentacles (Piraino et al. 2008). Furthermore stenoteles are present at the apical region of the hypostome. In contrast to the original description of S. tuuli, only a single nematocyst type was found within the tentacles, which we could not assign to any known capsule type. This capsule is characterised by an isodiametric, atrichous thread, which in undischarged state shows two large loops and a small one. The length of this coiled thread corresponds to the observed length of threads under discharged condition (3.3–3.8 times the capsule length) which indicates full extrusions. These observations could point to the capsule type isorhiza whereas the appearance of the undischarged capsule would be rather atypical. If the extruded part was interpreted as a shaft this could point to a macrobasic mastigophore but a continuing thread could not be detected neither in undischarged nor in discharged capsules. Alternatively, this capsule may be interpreted as a modified desmoneme. Piraino et al. (2008) reported a third capsule type, i.e. microbasic mastigophores, which was found within the tentacles and rarely on the body column. Consistently we found nematocysts sparsely distributed along the body column, but in this study no detailed insights into the capsule morphology could be gained. We could not find this type of capsule within the tentacles, which may indicate that they are present only in very low numbers within this part of the body.
Unlike originally reported for S. tuuli, the tentacles of the hydroids were found to be highly extensible, and flagella were observed to cover the whole body column up to the tentacle formation zone. Interestingly, the late bud of S.tuuli does not possess a distinct aboral part which has to be developed after detachment. In our view varying shape of the aboral end reflects, in part, different developmental stages of the animals characterised by a differentially well-developed acellular mesolamella or mesoglea. Generally, changing of the body shape is in our view a rather slow mechanism. This corresponds to differentially well developed myoid processes in epidermal cells of the body column compared to epithelial cells of mobile tentacles and hypostome. An unfolding mechanism of the foot-like structure as described before (Piraino et al. 2008) could not be observed in this study. Observed differences to the original species description may point to the existence of a second species within the genus Sympagohydra. In our view, however, the inconsistencies found are more probably due to different modes of analysis. Sequence data from hydroids originating from various sites across the Artic Ocean as well as further comparative morphological and histological analyses, including in vivo observations, are needed for the sake of clarity.
Phylogenetic position of S. tuuli
Hydrozoa comprise two well-supported monophyletic clades—the Trachylina and the Hydroidolina (Collins et al. 2006). Traditionally, within the Hydroidolina the order Anthomedusae is formed of two clades Capitata and Filifera (Petersen 1990). Morphological criteria for the latter separation are e.g. the presence of stenoteles, which can be found in the capitate members and which are absent in filiferans (Bouillon et al. 2004). However, sequence based phylogenies question this dichotomy and the monophyly of Anthomedusae in general (Marques and Collins 2004; Collins et al. 2006). A recent analysis based on a concatenated dataset for the three ribosomal genes 16S, 18S and 28S gave within Hydroidolina support for four separate filiferan and two distinct capitate clades, the Capitata sensu stricto and the Aplanulata (Cartwright et al. 2008). The relationship of these clades among each other and the relation to the two further orders of Hydroidolina, the Leptomedusae and the Siphonophorae, are not clarified (Cartwright et al. 2008).
To get insights into the phylogenetic position of S. tuuli using molecular markers, we complemented the dataset of the previous study (Cartwright et al. 2008) with the available sequence data of S. tuuli. In both ML and MP analyses S. tuuli grouped with filiferan species of a lately introduced clade Gonoproxima, particularly to Lizzia blondina (Bougainivilliidae) and Rathkea octopunctata (Rathkeidae) (Fig. 8). This is a highly unlikely relationship not only because of the presence of stenoteles in S. tuuli which clearly indicates a relation to capitate species. The Gonoproxima are suggested to comprise filiferan families whose members bear gonophores on hydrocauli, pedicels or stolons as a synapomorphy (Schuchert 2001; Cartwright et al. 2008) whereas embryogenesis in S. tuuli is internal. The unexpected grouping in the phylogenetic analysis might be explained by a confined resolution of the chosen molecular markers, at least for the sequence data to date available for S. tuuli. It can be also possible that this finding is caused by an extensive sequence divergence within S. tuuli compared to capitate relatives. Finally, this outcome may partly reflect the evolving status of the molecular phylogenies and be a hint for a new clade to be discovered sharing filiferan and capitate features with S. tuuli being a representative. From the developmental point of view, the observation of a flagellated planula state which is traversed during internal embryogenesis would suggest a closer affiliation of S. tuuli to a new clade Capitata sensu stricto rather than to capitate species of a clade Aplanulata. In absence of a robust molecular phylogeny, at present we tend to place S. tuuli within the traditional clade Capitata as it was initially suggested (Piraino et al. 2008).
