Abstract
-
1.
The formation of sea ice impacts directly on the physical dynamics of water masses (e.g. wind stress at the sea surface) and air-sea exchange processes (e.g. vertical heat fluxes).
-
2.
The annual cycle of formation, consolidation and melting of sea ice has a major influence on the ecology of both the benthic and pelagic components of the Baltic Sea ecosystem.
-
3.
There is considerable inter-annual variation in the extent of sea ice in the Baltic Sea and thus in the size of the habitat for sympagic (ice-associated) microbial and metazoan communities as well as for larger organisms living on the ice, notably the ringed seal.
-
4.
There is a pronounced gradient in ice characteristics, from more saline ice in the south of the Baltic Sea to freshwater ice in the north. The former is more porous and supports more ice-associated biology than the latter.
-
5.
The Baltic sympagic communities consist mainly of prokaryotic and eukaryotic microbes (bacteria, diatoms, dinoflagellates, flagellates), ciliates and rotifers. These communities are recruited from the plankton when the ice forms, followed by an ice-adapted successional pattern with an expansion of substrate-bound pennate diatoms, which does not occur in the seawater beneath the ice.
-
6.
The sea-ice food webs inside the ice are truncated compared to the open-water food webs because organisms larger than the upper size limit of the brine channels are lacking in the internal sympagic communities.
-
7.
Global climate change decreases the extension and thickness of the sea ice as well as the length of the ice season, and therefore the seasonal effects that sea ice has on the Baltic Sea winter-spring ecosystem dynamics.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Baltic Sea gradient
- Brine channels
- Climate change
- Productivity
- Sea ice habitat
- Sympagic communities
- Truncated food web
1 Sea ice is an integral part of the Baltic Sea ecosystem
1.1 Ice shapes the ecology of the Baltic Sea
One of the major drivers of the seasonal dynamics in the ecology of the Baltic Sea is the formation of sea ice (cf. Figs. 2.17–2.19). Vast areas of the sea are covered each winter by an ice layer that restricts the penetration of light, reduces water mixing by winds and changes heat and momentum flux (Leppäranta and Myrberg 2009). These physical changes strongly impact the biology living under the ice since water circulation and mixing are so clearly altered. A good example is that river plumes extend under the ice to a much larger distance and with higher stability than in ice-free conditions. Under-ice river plumes not only alter the mixing properties of the waters, they also result in changed ice growth dynamics, and ice-associated (sympagic ) biological communities, with the underside of the ice being encased, in the extreme case, with a frozen freshwater layer (Granskog et al. 2006).
Baltic Sea ice reaches its maximum extent in February-March with a maximum thickness of ~0.8 m for landfast ice and 0.3–0.5 m for offshore ice, even during mild winters in the Bothnian Bay (Vihma and Haapala 2009). Further to the south, the thickness of the ice decreases. Many shallow coastal benthic habitats of the northern Baltic Sea are disturbed by ice scouring (cf. Sect. 11.2.5). The ice itself forms a temporary habitat for many plankton organisms: here they undergo a “lifestyle” shift from a viscous, sparsely populated liquid habitat to one comprised of a semi-solid matrix with liquid inclusions and dense communities of organisms (Thomas and Dieckmann 2010).
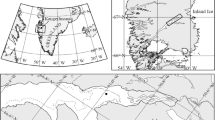
One of the most striking features of a winter Baltic Sea scene is how similar the sea-ice surface looks to the ice-covered Arctic and Southern Oceans (Fig. 9.1). However, while there are similarities between the biology associated with ice formed from the Baltic Sea water and that associated with thicker sea ice in the Arctic and Southern Oceans, findings from the frozen polar oceans cannot necessarily be transcribed to the frozen Baltic Sea and vice versa. This is mainly a result of the parent waters’ different salinities where the ice is formed.
Pack ice in the Baltic Sea and in the Arctic Ocean . On the surface it all looks the same, but underneath the snow the systems are quite different. (a) The Finnish research vessel Aranda in the Baltic Sea sea ice. (b) The Swedish ice-breaker Oden in the Arctic Ocean sea ice. Photo: (a) © Ilkka Lastumäki, (b) © Pauline Snoeijs-Leijonmalm
1.2 Sea ice and biogeochemical cycling
Sympagic communities , consisting of the organisms living within and on the sea ice, as well as in the seawater immediately below the ice, have been a source of study since the early days of polar exploration, and it is clear that they have a key role in the ecology of ice-covered waters (Thomas and Dieckmann 2002, 2010). This is also true of the Baltic Sea ice habitat. In the early days of sea-ice biological research, the scientists’ curiosity about the identity of the ice-associated organisms was probably the primary driver for many of the studies.
The ice itself has been considered a relatively impervious barrier between air and water. Nowadays we know that the ice compartment significantly contributes to biogeochemical cycling since biological and chemical processes within the ice and at the ice peripheries can considerably influence gas fluxes to and from the atmosphere as well as matter fluxes between the ice and the surface waters (Rysgaard et al. 2011).
The ice cover can also modify nutrient fluxes and heavy metal concentrations as well as those of other chemical pollutants. Chemical compounds can accumulate in snow and ice, following atmospheric deposition , and/or by organism uptake or regeneration within ice, followed by subsequent pulsed releases into the water column during spring melt (Granskog and Kaartokallio 2004). For example, for the Bothnian Bay it has been estimated that 5 % of the total annual flux of nitrogen and phosphorus and 20–40 % of the total annual flux of lead and cadmium may be deposited on to the ice fields from the atmosphere (Granskog et al. 2006).
2 Characteristics of Baltic Sea ice
2.1 Seasonal and spatial variation of the ice cover
Annually, sea ice covers an average of ~44 % of the Baltic Sea water surface area and the median maximum ice extent for the period 1961–2010 was 186,000 km2 (Fig. 9.2, Table 9.1). However, the interannual variability in the maximum ice extent is large as it ranges from 10 % to 100 % of the Baltic Sea water surface area (Vihma and Haapala 2009).
Map of the Baltic Sea, showing the probability of sea-ice coverage. The locations 1–12 denote the measuring stations for the ice statistics presented in Table 9.1. The annual duration of the sea-ice coverage for the time period 1961–2010 are plotted for Station 1 (Ajos), Station 4 (Valassaaret), Station 12 (Suursaari, Gogland in Russian) and Station 7 (Märket). The left-hand side of each annual bar indicates the first appearance of ice and the right-hand side indicates the final disappearance of ice. The dark-blue regions of each bar represent the period of permanent ice cover and the light-blue regions represent the period of temporary ice cover . The absence of a bar indicates no ice cover at the station. Figure based on ice data from the Finnish Ice Service. Figure: © Jouni Vainio
Ice formation begins along the coasts in the northernmost Bothnian Bay and in the easternmost Bothnian Sea, usually in November. Next to freeze is the shallow and narrow Norra Kvarken sill between the Bothnian Bay and the Bothnian Sea, followed by the coastal areas of the Bothnian Sea. In average winters, the ice covers the Gulf of Bothnia, the Archipelago Sea , the Gulf of Finland, the Gulf of Riga and the northern part of the Baltic Sea proper. The areas along the Polish and German coasts can also freeze over and in severe winters the southern Baltic Sea proper and the Belt Sea are also covered with ice.
Locally, ice formation usually starts within sheltered bays and around skerries (small rocky islands) and the ice edge moves outward from the coasts as the winter progresses (Fig. 9.3). Ice forms first at the inner skerries and bays where the water is often fresher and shallower than in the open Baltic Sea (and thus has a lower heat content), and where the ice cover can be anchored to islands and shoals. The landfast ice cover usually extends to the outer skerries, where the water depth is typically between 5 and 15 m. Along with increasing solar radiation in spring, the ice begins to melt starting from the south. The northern Baltic Sea proper is normally open by the beginning of April. By early May the sea ice is present only in the Bothnian Bay, and by early June it has usually also completely melted in the far north of the Baltic Sea.
In the early stages of ice formation when there is significant turbulence in the water, the first stages of ice formation may involve the formation of “pancake ice ”. The water movement induces the ice crystals to coalesce into small discs (“pancakes”) that become progressively larger. These can freeze together to form closed ice sheets . Under more quiescent conditions, more typical of coastal waters, the ice cover tends to be a rather more uniformly flat sheet ice that is cracked and fissured through larger scale water movements .
2.2 Ice formation
Ice formation in brackish waters with salinity below 24.7, such as the Baltic Sea water, resembles more the ice formation in freshwater lakes than that in the oceans. When brackish water cools down to the freezing point (which is determined by salinity and pressure), the temperature of the maximum density of fresh and brackish waters is reached prior to this (cf. Sect. 2.4.4, Fig. 2.17b). Consequently, vertical convection ceases.
Moreover, the physical processes at the ice/water interface that result in the salinity distribution in the solidifying sea-ice matrix may differ, partly because thermal convection at the ice/water interface is restricted as the result of the low salinity of the Baltic Sea water (Granskog et al. 2006). Even though the northern Baltic Sea has low surface-water salinity (cf. Fig. 2.15), at salinities higher than ~0.6 in the parent water (the water mass ice forms from), the ice structure resembles that of sea ice . Close to the mouths of rivers where large volumes of freshwater enter the coastal waters the ice often has typical freshwater ice properties .
2.3 Salinity and temperature define ice properties
The structure of sea ice differs from that of freshwater ice as some of the salts and other dissolved constituents of the parent water, which are not incorporated into the crystal lattices, are entrapped between the ice crystals and form a hypersaline liquid solution referred to as “brine”. Brine inclusions within sea ice form pockets and interconnected channels, and the brine concentration and corresponding brine volume are directly proportional to the ice temperature (Thomas and Dieckmann 2010).
In addition, brines move inside the ice sheet and across the ice/water interface due to gravity drainage and thermodynamic processes. For the establishment of a sympagic community, and the flux of water and/or gas through the ice or across the ice/water interface, the most important physical parameters are the ice porosity (i.e. the total volume of brine inclusions) and the permeability (the ability to transport fluid) of the ice. Sea ice is generally more porous than freshwater ice, the ice porosity being affected by the interaction of temperature, brine salinity and brine volume.
According to the “Law of fives ”, sea ice with a temperature of −5 °C and a bulk sea-ice salinity (melted sea ice including brine) of 5 has a brine volume of 5 % (Golden 2009), which makes it permeable because brine pockets and inclusions are then assumed to be interconnected. However, for the Baltic Sea the “Law of fives ” obviously does not hold true since the bulk sea-ice salinity does not reach 5, except in the very southernmost parts (Granskog et al. 2006). However, it can be calculated that for ice with a bulk salinity of 1, a temperature of −1 °C is needed for brine volumes to be large enough for Baltic Sea ice to become permeable (“Law of ones ”, Leppäranta and Manninen 1988).