Sympagohydra tuuli shows a unique combination of characters which separates it from known hydroids of the Capitata. A general motive in the sexual reproduction of various hydroids is the formation of gonophores. In S. tuuli sexual reproduction happens in the absence of such structures. Gonadal cell clusters develop in between gastro- and epidermis and gametes form apart from the epidermis which is clearly separated by the mesolamella (Figs. 3, 4). The latter is in contrast to the majority of hydrozoan representatives where gametes are generally of epidermal origin (Bouillon et al. 2004). Analysis of nematogenesis gives a hint for the presence of an interstitial cell population (Fig. 5), which is associated with the gastrodermis. The capitate hydroid Protohydra leukarti also lacks gonophores in its sexual reproduction and is known to produce a single egg. In contrast to S. tuuli, gametes in P. leukarti are of epidermal origin, the egg is expelled by a rupture of the body column and the parental polyp is reported to die after the egg is released (Westblad 1935).
Another character of S. tuuli is the clonal propagation via budding. In various hydroids and also in most meiobenthic capitate representatives, in contrast, asexual reproduction is realised by transverse fission, like in Cryptohydra thieli (Thomas et al. 1995), Psammohydra nana (Schulz 1950), Boreohydra simplex (Westblad 1937) and in the genus Protohydra, although for the latter the formation of lateral buds in rare cases has been described (Schulz 1952). Furthermore, there are no adhesive structures in Sympagohydra, which in capitate hydroids are generally present in the form of a pedal disc like in Psammohydranana (Schulz 1950) or in the form of a small epidermal disc for temporary attachment like in Protohydra leuckartii (Schuchert 2006). Finally, a flagellated epidermis is not known from adult hydroids of the Capitata.
Immigration of a meiobenthic ancestor into the channel system of sea ice is an imaginable scenario. However, to date we have no hints for a relationship of S. tuuli to known meiobenthic species. Considering the probably derived state of S. tuuli and in the absence of good hints from molecular data the closest relatives within the Hydroidolina in general remain elusive. In our view, the described combination of morphological and developmental features, like the mode of reproduction, the absence of adhesive structures, a flagellated epidermis used for locomotion in adult hydroids, location and genesis of gametes and nematocysts and the presence of a before uncharacterised nematocyst type within the tentacle region does not allow assignment of S.tuuli to the Protohydridae, like proposed before (Piraino et al. 2008), nor to any of the known capitate families. We suggest, instead of leaving the genus incerta sedis, the introduction of a new family Sympagohydridae and we propose the following taxonomy for the genus.
Taxonomy of Sympagohydra
-
Subclass Hydroidolina
-
Order Anthomedusae Haeckel 1879
-
Suborder Capitata Kühn 1913
-
Family Sympagohydridae fam. nov.
-
Genus Sympagohydra Piraino 2008.
Definition of Sympagohydridae
Small solitary polyps with three to four filiform (chordoid) tentacles and slightly extensible, proboscis-like hypostome. Aboral part with separated gastro- and epidermis with flagellated ectodermal cells. Type genus is Sympagohydra (Piraino et al. 2008).