For this reason, much of the biology of Baltic Sea ice is restricted to the bottommost part of ice floes , where dense accumulations of organisms give the ice a distinctive brownish colouration in the permeable layer (Fig. 9.4). The rest of the ice, the “internal ice habitat ” without visible colouration, has extremely low biological activity because it contains brine of too low a salinity to allow for the interconnection of fluids and permeability. The extent of the permeable layer varies in the different basins of the Baltic Sea as a function of brine salinity and temperature. Thus, as brine salinity and temperature decrease northwards, the permeable layer of the ice becomes narrower towards the north of the Baltic Sea (Fig. 9.5).
Schematic presentation of sea ice in three different sea areas of the northern Baltic Sea: (a) the Bothnian Bay, (b) the Bothnian Sea, (c) the western Gulf of Finland. Note the increasing size of the brine channels from (a) to (c). The figures show the snow layer (grey), the internal sea-ice layer with no or very low biological activity due to low ice porosity (blue) and the Biologically Active Layer in the lower part of the sea ice (yellow). P indicates the direction and size of the phosphorus flux in the different sea areas. Figure modified from Kuparinen et al. (2007)
2.4 Desalination
Sea ice contains a fraction of salts entrapped in so-called “brine channels ” and pockets between ice crystals . Brine channels form an interconnected system that allows brine movement inside the ice as well as brine transport from the ice to the underlying water. Since the major part of the sea ice volume consists of pure ice crystals, and all dissolved constituents, including salts, are in the brine fraction, the transport of saline brines out of an ice sheet causes desalination of the ice.
Ice salinity is a dynamic variable, governed by initial brine entrapment during ice formation and subsequent desalination processes. The initial brine entrapment depends on the salinity of the parent water and the growth velocity of the ice, with faster ice growth at lower temperatures allowing for more brine entrapment between the crystal lattice at the ice bottom and thus higher bulk ice salinity. The initial salt entrapment into growing ice has been measured to be smaller in the Baltic Sea than in marine sea ice (Uusikivi 2013). Once the ice is formed, several processes cause brine loss and thereby desalination.
In general, the desalination processes can be subdivided into those in colder ice during ice growth and those in warmer ice with increased ice porosity (Thomas and Dieckmann 2010). Cold-ice processes are related to pressure build-up within the brine channel system when the ice temperature changes, which can lead to brine expulsion from the upper surface of the ice, and the growth of frost flowers, or at the ice/water interface, brine loss to the underlying water. Other processes are based on gravity drainage, i.e. cold saline brines flowing downwards in the ice due to their higher density and brine channel flushing due to the pressure built up melt water accretion in the upper surface of the ice (Granskog et al. 2003a).
Warming of the sea ice leads to melting at the brine channel walls, enlargement of the brine channels and coalescence of isolated brine inclusions (Meese 1989; Weeks 1998). Ice warming thus increases both the ice porosity and the connectivity between the ice and the underlying seawater. The most efficient desalination processes are related to changes in ice porosity. Since the porosity of Baltic Sea ice is generally lower than that of marine sea ice due to the low salinity of the parent water (Meiners et al. 2002), it can be expected that desalination processes function differently. Quantitative information on desalination processes in Baltic Sea ice is virtually non-existent (Granskog et al. 2010), but measurements made by Uusikivi et al. (2006) suggest that the salinity fluxes from ice to seawater in the Baltic Sea are small compared to those from the sea ice formed in ocean waters.
3 Baltic sea ice as a habitat
3.1 Brine channels
For sympagic organisms, there are several habitats within and associated with the sea ice in which they thrive. Within the ice sheet , the brine channel system is the primary habitat of the ice biota (Fig. 9.5). This is a semi-enclosed system, consisting of partially interconnected small pockets and elongated vertical channels that form at ice-crystal junctions when the ice sheet grows. In the bottommost permeable layer , the brine channels are open to the underlying water and enable the movement of motile organisms into the ice. Not only the brine, but also the brine channel ice surfaces, are colonised by sympagic organisms , and many of the biological and chemical interactions in the ice system may be more like those found in aquatic biofilms than in pelagic systems .
The brine channel habitat is characterised by steep vertical gradients in temperature and salinity that can change on a diel scale following temperature changes. Salinity, pH, dissolved inorganic nutrients and dissolved organic matter within the brine channels change over seasonal scales following the succession of the sympagic communities . Also, the light field inside the ice may rapidly vary as a result of changes in the incoming solar radiation in combination with snow cover thickness. Inside the brine channels all biomass is confined to a space of 2–10 % of the total ice volume, and the brine channel habitat is typically densely packed with organisms compared to the underlying water. The maximum chlorophyll a (Chl a) concentrations, which may be regarded as a measure of the phototrophic biomass , can reach values of 800–2,000 µg Chl a L−1 in the brine fraction (Granskog et al. 2006). The brine channel diameter sets an approximate upper limit of 0.2 mm for organism body size (Weissenberger et al. 1992), although channels can be larger during the melting phase.
3.2 Spatial variability of ice properties
The other main sea ice habitat, besides brine channels, is the upper ice surface habitat, which consists of slush layers at the ice/snow interface and meltwater ponds on the ice. In addition, ice-bottom habitats occur at the ice/water interfaces, and these consist of a porous skeletal ice layer associated with relatively stable water layers immediately beneath the ice.
The spatial variability in sea-ice properties is controlled mainly by the bulk sea-ice salinity in the different parts of the Baltic Sea since the bulk salinity regulates the ice porosity and habitable space within the ice (larger space with higher salinity). Thus, the variability in ice properties on a subregional scale is largely controlled by the Baltic Sea salinity gradient (cf. Fig. 2.15, Meiners et al. 2002; Granskog et al. 2003a).
The regional- and local-scale variation in sea-ice properties in coastal areas is mainly caused by onshore-offshore gradients in salinity created by river water inflows (Granskog et al. 2005; Steffens et al. 2006; Piiparinen et al. 2010). However, even on small scales (tens of metres) the variability in ice properties can be as large as that on local or regional scales (Steffens et al. 2006). The bulk salinity of the ice bottom typically reflects the inshore-offshore salinity gradients in the ice parent water. It has been suggested that this controls the amount and distribution of sea-ice phototrophic biomass (as Chl a ) and the composition of the sympagic communities in the Baltic Sea (Kaartokallio et al. 2007; Piiparinen et al. 2010), as well as in other non-polar sea ice-covered areas (Thomas and Dieckmann 2010).
3.3 Landfast ice versus pack ice
The fundamental differences between landfast ice and pack ice (Fig. 9.3) are the location of formation (coast versus open sea) and the dynamics (drift/ridging/rafting versus stability). This is expected to result in different sympagic communities in these two classes of ice. As drifting pack ice is transported by wind and currents, the sympagic community represents the species composition at the site of ice formation rather than a community typical of the area to where the ice has drifted.
Thus, at the same latitude , the landfast ice , which remains more or less at the same spot from ice formation to spring thaw, may differ greatly in biological properties from the pack ice in the same area. In the Bothnian Bay, the low-biomass early stages of sympagic communities were found to be similar in both types of ice (Piiparinen et al. 2010; Rintala et al. 2010b). However, with the advancing ice season , the communities start to deviate, especially in terms of chlorophyte , ciliate, and rotifer biomass. Chlorophytes (e.g. Chlamydomonas sp. and Dictyosphaerium sp.) show decreasing trends from landfast to pack ice, while ciliates (e.g. Lacrymaria rostrata and Strombidium sp.) and rotifers (mainly Synchaeta cf. littoralis ) show the opposite trend (Meiners et al. 2002; Piiparinen et al. 2010; Rintala et al. 2010b).
3.4 High amounts of snow-ice are typical of the Baltic Sea
As a consequence of the relatively heavy snow load on Baltic Sea ice , the weight of the snow frequently submerges the ice surface. Thus, the seawater floods the upper ice surface and “snow-ice ” is formed when the flooded layers freeze (Granskog et al. 2003b). The superimposed ice is the ice formed from the freezing of the snow melt during melt-freeze cycles brought about by short-term warm weather events (Fig. 9.6), and is a favourable habitat for sympagic communities . This habitat type is especially important in the low-salinity Bothnian Bay, where the low brine volumes inside the ice greatly constrain the habitability of the ice (Fig. 9.5).
The development of an ice cover at Santala Bay (Gulf of Finland) during winter 1999. The snow/granular ice interface is shown as the reference level (0 cm). Measurements of δ18O showed that the snow-ice layer consisted of a mixture of ice and seawater while the superimposed ice was formed from melted snow alone. Figure modified from Kawamura et al. (2001)
In the Bothnian Bay , the nutrient-rich and well-illuminated snow-ice habitat is primarily occupied by chain-forming centric diatoms , with Melosira arctica (Fig. 9.7) as the dominant species (Piiparinen et al. 2010; Rintala et al. 2010b). A dominance of centric diatoms in the ice-surface layer has also been observed in the Gulf of Finland, but here the dominant species is Chaetoceros wighamii (Kaartokallio et al. 2007). The contribution of centric diatoms to the total sympagic biomass generally decreases from the Bothnian Bay to the Gulf of Finland, possibly due to the decrease of snow-ice (Rintala et al. 2010b).
3.5 Rafting and ridging
Currents or winds often push undeformed ice, pancake ice and larger ice floes around so that they slide over each other, a process known as rafting. Thicker sea ice may fracture and pile up under the influence of strong winds and pressure in the ice, forming ridges on the ice surface. The formation of pressure ridges is a common phenomenon in the pack ice of the Baltic Sea and they are typically 3–5 m thick (Kankaanpää 1997). However, freely floating ridges of up to 25 m thick have been observed (Haapala et al. 2015).
In addition to an increase in ice thickness and changes in the ice structure profiles, ridges often increase the sympagic biomass in the ice. Peaks of chlorophyll a and other biomass estimates in the centre of rafted sea ice are typical signs of rafting. These biomass peaks originate from the bottom-ice communities of the overlying ice floe and/or the surface-ice communities of the underlying ice floe (Rintala et al. 2010b). Occasionally the dynamic forces in a pack ice field may flip ice floes, resulting in the bottom-ice communities becoming effective ice-surface communities and vice versa. Thus, dynamic events in the pack ice may subject sympagic communities to changed physico-chemical conditions under which e.g. irradiation and/or nutrients reaching the organisms may be reduced or increased.
4 Productivity in Baltic Sea ice
4.1 Factors that regulate growth of sympagic organisms
The key factors regulating the growth and succession of autotrophic sympagic organisms are light, salinity and nutrients, while for heterotrophic organisms the key factors are salinity and organic matter . In general terms, the main nutrient supply in the internal sea ice habitat consists of the initial nutrients incorporated when the ice once formed. In older ice brine channels , exchange can result in the transport of nutrients across the ice/water interface. Concentrations of nitrogenous inorganic nutrients in melted sea ice are typically higher than those in the under-ice water, whereas dissolved inorganic phosphate concentrations show an opposite trend (Kuparinen et al. 2007).
The snow cover on the ice accumulates nutrients carried by precipitation , and these nutrients can be transported down into the ice sheet through snow-melting during warm weather (Granskog et al. 2003a; Granskog and Kaartokallio 2004). The recycling of nutrients from allochthonous (transported into the system) and autochthonous (produced within the system) biomass in the sea ice, through decomposition and nutrient regeneration carried out by sympagic heterotrophs , can also be an important source of nutrient supply inside the ice. Here the main actors are heterotrophic bacteria , which presumably degrade both particulate and dissolved organic matter and regenerate nutrients, as in other aquatic systems. However, phagotrophic protists are also likely to be key nutrient regenerators in the sea-ice environment (Kaartokallio 2004).
4.2 Autotrophic biomass and primary production
About 85 % of the sympagic communities’ total biomass in the Baltic Sea consist of microalgae (Figs. 9.8 and 9.9). Autotrophic growth in Baltic Sea ice is sequentially light-limited and nutrient-limited as winter progresses, as in polar sympagic communities . Phosphorus is thought to be the most important single limiting nutrient (Haecky et al. 1998; Kuosa and Kaartokallio 2006).
Community composition in sea ice and seawater based on the examination of integrated samples taken at three ice stations in the Bothnian Bay (BB) and three ice stations in the Gulf of Finland (GF) in March 2000. (a) Community composition in 23–30 cm thick sea ice after melting in the laboratory. (b) Community composition in the water column immediately under the ice (at a 0–10 m water depth ). The numbers above the bars denote the total biomass in µg C L−1. Figure modified from Meiners et al. (2002)
Examples of microorganisms found in sea ice: (a) the dinoflagellate Heterocapsa arctica subsp. frigida , (b) the dinoflagellate Scrippsiella hangoei , (c) a ciliate, (d) the prasinophyte Pyramimonas sp., (e) the dinoflagellate Scrippsiella hangoei during its transformation into a pellicular cyst when the cell has already shredded the flagellate and is withdrawn from its theca, (f) the cyanobacterium Nodularia spumigena , not a sympagic species (g) a diatom chain of Skeletonema sp. cells, (h) two diatom chains of Pauliella taeniata cells seen from different angles. All images show organisms from Baltic Sea ice samples or from cell cultures isolated from Baltic Sea ice samples. All images were taken under light microscopy , except for (b), which was taken with scanning electron microscopy (SEM). Photo: © Janne-Markus Rintala
Succession of eukaryotic community composition based on sequencing of the 18S rRNA gene from integrated samples of the Gulf of Finland and the Gulf of Bothnia. OTU = operational taxonomic unit . Figure modified from Majaneva et al. (2012)
Model simulation of the thickness of the snow layer (grey), the internal sea-ice layer with no or very low biological activity (blue) and the Biologically Active Layer in the sea ice (yellow) during the ice season 1999–2000 at Santala Bay in the Gulf of Finland. Figure modified from Tedesco et al. (2010)
Modelled sea-ice and pelagic chlorophyll a concentrations during the ice season and the pelagic spring bloom in April-May at Santala Bay (Gulf of Finland), showing two simulations. Ref = winter 1999–2000 with 138 days below the freezing point of the brackish Baltic Sea water and an ice cover , yielding a pelagic bloom dominated by diatoms. B2 = IPCC climate change scenario B2 SRES with only six days below the freezing point of the brackish Baltic Sea water and no ice cover, yielding a pelagic bloom dominated by flagellates. Figure: © Letizia Tedesco
The in situ level of primary production in sea ice is difficult to quantify due to the lack of suitable methods (Box 9.1). Haecky and Andersson (1999) estimated that the ice-algal production (~0.1 g C m−2) accounted for only ~1 % of the total annual open-sea production (ice and pelagic) and ~10 % of the total open-sea production during the ice-cover season. However, photosynthetic parameters measured in Baltic Sea ice point to a highly variable, but at times very active, primary productivity in all parts of the Baltic Sea (Piiparinen et al. 2010; Rintala et al. 2010b; Piiparinen and Kuosa 2011).
Box 9.1: Sampling of Sympagic Communities
Ice coring
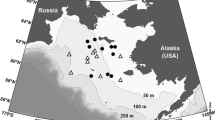
Sampling of sea ice presents some intriguing difficulties because, in order to investigate the organisms living in the ice itself, the habitat has to be destroyed (Box Fig. 9.1). This means melting the ice. Sampling the water column under the ice is not quite as problematic, but it is nonetheless not a trivial undertaking if the ice is thick. The polar, lake and Baltic Sea ice is sampled mainly by using ice corers that are power-driven, or on thin ice (<50 cm), hand-held. Most commercially available corers produce cores with diameters around 10 cm (Box Fig. 9.1a). During the ice core removal, the core is compromised in that the brine it contains is drained out. There is no solution to this problem, and draining is largest when the ice is warm and brine channels are wide and strongly interconnected. Other problems involve the warming up and melting of the ice core and a change in the light environment. Thus, the highest priority is to sample the ice before these environmental changes induce alterations to the biology and/or chemistry of the ice that are too significant. Traditionally, the retrieved ice core is sectioned (using ice saws) into ice horizons (slices typically 2–10 cm wide) as soon as possible after retrieval. Each of the sections is immediately placed in a clean plastic container that can be closed for safe return to the laboratory (Box Fig. 9.1e). Usually, replicate cores are taken at the same sampling site, since there is often not enough volume of ice in one core to perform the standard suite of measurements that are typically carried out.
Sampling of ice and seawater in the northern Baltic Sea. (a) A scientist operating an ice corer to make a hole in the ice and retrieve an ice core. (b) Sampling of under-ice seawater through the hole made by the ice corer. (c) Treatment of an ice core in the field. Holes are drilled and the temperature inside the ice is measured. (d) Salinity and other parameters are measured in the water under the ice. (e) The ice core is subdivided into slices and each slice is put into a separate container. Photo: © David N. Thomas
Studies under the ice
The ice core collection leaves a convenient hole in the ice through which water-sampling bottles can be operated for the purpose of taking under-ice seawater samples (Box Fig. 9.1b). The hole can be also used to deploy other equipment such as small CTDs (to measure conductivity , temperature and water depth), video/camera equipment (to examine the underside of the ice cover ) and light sensors (to measure the amount and quality of the light penetrating through the ice and snow covers ).
Processing of ice cores
There has been much debate over the years concerning how to best treat these sections for subsequent analyses. Rintala et al. (2014) have shown that probably the best method for most usual biological and chemical analyses of Baltic Sea ice is to melt the ice sections as rapidly as possible at room temperature, while gently shaking the sample to keep it cool. If the sample is subsampled just as the last ice melts, the temperature of the whole sample should still be only just above the freezing point . The standard suite of measurements that are typically carried out include measurements of ice crystal structure, salinity, temperature, chlorophyll a , dissolved inorganic and organic constituents, bacterial, algal and other protists' enumeration and activities. It is of key importance to protect the cores from contamination by seawater and/or by material from clothing and hands. Therefore, the cores should be handled as little as possible.
The average biomass of the phototrophic part of the sympagic communities during the ice season is generally higher in the Gulf of Finland than in the Gulf of Bothnia. Typical chlorophyll a concentrations in Gulf of Finland ice are up to ~5.5 mg m−2, but only up to ~2.2 mg m−2 in Gulf of Bothnia ice (Granskog et al. 2006). The under-ice water has typically a lower phototrophic biomass than the ice, although a high phototrophic biomass (>200–300 µg Chl a L−1) can occur during bloom conditions under the ice just before ice break-up (e.g. Stations GF 74 and GF 81 in Fig. 9.8b). Pressure ridges may also be hotspots of phototrophic biomass accumulation as the biomass in the keel ice blocks and interstitial water has been reported to be high (up to 50 µg Chl a L−1, Kuparinen et al. 2007), even in comparison with intense phytoplankton blooms in open Baltic Sea waters.
In the Bothnian Bay, the maximum phototrophic biomass is restricted to the ice bottom in both landfast ice and pack ice (Kuparinen et al. 2007, Rintala et al. 2010b). Also, in the Gulf of Finland, the maximum phototrophic biomass is typically found in the lowermost ice section where phosphorus accumulates (Granskog et al. 2005). However, in the Norra Kvarken area between the Bothnian Bay and the Bothnian Sea, both the major nutrient regeneration activity and the major ice phototrophic spring bloom were found in the interior ice layers (Norrman and Andersson 1994; Haecky et al. 1998), although ice-bottom maxima may also occur in this region (Haecky and Andersson 1999; Kaartokallio 2004).
Both surface and interior phototrophic biomass maxima have also been found in coastal ice in the Gulf of Bothnia and the Gulf of Finland (Granskog et al. 2005; Rintala et al. 2006; Kaartokallio et al. 2007; Piiparinen et al. 2010). As the research progresses, evidence regarding the occurrence of surface biomass maxima is accumulating (Piiparinen et al. 2010; Rintala et al. 2010b). Those combined observations show vertical biomass distribution patterns in the Baltic Sea, whereby the Bothnian Bay ice displays blooms that are restricted to the ice bottom and generally lower phototrophic biomass. More to the south, the location of the sympagic biomass maximum is variable, and the biomass is generally higher. It is probable that the availability of nutrients and their transport from the underlying water to the ice, as well as nutrient regeneration inside the ice, are decisive factors for biomass development when habitat space is available.
4.3 Bacterial biomass and activity
About 8 % of the total biomass of the sympagic communities in the Baltic Sea consist of bacteria (Fig. 9.8a). Heterotrophic bacteria in Baltic Sea ice also show vertical biomass distribution patterns that are analogous to those of the phototrophic part of the sympagic communities. In general, bacterial biomass and production in the ice are lowest in the Bothnian Bay , whereas the sea ice in the Norra Kvarken area and Gulf of Finland shows a higher bacterial biomass and higher production rates (Kuparinen et al. 2007).