Convergent evolution in interstitial cnidarians
In S. tuuli locomotion is performed by movement of the flagella. So far with the Halammohydridae and the Otohydridae only two hydrozoan families of meiobenthic cnidarians are described in which flagella are used for locomotion in the adult. Members of both families have medusal characters, e.g. statocysts, and are, based on morphological and developmental criteria, combined within the taxon Actinulida (Swedmark and Teissier 1959). The Actinulida are systematically placed within the sister taxon to the Hydroidolina—the Trachylina. Newest molecular data support this systematic position for Halammohydra sp. (Collins et al. 2008). Although a common origin for Actinulida and Sympagohydra can therefore be excluded, the locomotory and the reproductive analogy from S. tuuli especially to Otohydra vagans are striking. While in Halammohydra sp. also the tentacles are used for moving within the interstitium (Remane 1927), movement by means of flagellar beating seems to be the exclusive way of locomotion in the genus Otohydra (Swedmark and Teissier 1958) paralleling Sympagohydra. Both S.tuuli and O. vagans miss ectodermal adhesive structures for attachment.
While the Halammohydridae form a single egg, which is afterwards attached to the substrate (Remane 1927), in O. vagans embryogenesis is internalised and the animals show a viviparous breeding behaviour (Swedmark and Teissier 1958). Viviparity in S. tuuli like in O. vagans is indicated but remains to be shown. Depending on the monophyly of Actinulida, movement mediated by flagella in adult interstitial hydrozoans therefore has been developed independently for two or even three different times on the way into the interstitia of the sediments or the brine channel system of sea ice. Flagella as a general larval feature may have been kept in independent paedomorphic processes. Interestingly, in the actinulid species movement has been described to be with the aboral end ahead (Remane 1927, Swedmark and Teissier 1958). We could show that S. tuuli is capable to directional changes via inverting flagellar beating, suggesting perfect navigation in the three-dimensional environment of the brine channel system in sea ice. Remane (1927), analogously to the observations here, reports rotation of Halammohydra around the longitudinal axis when rising in the water column.
Role of Sympagohydra in the sympagic ecosystem
Initially, S.tuuli was reported from Arctic coastal fast ice at different sites at Barrow, Chuchki Sea (Bluhm et al. 2007). S.tuuli was recently also found in coastal fast ice as well as in pack ice in the Western Canadian Arctic, near Banks Island (M. Kramer unpublished). In the present study hydroids were found in pack ice at various sites within the central Arctic Ocean extending the known geographical distribution. Considering large scale drift patterns of sea ice within the Arctic Ocean this indicates a circumpolar occurrence of the species. Findings of the hydroids in coastal fast ice with low water depth suggests bentho-sympagic coupling and infiltration processes at different times of the year (Bluhm et al. 2007). However, great water depths at the various sites in the central Arctic Ocean contradict this idea. Occurrence of reproducing individuals and the pronounced tolerance towards changing temperatures and salinities rather support the view that S. tuuli is a truly sympagic species, although occurrence of an individual in a sample from sub-ice water in an area with new-ice formation indicates that hydroids leave the ice at least temporarily.
In the present study we give the first direct evidence of predation within sea ice, as we could directly demonstrate sympagic copepod nauplii and rotifers to be part of the prey spectrum of S. tuuli. A potential importance of heterotrophs in sympagic food webs had been recognised before (Gradinger 1999; Werner 2006), and Bluhm et al. (2007) hypothesized the role of S. tuuli as top predator due to correlations of abundances with those of polychaete larvae and copepods. Nevertheless, except for few studies on some sympagic copepods species (Hoshiai et al. 1987, Schnack-Schiel et al. 1995, 2004) and one study of gut contents including sympagic meiofauna (Grainger and Hsiao 1990), there have been no specific experimental or analytical studies concerning the feeding ecology of sympagic meiofauna so far.
Predatory impact of a benthic hydroid was shown for Protohydra leuckartii which regulates directly the density of accessory species (Heip and Smol 1976). The high trophic level of sympagic hydroids in combination with their Arctic-wide distribution suggests an essential role in the sympagic food web of Arctic sea ice.
References
Arrigo KR, Thomas DN (2004) Large scale importance of sea ice biology in the Southern Ocean. Antarct Sci 16(4):471–486. doi:https://doi.org/10.1017/S0954102004002263
Bluhm BA, Gradinger R, Piraino S (2007) First record of sympagic hydroids (Hydrozoa, Cnidaria) in Arctic coastal fast ice. Polar Biol 30:1157–1563. doi:https://doi.org/10.1007/s00300-007-0316-9
Bouillon J, Medel MD, Pages F, Gili JM, Boero B, Gravili C (2004) Fauna of the Mediterranean Hydrozoa. Sci Mar Barc 68(Suppl. 2):1–448
Cartwright P, Evans NM, Dunn CW, Marques AC, Miglietta MP, Schuchert P, Collins AG (2008) Phylogenetics of Hydroidolina (Hydrozoa: Cnidaria). J Mar Biol Assoc UK. doi:https://doi.org/10.1017/S0025315408002257
Collins AG, Bentlage B, Lindner A, Lindsay D, Haddock SHD, Jarms G, Norenburg JL, Jankowski T, Cartwright P (2008) Phylogenetics of Trachylina (Cnidaria: Hydrozoa) with new insights on the evolution of some problematical taxa. J Mar Biol Assoc UK. doi:https://doi.org/10.1017/S0025315408001732
Collins AG, Schuchert P, Marques AC, Jankowski T, Medina M, Schierwater B (2006) Medusozoan phylogeny and character evolution clarified by new large and small subunit rDNA data and an assessment of the utility of phylogenetic mixture models. Syst Biol 55:97–115. doi:https://doi.org/10.1080/10635150500433615
Cunningham CW, Buss LW (1993) Molecular evidence for multiple episodes of paedomorphosis in the family Hydractiniidae. Biochem Syst Ecol 21:57–69. doi:https://doi.org/10.1016/0305-1978(93)90009-G
Folmer O, Black M, Hoen W, Lutz R, Vrijenhoek R (1994) DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biol Biotechnol 3:294–299
Garrison DL, Buck KR (1986) Organism losses during ice melting: a serious bias in sea ice community studies. Polar Biol 6(4):237–239. doi:https://doi.org/10.1007/BF00443401
Giere O (1993) Meiobenthology: the microscopic fauna in aquatic sediments. Springer, Berlin
Gradinger R (1999) Integrated abundance and biomass of sympagic meiofauna in Arctic and Antarctic pack ice. Polar Biol 22(3):169–177. doi:https://doi.org/10.1007/s003000050407
Gradinger R, Meiners K, Plumley G, Zhang Q, Bluhm BA (2005) Abundance and composition of the sea-ice meiofauna in off-shore pack ice of the Beaufort Gyre in summer 2002 and 2003. Polar Biol 28(3):171–181. doi:https://doi.org/10.1007/s00300-004-0674-5
Grainger EH, Hsiao SIC (1990) Trophic relationships of the sea ice meiofauna in Frobisher Bay, Arctic Canada. Polar Biol 10(4):283–292. doi:https://doi.org/10.1007/BF00238427
Heip C, Smol N (1976) On the importance of Protohydra leuckarti as a predator of meiobenthic populations. In: Persoone G, Jaspers E (eds) Proceedings of the 10th European marine biology symposium, vol 2, Population dynamics of marine organisms in relation with nutrient cycling in shallow waters. Universa Press, Wetteren, pp 285–296
Hemmrich G, Anokhin B, Zacharias H, Bosch TCG (2007) Molecular phylogenetics in Hydra, a classical model in evolutionary developmental biology. Mol Phylogenet Evol 44(1):281–290. doi:https://doi.org/10.1016/j.ympev.2006.10.031
Hoshiai T, Tanimura A, Watanabe K (1987) Ice algae as food of an Antarctic ice-associated copepod, Paralabidocera antarctica (I. C. Thompson). Proc NIPR Symp Polar Biol 1:105–111
Kiko R, Kramer M, Spindler M, Wägele H (2008) Tergipes antarcticus (Gastropoda, Nudibranchia): distribution, life cycle, morphology, anatomy and adaptation of the first mollusc known to live in Antarctic sea ice. Polar Biol. doi:https://doi.org/10.1007/s00300-008-0478-0