Bacterial biomass in the sea ice of the Norra Kvarken area is in the range of 1–8 µg C L−1. In the Gulf of Finland it is 4–10 µg C L−1 while production rates are 0.001–0.6 µg C L−1 h−1 and 0.03–1.1 µg C L−1 h−1, respectively (Kuparinen et al. 2007). The maximum bacterial production rates in the sympagic communities are similar to the maximum values measured in the Baltic Sea surface waters during plankton blooms at other times of the year (Kuparinen et al. 2007, 2011). Short turn-over times and high per-cell activity in sympagic bacteria , compared to the open-water bacteria, imply that sympagic bacteria have a high capacity to process organic carbon and to regenerate nutrients (Kuparinen et al. 2007).
4.4 Dissolved organic matter (DOM)
Bacterial growth in Baltic Sea ice is sequentially limited by nutrients and substrate as winter progresses (Kuosa and Kaartokallio 2006). This suggests that the bacteria depend on the production of autochthonous DOM by phototrophs. As opposed to polar sea ice , DOM concentrations in Baltic Sea ice are lower than in underlying waters because of the generally high concentrations of terrestrially-derived DOM in Baltic Sea water (cf. Sect. 15.2.6). However, the DOM concentrations in Baltic Sea ice are still higher than those in Arctic sea ice . The high DOM loading of Baltic Sea waters and ice result in the ice having quite different chemical and optical characteristics compared to those known from polar oceans (Granskog et al. 2006).
Inside Baltic Sea ice , the DOM is thought to originate both from material incorporated into the ice during its formation and from autochthonous matter produced by the organisms inhabiting the ice, the latter largely comprising carbohydrate-rich polysaccharides (Underwood et al. 2013; Krembs et al. 2011). Thus, the DOM in Baltic Sea ice has a complex origin, being partially terrestrial and partially produced within the ice (Stedmon et al. 2007). The complex origin of the ice DOM may lead to uncoupled dynamics of DOC (dissolved organic carbon ), DON (dissolved organic nitrogen ) and DOP (dissolved organic phosphorus), as shown by e.g. the lack of significant correlations between DOM and bacterial parameters in Baltic Sea ice (Kaartokallio et al. 2007).
5 Diversity of sympagic organisms
5.1 Composition of sympagic communities
Our knowledge of the taxonomic composition of sympagic communities has greatly increased over the last 20 years and new species continue to be described (Rintala et al. 2010a). This progress has been achieved by microscopy and conventional cell counts (Figs. 9.8 and 9.9), but also by molecular techniques (Fig. 9.10).
In general, molecular studies on sea-ice eukaryotic diversity in the Baltic Sea provide results similar to morphological studies, although the diversity resolved with molecular tools is generally higher than that produced by studies based on morphology . Several organism groups, such as heterotrophic flagellates , may be better represented in molecular studies. The reverse is true for other groups, such as haptophytes and diatoms, which are generally underrepresented in environmental molecular studies.
Based on molecular studies of Baltic Sea ice , the most common phototrophic groups (in the order of decreasing species richness ) are diatoms, dinoflagellates, chlorophytes , cryptophytes , pelagophytes , haptophytes, synurophytes , bolidophytes , dictyochophytes , eustigmatophytes and chrysophytes. The most species-rich groups of heterotrophic protists in Baltic Sea ice are ciliates, cercozoans, dinoflagellates, choanoflagellates , novel unnamed stramenopiles, chrysophytes, labyrinthulids and Telonema spp. (Majaneva et al. 2012).
Many of the frequently observed phototrophic sympagic taxa in the Baltic Sea, such as Melosira arctica , Pauliella taeniata and Peridiniella catenata , are also commonly found in association with Arctic sea ice . As in the Arctic Ocean , the ice near river mouths in the Baltic Sea has a clear freshwater component with abundant chlorophytes . However, not all organisms found in the sea ice are “truly” sympagic. For example, some colonial filamentous cyanobacteria typical of Baltic Sea summer blooms, such as Nodularia spumigena (Fig. 9.9f), may survive in Baltic Sea ice, possibly overwintering there (Rintala et al. 2010b).
5.2 Bacteria and viruses
Heterotrophic bacteria are the most abundant prokaryote group in the sea ice . They contribute 4–11 % (average 8 %) to the total biomass of the Baltic Sea sympagic communities (Fig. 9.8, Meiners et al. 2002). Picocyanobacterial cells may be abundant, but never exceed 0.1 % of the total sympagic biomass. Studies that focus on Baltic Sea ice bacteria have been performed in the Gulf of Bothnia, the Gulf of Finland and in the Kiel Bay (southwestern Belt Sea). In general, the bacterial diversity found in Baltic Sea ice is fairly similar to that of the sea ice in both the Arctic Ocean and the Southern Ocean. This suggests that the factors shaping the communities are the same despite the different geographical locations.
The bacteria in the sympagic communities develop from bacteria in the parent water as a result of physical, chemical and biological processes. At the beginning of winter, the bacteria seem to go through an adaptive phase. When sea ice forms, the bacterial numbers are relatively high and cells are dividing, but activities are lower than in the under-ice water. Another important phase change for the sympagic bacteria is a peak in bacterial production after an ice-algal bloom (Kaartokallio 2004; Kaartokallio et al. 2008).
Bacteria from α-Proteobacteria (e.g. the genus Loktanella ), β-Proteobacteria (e.g. the family Comamonadaceae ), and γ-Proteobacteria (e.g. the genera Colwellia , Psychromonas and Shewanella ), Bacteroidetes (e.g. the genus Flavobacterium ) and Actinobacteria have been recorded in Baltic Sea ice. The same bacterial classes and phyla occur in Baltic Sea water. The bacterial components of the sympagic communities apparently have successional patterns resulting from exchange processes at the ice/water interface, the maturity of the ice and the availability of substrate for ice algae (Kaartokallio et al. 2008; Eronen-Rasimus et al. 2015).
At the early stages of the ice formation (nilas and pancake ice ) the sympagic bacteria in the Gulf of Bothnia drift ice are reminiscent of those in the parent water with a dominance of Actinobacteria and α-Proteobacteria, whereas the older columnar ice supports typical sympagic communities with a dominance of Flavobacteriia and γ-Proteobacteria, which is similar to polar sea ice (Eronen-Rasimus et al. 2015). Dominant taxa in older columnar ice are known to be able to efficiently utilise high substrate concentrations, e.g. in conjunction with ice-algal blooms . The maturity of ice as a structuring factor regarding the bacterial component of the sympagic communities may thus be related to increased supply of autochthonous organic matter as a substrate rather than to the time elapsed from ice formation per se.
In a recent study, bacteria isolated from Baltic Sea ice were shown to affiliate with Flavobacterium gelidilacus , Shewanella baltica and Shewanella frigidimarina (Luhtanen et al. 2014). Flavobacterium and Shewanella are common sea ice bacterial genera (Thomas and Dieckmann 2010). In the isolated bacterial strains, a total of seven bacteria-infecting viruses (bacteriophages ) were found. These viruses represented the families Siphoviridae and Myoviridae with hosts belonging to the classes Flavobacteriia and γ-Proteobacteria (Luhtanen et al. 2014). The ecological significance of these viruses is still unknown, but since the host organisms are common sea-ice bacteria , the viruses can potentially modify the sympagic community dynamics in Baltic Sea ice.
5.3 Diatoms
In their recent synthesis of sympagic organisms from Arctic sea ice , Poulin et al. (2011) found 71 % of the 1,027 Arctic eukaryote taxa to be diatoms. Diatoms are also the dominant primary producers in and under Baltic Sea ice. Both pennate and centric diatoms usually dominate the sympagic community biomass with 10–71 % (average 33 %) and 6–57 % (average 29 %) of the total biomass, respectively (Fig. 9.8). The pennates Pauliella taeniata and Nitzschia spp., and the centrics Melosira arctica and Chaetoceros spp., are the most typical sea-ice diatoms in Baltic Sea ice (Table 9.2, Piiparinen et al. 2010; Rintala et al. 2010b; Majaneva et al. 2012). Pauliella taeniata and Melosira arctica are examples of glacial relicts , which today occur in association with sea ice both in the Baltic Sea and in the Arctic Ocean .
When morphological studies of the same sea-ice samples were compared to the molecular data, the molecular analyses showed a lower relative diatom richness compared to morphological analyses (Majaneva et al. 2012). It is likely that diatoms are better represented in light-microscopy studies because they generally have a larger cell size and more distinguishable morphological characters compared to other sympagic eukaryotes.
A striking difference between the community composition in the sea ice and that of the phytoplankton under the sea ice is the enormous difference in the abundances of raphe -bearing pennate diatoms such as Navicula and Nitzschia species (Table 9.2). This group is usually nearly absent in the phytoplankton, but may completely dominate the biomass during the sympagic community bloom period inside the ice (Box 9.2, Fig. 9.8).
Diatoms are not able to move in water (except for flagellated male gametes ), but pennate species that possess a raphe can glide over a surface (e.g. ice) at speeds >10 µm s−1 (Cohn and Witzell 1996). Motility is even an advantage inside the ice as the cells have the ability to actively expose themselves to optimal light conditions , i.e. they can move towards the light and they can also move away from supersaturating irradiance to avoid oxidative damage . Another advantage of motility is that the cells can move to more nutrient-rich microhabitats when nutrients are limiting growth.
Non-motile pennate chain-forming diatoms (e.g. Pauliella taeniata ) are more confined to the ice/water interface, using the ice for attachment of the chains, while the centric chain-forming diatoms Chaetoceros wighamii and Melosira arctica are dominant in surface layers composed of snow-ice (Kaartokallio et al. 2007; Piiparinen et al. 2010; Rintala et al. 2010b).
5.4 Flagellates
When diatoms do not dominate the sympagic community biomass, flagellates (especially dinoflagellates) usually become dominants in Baltic Sea ice (Kuosa and Kaartokallio 2006; Kaartokallio et al. 2007). Autotrophic flagellates contribute 8–38 % (average 23 %), and heterotrophic flagellates 2–17 % (average 7 %) to the total biomass (Meiners et al. 2002). Besides dinoflagellates, the flagellate group includes bolido-, chryso-, crypto-, dictyocho-, dino-, eustigmato-, hapto-, pelago- and prasinophytes and Cercozoa (Table 9.2, Spilling 2007; Piiparinen et al. 2010; Rintala et al. 2010b).