Marques AC, Collins AG (2004) Cladistic analysis of Medusozoa and cnidarian evolution. Invertebr Biol 123:23–42
Petersen KW (1990) Evolution and taxonomy in capitate hydroids and medusae (Cnidaria: Hydrozoa). Zool J Linn Soc-Lond 100:101–231. doi:https://doi.org/10.1111/j.1096-3642.1990.tb01862.x
Piraino S, Bluhm BA, Gradinger R, Boero F (2008) Sympagohydra tuuli gen. nov. and sp. nov. (Cnidaria: Hydrozoa) a cool hydroid form the Arctic sea ice. J Mar Biol Assoc UK. doi:https://doi.org/10.1017/S0025315408002166
Posada D, Crandall KA (1998) Modeltest: testing the model of DNA substitution. Bioinformatics 14(9):817–818. doi:https://doi.org/10.1093/bioinformatics/14.9.817
Remane A (1927) Halammohydra, ein eigenartiges Hydrozoon der Nord- und Ostsee. Z Morph U Ökol Tiere 7:643–677
Reynolds AS (1963) The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol 17:208–212. doi:https://doi.org/10.1083/jcb.17.1.208
Richardson KC, Jarett L, Finke EH (1960) Embedding in epoxy resins for ultrathin sectioning in electron microscopy. Stain Technol 35:313–323
Salvini-Plawen vL (1966) Zur Kenntnis der Cnidaria des nordadriatischen Mesopsammon. In: VI. Meeresbiologisches Symposium. Veröffentlichungen des Instituts für Meeresforschung in Bremerhaven, Sonderband 2:165–186
Schnack-Schiel SB, Thomas D, Dieckmann GS, Eicken H, Gradinger R, Spindler M, Weissenberger J, Mizdalski E, Beyer K (1995) Life cycle strategy of the Antarctic calanoid copepod Stephos longipes. Prog Oceanogr 36(1):45–75. doi:https://doi.org/10.1016/0079-6611(95)00014-3
Schnack-Schiel SB (2003) The macrobiology of sea ice. In: Thomas DN, Diekmann GS (eds) Sea ice. Introduction to its physics, chemistry, biology and geology. Blackwell, Oxford, pp 211–239
Schnack-Schiel SB, Dieckmann GS, Kattner G, Thomas DN (2004) Copepods in summer platelet ice in the eastern Weddell Sea, Antarctica. Polar Biol 27(8):502–506. doi:https://doi.org/10.1007/s00300-004-0613-5
Schuchert P (2001) Hydroids of Greenland and Iceland (Cnidaria, Hydrozoa). Medd Gronl Biosci 53:1–184
Schuchert P (2006) The European athecate hydroids and their medusae (Hydrozoa, Cnidaria): Capitata. Part 1. Rev Suisse Zool 113:325–410
Schulz E (1950) Psammohydra nana, ein neues solitäres Hydrozoon in der westlichen Beltsee (Studien an Hydrozoa, II). Kiel Meeresforsch 7(2):122–137
Schulz E (1952) Ungeschlechtliche Fortpflanzung durch Knospung bei Protohydra leuckarti Greef (Studien an Hydrozoa, III). Kiel Meeresforsch 7:68–69
Schünemann H, Werner I (2005) Seasonal variations in the distribution patterns of sympagic metazoans in Arctic pack ice. Mar Biol (Berl) 146:1091–1102. doi:https://doi.org/10.1007/s00227-004-1511-7
Spreen G, Kaleschke L, Heygster G (2008) Sea ice remote sensing using AMSR-E 89-GHz channels. J Geophys Res 113:C02S03. doi:https://doi.org/10.1029/2005JC003384
Swedmark B, Teissier G (1958) Otohydra vagans n. g., n.sp., hydrozoaire des sables, apparanté aux Halammohydridées. C R Acad Sci Paris 247(2):238–240
Swedmark B, Teissier G (1959) Halammohydra et Otohydra, Hydrozoaires de la microfaune des sables et l’ordre des Actinulides. Proceedings XV Int Congr Zool, pp 330–331
Swedmark B (1964) The interstitial fauna of marine sand. Biol Rev Camb Philos Soc 39:1–42. doi:https://doi.org/10.1111/j.1469-185X.1964.tb00948.x
Swofford DL (1998) PAUP phylogenetic analysis using parsimony (and other methods). Sinauer, Sunderland
Thomas MB, Edwards NC, Higgins RP (1995) Cryptohydra thieli n. gen., n. sp.: a meiofaunal marine hydroid (Hydroida, Athecata, Capitata). Invertebr Biol 114:107–118. doi:https://doi.org/10.2307/3226883
Weissenberger J, Dieckmann G, Gradinger R, Spindler M (1992) Sea ice: a cast technique to examine and analyze brine pockets and channel structure. Limnol Oceanogr 37:179–183
Werner I (2006) Seasonal dynamics, cryo-pelagic interactions and metabolic rates of Arctic pack-ice and under-ice fauna: a review. Polarforschung 75(1):1–19
Westblad E (1935) Neue Beobachtungen über Protohydra. Zool Anz 111:152–158
Westblad E (1937) Boreohydra simplex n. gen., n. sp., ein Solitärpolyp von der norwegischen Küste. Arkiv Zool 29:1–6
Zwickl DJ (2006) Genetic algorithm approaches for the phylogenetic analysis of large biological sequence datasets under the maximum likelihood criterion. Ph.D. dissertation, The University of Texas at Austin
Acknowledgments
We thank Thomas C.G. Bosch for providing excellent laboratory facilities and him and Iris Werner for generous support. Furthermore we thank both for critically reading and improving the manuscript. We also thank Peter Schuchert for comments on the manuscript, discussion and helpful advises, Boris Anokhin for discussion, help with nematocyst analysis and the stenotele drawing, Antje Thomas for help with histology, Stefano Piraino for sharing unpublished data, Alice Schneider for help in the field, Gunnar Spreen for providing the map of sampling sites, Kristina Barz for sharing digital devices, the captain and crew of RV “Polarstern” for professional support, Sebastian Fraune for help with molecular phylogenetic analysis, Dirk Brandis and Sievert Lorenzen for useful comments. This work was supported, in part, by grants to Iris Werner from the Deutsche Forschungsgemeinschaft (WE 2536/11-1).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by U. Sommer.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplement S1.
Phylogenetic hypothesis among 112 hydrozoan taxa using themaximum likelihood criterion on a concatenated dataset comprising partial 16S, 18Sand 28S ribosomal sequence information. Bootstrap values greater than 50 areindicated. Estimated parameters of the assumed model GTR+I+G were: A-C, 0.8877; AG,2.9294; A-T, 1.6218, C-G, 0.8694; C-T, 5.2844; G-T, 1.0000; assumed proportion ofinvariant sites: 0.5775, gamma shaped parameter 0.6086. (6.96 MB)
Supplement S2.
File containing the data matrix used in the phylogenetic analysis and consensus tree of the ML analysis. (TXT 830 kb)
V1. Bending of proboscis-like hypostome. Two tentacles are visible. Gastrodermal cells contain vacuoles with reddish prey pigment. (WMV 4911 kb)
V2. Locomotion of Sympagohydra tuuli. Beating flagella on epidermal cells of the body column. (WMV 7177 kb)
V3. Locomotion of Sympagohydra tuuli. Beating flagella facilitate gliding movement. Tentacles can be seen at oral end of the animals. Beating mode and direction of gliding can be changed. (AVI 3012 kb)
V4. Locomotion of Sympagohydra tuuli. Animals rotate around their longitudinal axis when rising in the water column. (WMV 5255 kb)
Rights and permissions
About this article
Cite this article
Siebert, S., Anton-Erxleben, F., Kiko, R. et al. Sympagohydra tuuli (Cnidaria, Hydrozoa): first report from sea ice of the central Arctic Ocean and insights into histology, reproduction and locomotion. Mar Biol 156, 541–554 (2009). https://doi.org/10.1007/s00227-008-1106-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-008-1106-9