The smaller flagellates are usually well-represented in molecular data but in light-microscopy studies they are often combined into a generic group of “auto- and heterotrophic flagellates” due to their small size and general lack of distinct visible features. Among the Cercozoa (Rhizaria ), especially abundant in Baltic Sea ice, are members of the genera Cryothecomonas and Protaspis (Ikävalko 1998; Majaneva et al. 2012).
Some phototrophic flagellates are capable of mixotrophic modes of nutrition, meaning that they can combine autotrophy and heterotrophy both by taking up inorganic carbon, nitrogen and phosphorus directly from the water and by utilising organic nutrients, e.g. via phagotrophy (both phagocytosis and pinocytosis ). Extracellular enzymes have been successfully used to detect the occurrence of mixotrophy in aquatic organisms and these enzymes have also been found in the sympagic communities of the Baltic Sea (Rintala 2009). The capability of mixotrophy provides any organism with a competitive advantage over strict autotrophs and heterotrophs , e.g. when sudden changes in snow cover have a large impact on the incoming solar radiation .
Some flagellate species possess unique survival strategies in the form of resting stages , which can enable a population to survive unfavourable environmental conditions. An example of the successful use of a resting stage in sympagic communities is the formation of cysts of the dinoflagellate Scrippsiella hangoei in Baltic Sea ice (cf. Fig. 9.9e, Rintala et al. 2007). It is evident that encystment provides Scrippsiella hangoei populations with a possibility to survive prolonged periods of low irradiance , which are common in the sea ice (especially when it is snow-covered).
5.5 Ciliates
The main heterotrophic protists in Baltic Sea ice, besides heterotrophic flagellates , are ciliates of various cell sizes . Ciliates seem to be more important in Baltic Sea ice than in other non-polar ice-covered areas (Thomas and Dieckmann 2010), probably reflecting the paucity of metazoans . The present data are still limited, but the ciliate component of the Baltic Sea sympagic communities seems to be dominated by species of the genus Strombidium . Molecular analyses have confirmed the dominance of Strombidiidae and also identified the genus Lacrymaria and several unidentified ciliates as common (Majaneva et al. 2012).
As in the Arctic sea ice , most of the ciliates are relatively small (20–80 µm), which implies their possible role as grazers of small particles, including bacteria. Mixotrophic ciliate species have not been recorded in the Baltic Sea sympagic communities , except for Mesodinium rubrum (Kaartokallio et al. 2007; Rintala et al. 2010b). A notable feature of the ice-associated ciliate fauna is the growth of large ciliates ( Bursaria sp.) under the ice (Kaartokallio 2004, Kaartokallio et al. 2007; Rintala et al. 2010b). These large species obviously graze on dinoflagellates, which is otherwise not common for Baltic Sea ciliates.
5.6 Metazoans
In contrast to polar areas where a diverse metazoan fauna occurs in sea ice (Thomas and Dieckmann 2010), the metazoans represented in Baltic Sea ice are limited to rotifers and the nauplii stages of copepods (Meiners et al. 2002; Werner and Auel 2004). Metazoans contribute less than 4.3 % (average 1.0 %) to the total biomass of the sympagic communities (Meiners et al. 2002). Typical taxa encountered in Baltic Sea ice are the rotifers Keratella spp. and Synchaeta spp., and nauplii of the copepod Acartia bifilosa (Table 9.2).
Rotifer biomass can make up as much as 30 % of the total sympagic community biomass during the low-productive winter period, but is reported to be in the range of 1–7 % during ice-algal bloom periods (Meiners et al. 2002; Granskog et al. 2006). It is possible that the sea ice in the Baltic Sea functions as an overwintering habitat and feeding ground for rotifers and copepod nauplii, as well as a reservoir for rotifer resting eggs (Granskog et al. 2006). The underside of the ice is also an important feeding ground in winter for calanoid copepod species dominant in the Baltic Sea, such as Acartia bifilosa, and populations of this species have been shown to reproduce, grow and develop under the ice cover (Werner and Auel 2004).
5.7 Fungi and parasitic protists
Generally , fungi are an important group in the recycling of organic matter , and they occur in high abundances in both Baltic Sea water and ice. The presence of sympagic microscopic fungi, consisting of unicellular Ascomycota (e.g. Eurotium molds and Debaryomyces yeasts ) and Basidiomycota (e.g. Rhodotorula yeasts) associated with Baltic Sea ice, has been confirmed with molecular techniques (Fig. 9.10, Majaneva et al. 2012). Also chytrids and Graphiola and Sclerotium species have been found within the sea ice, suggesting that besides being saprophytic, some fungi in the ice may be parasitic. Other parasites in Baltic Sea ice include members of the endosymbiont dinoflagellate order Syndiniales (Majaneva et al. 2012). Parasites of protists and animals are good examples of organisms that are generally easier to detect with molecular techniques than through direct microscopic observation.
6 Sea-ice food webs
6.1 The food webs in Baltic Sea ice are truncated
Due to space limitation in the brine channels, the internal sea-ice food webs are truncated compared to the open water food webs. The reason for the occurrence of these truncated food webs is that organisms larger than the upper size limit of the brine channels are lacking from the sympagic communities . In Baltic Sea ice , where low ice porosity and small brine channel diameter severely restrict the upper size of organisms in brine channels, the largest metazoan animals are occasional copepod nauplii . The absence, or low numbers, of metazoans simplifies the food webs by lowering the number of trophic interactions .
However, describing sea-ice food webs and their function is challenging due to sampling difficulties (Box 9.1), the complex dynamics of the physical environment exerting control over biological communities (Zhou et al. 2014) and an open boundary with the underlying water food webs that allows the exchange of matter and ecological interactions across it. Different “short circuits” in the organic matter and energy flows are suggested to be typical of microbial food webs inside the sea ice. These include herbivory by ciliates and flagellates, ciliate bacterivory and the direct utilisation of DOM by heterotrophic flagellates . Of these, at least direct utilisation of DOM by flagellates and ciliate grazing over several size classes has been suggested to be functional in the Baltic Sea sympagic communities (Haecky and Andersson 1999; Kaartokallio 2004).
The ice food web is characterised by the importance of heterotrophic bacteria as they are able to recycle DOM and serve as prey for both flagellates and small ciliates (Kaartokallio 2004). In Baltic Sea ice , DOM comprises both allochthonous (originating from parent seawater) and autochthonous components (produced by ice algae) (Stedmon et al. 2007). As in polar sea ice , they can be considered a major link between primary and secondary producers (Gradinger et al. 1992). Bacteria are able to directly utilise DOM for their growth and benefit from the generally high substrate availability in the sea ice environment. Ice bacteria can then serve as a food source for flagellates and small ciliates capable of ingesting bacteria-sized prey (Kaartokallio 2004).
The sparse data available suggest that there are differences in the sea-ice food web structure and nutrient dynamics between the subregions of the Baltic Sea. Auto- and heterotrophic flagellates seem to be more dominant in the ice food webs in the Gulf of Bothnia compared with those in the Gulf of Finland (Haecky and Andersson 1999; Meiners et al. 2002; Kaartokallio 2004). The parent-water nutrient concentrations affect the amount of nutrients entrapped during ice formation. Nutrients entrapped in ice, and the subsequent regeneration of this pool, are probably more important in the Gulf of Bothnia than in the Gulf of Finland (Haecky and Andersson 1999; Kaartokallio 2004; Granskog et al. 2005; Piiparinen et al. 2010). In the Gulf of Finland, phosphorus accumulates in the lower ice layers typically supporting the maximum algal biomass (Granskog et al. 2005), whereas in the Gulf of Bothnia the ice algal spring bloom is located in the interior ice layers (Norrman and Andersson 1994; Haecky et al. 1998) with the highest nutrient regeneration activity (Kaartokallio 2001).
6.2 Seasonal succession of autotrophy and heterotrophy
The typical successional pattern of the sympagic communities in Baltic Sea ice is analogous to that in polar sea ice . The initial colonisation during sea ice formation is followed by a low-productivity mid-winter stage, a bloom of the sea ice phototrophs and finally a heterotrophy -dominated stage late in the season (Box 9.2). Biomass accumulation of the sea ice phototrophs generally follows the seasonal increase in solar radiation beginning at the transition of winter and spring and lasts until the onset of ice melt (Norrman and Andersson 1994; Haecky and Andersson 1999). The relative importance of heterotrophic and autotrophic processes in the sea ice changes during ice season progress, and is driven by changes in incoming solar radiation. The growth of sea ice algae and bacteria is sequentially limited by light, nutrients and substrate (for bacteria) as the ice season progresses (Haecky et al. 1998; Kuosa and Kaartokallio 2006).
Box 9.2: Succession in sympagic communities
Spational and seasonal changes of the sea ice habitat
Ice formation, ice growth, ice melting and community succession follow seasonal changes in solar angle and air temperature (Box Fig. 9.2). The timing of these processes depends on latitude and weather conditions (cf. Fig. 9.2). The coldest months are January and February. Sea ice formation begins in January and the ice cover continues to grow downwards through February and reaches its maximum thickness in March. Ice melting starts in early April and ice breaks up and finally disappears between mid-April and May. Low-saline river waters and ice-melt water are fresher and therefore lighter than the brackish under-ice water (cf. Sect. 1.2.3), and can form distinct freshwater layers under the ice (Box Fig. 9.2). Snow accumulates on top of the ice and its weight can submerge the ice, leading to snow-ice formation as the slush layer freezes (cf. Fig. 9.6). Superimposed ice is ice formed from the freezing of melted snow during melt-freeze cycles brought about by short-term warm weather events, e.g. when a diminishing snowpack enhances the amount of solar radiation energy that is able to pass into the ice.
A schematic presentation showing the major successional stages of sympagic communities during the ice season in the Baltic Sea. The size of the symbols denoting a group of organisms (diatoms, dinoflagellates, bacteria, ciliates and rotifers ) is roughly proportional to the biomass of those groups within each successional stage. The total biomass of the ice organisms also varies between phases, with Phase A having the lowest and Phase B the highest biomass. PAR = photosynthetically active radiation , UVR = ultraviolet radiation . Figure: © Hermanni Kaartokallio
Three successional stages in the sympagic communities are distinguished:
-
Phase A is a low-productivity winter stage with only a low amount of solar radiation entering the ice; accordingly, biomass is low. The dominance of rotifers early in the season in thin young ice also occurs in the Arctic (Friedrich and De Smet 2000). This is possibly due to the effective entrapment of pelagic rotifers (or their eggs) from the parent water mass during ice formation, or their active migration into the ice from under-ice water. Rotifers are known to effectively utilise detritus as a food source, which explains their growth in this low-productivity phase.
-
Phase B starts when the amount of light increases after the mid-winter minimum and the ice-algal bloom period with high autotrophic productivity and biomass formation sets in. This phase is characterised by a strong dominance of autotrophic organisms in the total organism standing stocks . Diatoms and dinoflagellates dominate the biomass.
-
Phase C is the post-bloom phase, which starts when the ice-algal bloom is terminated. This phase is characterised by high heterotrophic productivity, with rotifers and heterotrophic bacteria being the most important organism groups in terms of biomass.
Phosphorus is probably the most important single limiting nutrient for ice-associated algae (Haecky and Andersson 1999; Granskog et al. 2005; Kuosa and Kaartokallio 2006). The spring ice phototrophic blooms typically occur in March in the Gulf of Finland and in March-April in the Gulf of Bothnia. In the Gulf of Finland, the occurrence of another, minor phototrophic biomass maximum during a low-light period in January under snow-free ice has also been reported (Kaartokallio 2004). The significance of heterotrophic processes in carbon cycling increases during late-bloom and post-bloom situations towards the end of the sea ice season .
6.3 Under-ice microalgal blooms
Under-ice microalgal blooms starting at the ice/water interface before ice-breakup, and facilitated by a stable melting-water layer under the ice, were reported from the southwestern coast of Finland (Spilling 2007). These blooms are assumed to contribute to the onset of the major phytoplankton spring bloom after ice break-up . In the Gulf of Finland these blooms are dominated by phototrophic dinoflagellates (Spilling 2007). Dense under-ice algal blooms dominated by dinoflagellates may also occur due to river plumes under the ice over the whole ice season (Larsen et al. 1995).
Quite specific circumstances are required to produce these blooms: there has to be a layer of low-saline water under the ice and the ice should be free of snow. The blooms can be very patchy and are concentrated to the upper few cm of the water column due to the active movement of the dinoflagellates, or as a result of very shallow vertical salinity gradients. A large haptophyte , Chrysochromulina birgeri , can also form under-ice blooms similar to those of the dinoflagellates. The distribution of these blooms, their effects on winter productivity and implications for the survival of metazoans are not yet fully understood (Spilling 2007).
6.4 Do sea-ice diatoms “seed” the pelagic spring bloom?
Most of the sympagic diatoms sink rapidly, almost immediately after the ice melt, but there is a difference in the dominant pelagic spring-bloom diatom species, depending on whether or not the spring bloom is formed after an ice-free or an ice-covered sea in winter (Haecky et al. 1998). The spring bloom of ice-free locations is dominated by centric diatoms such as Skeletonema marinoi and Thalassiosira baltica , whereas the blooms following ice-covered areas are dominated by Pauliella taeniata , Chaetoceros wighamii and Nitzschia frigida . The latter two species are thought to be introduced into the pelagic zone from the ice, but Pauliella taeniata could also originate from sediments (Piiparinen et al. 2010).
7 Ice and light
7.1 Ice optical properties
Light is the key controlling factor in determining the seasonal development of the sympagic phototrophs. Along with the seasonal development of incoming solar radiation due to changing solar zenith and day length, the light regime in ice is strongly dependent on the surface characteristics, which affect the albedo (the fraction of solar energy reflected from the Earth back to space) and the attenuation coefficients (i.e. the attenuation of light with depth by absorption and scattering). For example, dry surfaces (including ice) efficiently reflect solar radiation, whereas wet surfaces retain more radiation than they reflect (Rasmus et al. 2002; Ehn et al. 2004).
Due to the highly scattering nature of snow and the inclusion of variable amounts of absorptive impurities (e.g. soot, dust), the attenuation coefficients are generally higher for snow than for bare ice. Thus, the presence of a snow cover may greatly reduce the amount of light reaching the sea ice, depending on the thickness of the snow cover as well as on other properties of snow. Similarly to snow, the transmission of light in the sea ice is also changed by scattering (air bubbles, brine pockets), and by absorption by the biota, other particles and DOM.
Ultraviolet radiation (UVR, 280–400 nm) attenuates faster than photosynthetically active radiation (PAR, 400-700 nm) and thus most of the transmitted light is in the PAR band (Rasmus et al. 2002; Ehn et al. 2004). Some characteristics of Baltic Sea ice optical properties are different from those of Arctic sea ice . In the Baltic Sea, the typically large snow-ice fraction in the surface ice enhances scattering, while large amounts of atmospheric fallout, DOM and POM reduce the transmittance by absorption at shorter wavelengts. Therefore the maximum transmittance wavelength is shifted from 500–550 nm in the Arctic Ocean to 562–570 nm in the Baltic Sea (Ehn et al. 2004; Uusikivi et al. 2010).
As in all aquatic systems, the ability of phototrophs to increase their cellular Chl a content in response to low irradiances , and to decrease it at high irradiances (photoacclimation ) is a common phenomenon in Baltic Sea ice phototrophs (Rintala et al. 2006; Piiparinen and Kuosa 2011). The photosynthetic components of the Baltic Sea sympagic communities are not as strongly dark-adapted as their polar counterparts, possibly due to the relatively thin snow and ice cover , the rapid changes in snow-cover thickness, and the shorter period of low irradiance in mid-winter (Piiparinen et al. 2010; Rintala et al. 2010b).
7.2 Ultraviolet radiation
Sympagic organisms are potentially more susceptible to UVR than planktonic organisms due to limited vertical movement inside the ice and the occasionally high O2 concentrations in the brine (mainly from photosynthesis ), which induces the formation of reactive oxygen species (ROS) in the presence of UVR. High-energy UV photons impair cell’s normal functions, either directly or indirectly through formation of ROS, by promoting damage in DNA, RNA, proteins and membranes.
However, organisms can partially cope with the harmful effect of UVR by generic DNA repair and by the synthesis of UV-absorbing compounds, pigments and antioxidants in the cell. One specific group of UV-screening compounds, found in many algae and cyanobacteria , are the mycosporine-like amino acids (MAAs), which absorb between 309 and 362 nm (Uusikivi et al. 2010; Piiparinen et al. 2015). The stronger attenuation of shorter wavelengths in ice results in UV effects being concentrated mostly in the sympagic communities in the top 10 cm of sea ice . The high concentrations of the MAAs palythine and shinorine reported from the surface layer of snow-free ice indicate that the sympagic organisms in the Baltic Sea need to protect themselves against UVR (Uusikivi et al. 2010).
The few existing studies on the effects of UVA (315–400 nm) on bacteria and phototrophs in Baltic Sea ice indicate that UVR (which in sea ice consists mainly of UVA) shapes the vertical distribution of organisms in the ice column to some degree (Piiparinen and Kuosa 2011). This seems to apply especially to chlorophytes and pennate diatoms , which increased in biomass in the surface layers of snow-free ice when UVA was experimentally filtered off (Fig. 9.11), whereas exposure to UVA had the opposite effect on these two algal groups. On the other hand, centric diatoms showed sensitivity to both PAR and PAR + UVA and concentrated in deeper ice layers under these exposure regimes.
Bacterial production is closely linked to changes in algal biomass and species composition, indicating that the UV-effects may extend throughout the sea-ice food web . The bacteria in the surface ice are also affected by UVA: α-, β-, and γ-Proteobacteria are sensitive to UVA whereas the Flavobacteriia seem to be UVA-resilient. When snow covers the sea ice it provides efficient protection against UVR for sympagic organisms , but at the same time it reduces the PAR intensity.
8 Modelling the Baltic Sea ice system
8.1 Climate change in the Baltic Sea Area
The global air temperature is increasing (cf. Fig. 2.28; Stocker et al. 2013), and the Baltic Sea Area is no exception. The importance of sea ice for the functioning of the Baltic Sea ecosystem needs to be well understood since the sea ice is expected to disappear from most of the basins in the near future. Over the past century the air temperature in the Baltic Sea Area has increased by 0.7 °C, which exceeds the global average of 0.5 °C (BACC Author Team 2015). The temperature trend in the Baltic Sea surface waters over the time period of 1982–2010 indicates a warming rate of 0.063–0.078 °C per year (Baker-Austin et al. 2013). The increasing temperature shrinks the extension of Baltic Sea ice , as already observed e.g. at the ice monitoring stations Märket and Suursaari (Fig. 9.2).
The increase of both air temperature and precipitation is predicted to continue over the coming decades. A projection of the B2 SRES climate change scenario, a mild IPCC climate change scenario (Stocker et al. 2013), to the northeastern Baltic Sea, shows an increase in the air temperature by ~3 °C in spring and autumn, ~4 °C in summer and ~4.5 °C in winter in the time slice 2071–2090 (BACC Author Team 2015). The same scenario predicts an increase of precipitation in the northeastern Baltic Sea by ~5 % in summer, ~ 10 % in autumn, ~15 % in spring and ~ 20 % in winter.
Therefore, the largest changes, both in temperature and in precipitation, are projected to occur in winter, whereby the ice cover will be reduced, both in time and space. The ice-covered area in the Baltic Sea is estimated to shrink by ~45,000 km2 for each 1°C increase in the average temperature. With an increase of 1 °C only the northernmost part of the Gulf of Bothnia and the easternmost part of the Gulf of Finland would freeze during mild winters and the ice would be thinner and more easily movable. With an air temperature increase of ~4 °C in winter, the Baltic Sea would become completely ice-free (Omstedt and Hansson 2006).
8.2 Sea ice is a challenge for modellers
To understand and predict the consequences of global climate change and other non-climate stressors, the development of ecosystem modelling applications that can act as a decision support system (tool) for policy makers has become a major task for the scientific community . Assessing the qualitative and quantitative role of the sea ice is a challenge facing ecosystem modellers of polar and subpolar regions. For example, how will the absence of an ice cover affect primary production, and ultimately fish production , in these regions?
Very few studies have dealt with modelling of the sea-ice habitat (Tedesco and Vichi 2014), and modelling of sea ice biota is a challenge, firstly because there is a scarcity of observational data and secondly because of the complexity of the system. In situ observations of spatial and temporal variability of the sea-ice properties and processes are needed for model development and evaluation, but usually only point data with small spatial resolution are available. Thus, while the principal biological and ecological processes that characterise a certain sea-ice area may be known, the variability of the biogeochemical properties remains still largely unknown, which lowers the reliability of a model.
Compared to ocean biogeochemical models , those developed for sea-ice biogeochemistry are more complex as they include also ice physics, specific light parameterisations and specific fluxes at the ice/water interface. Sea-ice models may differ in complexity in terms of resolution and biology. A single-layer model of a preset ice thickness has a lower resolution than a multi-layer model with several sea-ice layers . The number and type of biological tracers, functional groups and ecological and physiological processes affect model complexity. For example, a silica -based model representing only one group of primary producers (diatoms) is less complex than a multi-nutrient-based model with several living components.
8.3 A biogeochemical model of Baltic Sea ice
A sea ice biogeochemical model considering the specific physical, chemical and biological characteristics of Baltic Sea ice has been developed by Tedesco et al. (2009, 2010). In the seasonal ice of the Baltic Sea, the bottommost part usually is the most productive layer, and the concept of the “Biologically Active Layer ” (Fig. 9.5) is suitable to represent the vertical resolution of the sea ice biogeochemical model.
The model includes the state variables: nitrogen, phosphorus, silica, chlorophyll a and carbon in a flexible stoichiometry framework as well as two functional groups of algae (adapted diatoms and surviving sea ice algae), sea ice bacteria, sea-ice fauna, sea-ice organic matter (dissolved and particulate) and gases such as oxygen and carbon dioxide (Fig. 9.12). The physical part of the model considers only two sea-ice layers , the Biologically Active Layer and the rest of the sea ice with no, or very low, biological activity. Included are several snow layers that describe the different characteristics of the upper layers, such as freshly deposited snow , compacted snow , snow-ice or superimposed ice (Tedesco et al. 2009, 2010).
8.4 A climate change scenario for the Baltic Sea
The Baltic Sea ice biogeochemical model of Tedesco et al. (2009) was first used to simulate the ice season 1999–2000 at Santala Bay in the Gulf of Finland (Tedesco et al. 2010). The Biologically Active Layer showed a dynamic thickness and biological production during the ice season that compared well with the available physical and biochemical observations (Fig. 9.13). In particular, the model was able to reproduce a rather mild winter during which the sympagic community appeared to be very active throughout most of the ice season.
When the B2 SRES climate change scenario (Stocker et al. 2013) was applied to Santala Bay in the Baltic Sea ice biogeochemical model of Tedesco et al. (2010), only six days during the whole of winter were projected to be below the freezing point of the brackish Baltic Sea water. In comparison, the ice season of 1999–2000 had 138 days below the freezing point at Santala Bay. Six days is not enough for the sea ice to become firm and persistent.
Without an ice cover , the pelagic phytoplankton bloom was projected to occur later than in the reference simulation of 1999–2000 (Fig. 9.14). Furthermore, the biomass of the pelagic spring bloom following an ice-free winter was projected to be larger with community composition to be dominated by flagellates instead of diatoms. This flagellate bloom consists mainly of dinoflagellates, which are more efficient in warmer waters and currently already compete with diatoms in large areas of the Baltic Sea during the pelagic spring bloom (Spilling 2007). The change in the biomass and composition of the phytoplankton bloom will presumably affect the composition and timing of the zooplankton peak, and bacterial dynamics are expected to change as well. In general, the whole food web is projected to change, ultimately impacting top predator populations.
More extensive models, i.e. encompassing the entire ice-covered Baltic Sea and its biology, are needed. Their construction requires more field observations and the development of new parameterisations for sea ice physical, chemical and biological processes at large spatial scales. Large-scale models should also include the biogeochemical importance of ice movement as thinner ice is more readily deformed by winds, which may result in increased roughness of the ice and rafting and ridging among ice floes (Stirling et al. 2008; Löptien et al. 2013). Currently, e.g. pressure ridges are missing in all sea-ice models existing worldwide, and this most likely leads to an underestimation of the sea ice biological production.
9 Life on the Baltic Sea ice
In the Arctic Ocean , four ice-obligate marine mammals occur: the ringed seal Pusa hispida , the walrus Odobenus rosmarus , the bearded seal Erignathus barbatus and the polar bear Ursus maritimus (Moore and Hunting ton 2008). In the Baltic Sea there is only one, the ringed seal (Fig. 9.15, cf. Box 4.13). In the Baltic Sea, The ringed seal occupies the seasonal sea ice from the time of ice formation until ice break-up , including the breeding season. One of the critical periods in the ringed seals' life is the “subnivean period ” in lairs under the snow.
Already in the 1950s it was hypothesised that the Archipelago Sea subpopulation of the ringed seal had declined due to the mild winters in the 1930s; later findings confirmed the correlation between high pup mortality and restricted ice coverage (Meier et al. 2004). This is because successful breeding depends on the seals’ ability to carve lairs in the snow and the subsequent (about a month long) period in March and early April during which pup survival depends on stable ice conditions, as the pups still live under the protection of lairs.
Because of the projected climate change , in the future the breeding of all the southern subpopulations of the Baltic ringed seal will only be possible in exceptionally cold years. Only the Bothnian Bay subpopulation is likely to survive since the present climate change scenarios still predict an annual two-month long ice-cover period here, which is the minimum time required to sustain a ringed seal population (Meier et al. 2004).
10 Review questions
-
1.
What are the characteristics of the three main habitats of sympagic communities?
-
2.
What is the major driver of the seasonal succession in sympagic communities?
-
3.
What limits primary production in Baltic Sea ice?
-
4.
How do the main Baltic Sea ice habitats differ from those in the Arctic Ocean, and what are the biological consequences of these differences?
-
5.
Which factors affect the transmission of light in sea ice?
11 Discussion questions
-
1.
What impacts on Baltic Sea ice are predicted by climate change scenarios and what consequences of those impacts can you envisage for the Baltic Sea ecology?
-
2.
Why is modelling an important tool in studying sea ice?
-
3.
Why are diatoms the dominating algal group in sea ice?
-
4.
Can the ice cover affect the productivity of the Baltic Sea in other seasons?
-
5.
Why are bacteria in sea ice able to grow almost as fast as they do in the open water in summer?
References
BACC Author Team (2015) Second assessment of climate change for the Baltic Sea basin. Regional climate studies. Springer, Berlin 501 pp
Baker-Austin C, Trinanes JA, Taylor NGH, Hartnell R, Siitonen A, Martinez-Urtaza J (2013) Emerging Vibrio risk at high latitudes in response to ocean warming. Nature Climate Change 3:73–77
Cohn SA, Witzell RE (1996) Ecological considerations of diatom cell motility. I. Characterization of motility and adhesion in four diatom species. Journal of Phycology 32:928–939
Ehn J, Granskog MA, Reinart A, Erm A (2004) Optical properties of melting landfast sea ice and underlying seawater in Santala Bay, Gulf of Finland. Journal of Geophysical Research 109(C09003):1–12
Eronen-Rasimus E, Lyra C, Rintala JM, Jürgens K, Ikonen V, Kaartokallio H (2015) Ice formation and growth shape bacterial community structure in Baltic Sea drift ice. FEMS Microbiology Ecology 91:13. doi: 10.1093/femsec/fiu022
Friedrich C, De Smet WH (2000) The rotifer fauna of Arctic sea ice from the Barents Sea, Laptev Sea and Greenland Sea. Hydrobiologia 432:73–89
Golden KM (2009) Climate change and the mathematics of transport in sea ice. Notices of the American Mathematical Society 56:562–584
Gradinger R, Spindler M, Weissenberger J (1992) On the structure and development of Arctic pack ice communities in Fram Strait: a multivariate approach. Polar Biology 12:727–733
Granskog MA, Kaartokallio H (2004) An estimation of the potential fluxes of nitrogen, phosphorus, cadmium and lead from sea ice and snow in the northern Baltic Sea. Water, Air and Soil Pollution 154:331–347
Granskog MA, Kaartokallio H, Kuosa H (2010) Sea ice in non-polar regions. In: Thomas DN, Dieckmann GS (eds) Sea ice, 2nd edn. Wiley-Blackwell Publishing, Oxford, pp 531–577
Granskog MA, Kaartokallio H, Kuosa H, Thomas DN, Vainio J (2006) Sea ice in the Baltic Sea: a review. Estuarine, Coastal and Shelf Science 70:145–160
Granskog MA, Kaartokallio H, Shirasawa K (2003a) Nutrient status of Baltic Sea ice: evidence for control by snow-ice formation, ice permeability and ice algae. Journal of Geophysical Research 108:3253–3256
Granskog MA, Kaartokallio H, Thomas DN, Kuosa H (2005) The influence of freshwater inflow on the inorganic nutrient and dissolved organic matter within coastal sea ice and underlying waters in the Gulf of Finland (Baltic Sea). Estuarine, Coastal and Shelf Science 65:109–122
Granskog MA, Martma TA, Vaikmäe RA (2003b) Development, structure and composition of landfast sea ice in the northern Baltic Sea. Journal of Glaciology 49:139–148
Haecky P, Andersson A (1999) Primary and bacterial production in sea ice in the northern Baltic Sea. Aquatic Microbial Ecology 20:107–118
Haecky P, Jonsson S, Andersson A (1998) Influence of sea ice on the composition of the spring phytoplankton bloom in the northern Baltic Sea. Polar Biology 20:1–8
Hällfors G, Niemi Å (1974) A Chrysochromulina (Haptophyceae) bloom under the ice in the Tvärminne archipelago, southern coast of Finland. Memoranda Societatis pro Fauna et Flora Fennica 50:89–104
Haapala JJ, Ronkaonen L, Schmelzer N, Sztobryn M (2015) Recent change—sea ice. BACC Author Team. Second assessment of climate change for the Baltic Sea basin. Regional Climate Studies, Springer, Berlin, pp 145–153
Huttunen M, Niemi Å (1986) Sea-ice algae in the northern Baltic Sea. Memoranda Societatis pro Fauna et Flora Fennica 62:58–62
Ikävalko J (1998) Further observations on flagellates within sea ice in northern Bothnian Bay, the Baltic Sea. Polar Biology 19:323–329
Ikävalko J, Thomsen HA (1997) The Baltic Sea ice biota (March 1994): a study of the protistan community. European Journal of Protistology 33:229–243
Kaartokallio H (2001) Evidence for active microbial nitrogen transformations in sea ice (Gulf of Bothnia, Baltic Sea) in midwinter. Polar Biology 24:21–28
Kaartokallio H (2004) Food-web components, and physical and chemical properties of Baltic Sea ice. Marine Ecology Progress Series 273:49–63
Kaartokallio H, Kuosa H, Thomas DN, Granskog MA, Kivi K (2007) Changes in biomass, composition and activity of organism assemblages along a salinity gradient in sea ice subjected to river discharge. Polar Biology 30:186–197
Kaartokallio H, Tuomainen J, Kuosa H, Kuparinen J, Martikainen P, Servomaa K (2008) Succession of sea-ice bacterial communities in the Baltic Sea fast ice. Polar Biology 31:783–793
Kankaanpää P (1997) Distribution, morphology and structure of sea ice pressure ridges in the Baltic Sea. Fennia 175:139–240
Kawamura T, Shirasawa K, Ishikawa N, Lindfors A, Rasmus K et al (2001) Time-series observations of the structure and properties of brackish ice in the Gulf of Finland. Annals of Glaciology 33:1–4
Krembs C, Eicken H, Deming JW (2011) Exopolymer alteration of physical properties of sea ice and implications for ice habitability, biogeochemistry and persistence in a warming climate. Proceedings of the National Academy of Sciences of the USA 108:3653–3658
Kuosa H, Kaartokallio H (2006) Experimental evidence on substrate and nutrient limitation of Baltic Sea sea-ice algae and bacteria. Hydrobiologia 554:1–10
Kuparinen J, Autio R, Kaartokallio H (2011) Sea ice bacteria have high growth rate, growth efficiency and preference for inorganic nitrogen sources in the Baltic Sea. Polar Biology 34:1361–1373
Kuparinen J, Kuosa H, Andersson A, Autio R, Granskog MA et al (2007) Role of sea-ice biota in nutrient and organic material cycles in the northern Baltic Sea. AMBIO 36:149–154
Larsen J, Kuosa H, Ikävalko J, Kivi K, Hällfors S (1995) A redescription of Scrippsiella hangoei (Schiller) comb. nov.—a “red tide” dinoflagellate from the northern Baltic. Phycologia 34:135–144
Leppäranta M, Manninen T (1988) The brine and gas content of sea ice with attention to low salinities and high temperatures. Finnish Institute for Marine Research, Helsinki, Report 2:1–14
Leppäranta M, Myrberg K (2009) Physical oceanography of the Baltic Sea. Springer, Berlin 378 pp
Löptien U, Mårtensson S, Meier HEM, Höglund A (2013) Long-term characteristics of simulated ice deformation in the Baltic Sea (1962-2007). Journal of Geophysical Research: Oceans 118:801–815
Luhtanen AM, Eronen-Rasimus E, Kaartokallio H, Rintala JM, Autio R, Roine E (2014) Isolation and characterization of phage-host systems from the Baltic Sea ice. Extremophiles 18:121–130
Majaneva M, Rintala JM, Piisilä M, Fewer DP, Blomster J (2012) Comparison of wintertime protists and fungi from sea ice and open water in the Baltic Sea. Polar Biology 35:875–889
Meese DA (1989) The chemical and structural properties of sea ice in the southern Beaufort Sea. Cold Regions Research and Engineering Laboratory Monograph 1989 25:1–144
Meier HEM, Döscher R, Halkka A (2004) Simulated distributions of Baltic Sea ice in warming climate and consequences for the winter habitat of the Baltic ringed seal. AMBIO 33:249–256
Meiners K, Fehling J, Granskog MA, Spindler M (2002) Abundance, biomass and composition of biota in Baltic Sea ice and underlying water (March 2000). Polar Biology 25:761–770
Moore SE, Huntington HP (2008) Arctic marine mammals and climate change: impacts and resilience. Ecological Applications 18:157–165
Norrman B, Andersson A (1994) Development of ice biota in a temperate sea area (Gulf of Bothnia). Polar Biology 14:531–537
Omstedt A, Hansson D (2006) The Baltic Sea ocean climate system memory and response to changes in the water and heat balance components. Continental Shelf Research 26:236–251
Piiparinen J, Enberg S, Rintala JM, Sommaruga R, Majaneva M et al (2015) The contribution of mycosporine-like amino acids, chromophoric dissolved organic matter and particles to the UV protection of sea-ice organisms in the Baltic Sea. Photochemical and Photobiological Sciences 14:1025–1038
Piiparinen J, Kuosa H (2011) Impact of UVA radiation on algae and bacteria in Baltic Sea ice. Aquatic Microbial Ecology 63:75–87
Piiparinen J, Kuosa H, Rintala JM (2010) Winter-time ecology in Bothnian Bay, Baltic Sea: nutrients and algae in fast-ice. Polar Biology 33:1445–1461
Poulin M, Daugbjerg N, Gradinger R, Ilyash L, Ratkova T, von Quillfeldt C (2011) The Pan-Arctic biodiversity of marine pelagic and sea-ice unicellular eukaryotes: a first-attempt assessment. Marine Biodiversity 41:13–28
Rasmus K, Ehn J, Granskog MA, Kärkäs E, Leppäranta M et al (2002) Optical measurements of sea ice in the Gulf of Finland. Nordic Hydrology 33:207–226
Rintala (2009) A systematic-ecological approach to Baltic Sea ice studies of algae and protists. University of Helsinki, Walter and Andrée de Nottbeck Foundation Scientific Reports 34:1–48 [PhD Thesis]
Rintala JM, Hällfors H, Hällfors S, Hällfors G, Majanerva M, Blomster J (2010a) Heterocapsa arctica subsp. frigida subsp. nov. (Peridiniales, Dinophyceae)—description of a new dinoflagellate and its occurrence in the Baltic Sea. Journal of Phycology 46:751–762
Rintala JM, Piiparinen J, Ehn J, Autio R, Kuosa H (2006) Changes in phytoplankton biomass and nutrient quantities in sea ice as responses to light/dark manipulations during different phases of the Baltic winter 2003. Hydrobiologia 554:11–24
Rintala JM, Spilling K, Blomster J (2007) Temporary cyst enables long term dark survival of Scrippsiella hangoei (Dinophyceae). Marine Biology 152:57–62
Rintala JM, Piiparinen J, Uusikivi J (2010b) Drift-ice and under-ice water communities in the Gulf of Bothnia (Baltic Sea). Polar Biology 33:179–191
Rintala JM, Piiparinen J, Blomster J, Majaneva M, Müller et al (2014) Fast direct melting of brackish sea-ice samples results in biologically more accurate results than slow buffered melting. Polar Biology 37:1811–1822
Rysgaard S, Bendtsen J, Delille B, Dieckmann GS, Glud RN et al (2011) Sea ice contribution to the air-sea CO2 exchange in the Arctic and Southern Oceans. Tellus B 63:823–830
Spilling K (2007) Dense sub-ice bloom of dinoflagellates in the Baltic Sea, potentially limited by high pH. Journal of Plankton Research 29:895–901
Stedmon CA, Thomas DN, Granskog M, Kaartokallio H, Papadimitriou S, Kuosa H (2007) The characteristics of dissolved organic matter in Baltic coastal sea ice: allochthonous or autochthonous origins? Environmental Science and Technology 41:7273–7279
Steffens M, Granskog MA, Kaartokallio H, Kuosa H, Luodekari K et al (2006) Spatial variation of biogeochemical properties of landfast sea ice in the Gulf of Bothnia (Baltic Sea). Annals of Glaciology 44:80–87
Stirling I, Richardson E, Thiemann GW, Derocher AE (2008) Unusual predation attempts of polar bears on ringed seals in the southern Beaufort Sea: possible significance of changing spring ice conditions. Arctic 61:14–22
Stocker TF, Qin D, Plattner GK, Tignor M, Allen SK et al (eds) (2013) The physical science basis—contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge 1535 pp
Tedesco L, Vichi M (2014) Sea ice biogeochemistry: a guide for modellers. PLoS ONE 9(2):e89217
Tedesco L, Vichi M, Haapala J, Stipa T (2009) An enhanced sea ice thermodynamic model applied to the Baltic Sea. Boreal Environment Research 14:68–80
Tedesco L, Vichi M, Haapala J, Stipa T (2010) A dynamic biologically-active layer for numerical studies of the sea ice ecosystem. Ocean Modelling 35:89–104
Thomas DN, Dieckmann GS (2002) Antarctic sea ice—a habitat for extremophiles. Science 295:641–644
Thomas DN, Dieckmann GS (eds) (2010) Sea ice, 2nd edn. Wiley-Blackwell Publishing, Oxford 621 pp
Underwood GJC, Salam SN, Michel C, Niemi A, Norman L et al (2013) Broad-scale predictability of carbohydrates and exopolymers in Antarctic and Arctic sea ice. Proceedings of the National Academy of Sciences of the USA 110:15734–15739
Uusikivi J (2013) On optical and physical properties of sea ice in the Baltic Sea. Report Series in Geophysics 73:1–49
Uusikivi J, Ehn J, Granskog MA (2006) Direct measurements of turbulent momentum, heat and salt fluxes under landfast ice in the Baltic Sea. Annals of Glaciology 44:42–46
Uusikivi J, Vähätalo AV, Granskog MA, Sommaruga R (2010) Contribution of mycosporine-like amino acids and colored dissolved and particulate matter to sea ice optical properties and ultraviolet attenuation. Limnology and Oceanography 55:703–713
Vihma T, Haapala J (2009) Geophysics of sea ice in the Baltic Sea: a review. Progress in Oceanography 80:129–148
Weeks WF (1998) Growth conditions and the structure and properties of sea ice. In: Leppäranta M (ed) Physics of ice-covered seas. Department of Geophysics, University of Helsinki, Helsinki, pp 25–104
Weissenberger J, Dieckmann GS, Gradinger R, Spindler M (1992) Sea ice: a cast technique to examine and analyse brine pockets and channel structure. Limnology and Oceanography 37:179–183
Werner I, Auel H (2004) Environmental conditions and overwintering strategies of planktonic metazoans in and below coastal fast ice in the Gulf of Finland (Baltic Sea). Sarsia 89:102–116
Zhou J, Delille B, Kaartokallio H, Kattner G, Kuosa H et al (2014) Physical and bacterial controls on inorganic nutrients and dissolved organic carbon during a sea ice growth and decay experiment. Marine Chemistry 166:59–69
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Thomas, D.N. et al. (2017). Life associated with Baltic Sea ice. In: Snoeijs-Leijonmalm, P., Schubert, H., Radziejewska, T. (eds) Biological Oceanography of the Baltic Sea. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-0668-2_9
Download citation
DOI: https://doi.org/10.1007/978-94-007-0668-2_9
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-0667-5
Online ISBN: 978-94-007-0668-2
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)