Abstract
Key message
Sugarcane transgenic overexpressing EaGly III from Erianthus arundinaceus showed enhanced water deficit stress tolerance.
Abstract
Methylglyoxal (MG), an α-ketoaldehyde formed from either glycolysis or TCA cycle, is capable of causing total cellular damage via the generation of reactive oxygen species (ROS), advanced glycation end products (AGEs) and nucleic acid degradation. Glyoxalase pathway is a ubiquitous pathway known for detoxification of MG, involving key enzymes glyoxalase I (Gly I) and glyoxalase II (Gly II). Recently, a novel and an additional enzyme in glyoxalase pathway, viz., glyoxalase III (Gly III), has been discovered which possesses DJ-1/PfpI domain recognized for detoxifying MG in a single step process without requirement of any coenzyme. In the present study, a Gly III gene isolated from Erianthus arundinaceus, a wild relative of sugarcane, overexpressed in commercially cultivated sugarcane hybrid Co 86032 was assessed for drought tolerance. Morphometric observations revealed that transgenic sugarcane overexpressing EaGly III acquired drought tolerance trait. Oxidative damage caused by triggering generation of ROS has been determined to be low in transgenic plants as compared to wild type (WT). Transgenics resulted in higher relative water content, chlorophyll content, gas exchange parameters, photosynthetic efficiency, proline content and soluble sugars upon water deficit stress. In addition, higher and stable level of superoxide dismutase and peroxidase activities were observed along with minimal lipid peroxidation during drought stress signifying the tolerance mechanism exhibited by transgenic events. There was no significant structural change observed in the root anatomy of transgenic plants. Altogether, EaGly III gene could be considered as a potential candidate for conferring water deficit stress tolerance for sugarcane and other agricultural crops.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diminishing area of cultivation land for agriculture together with adverse environmental distresses, such as, water scarcity and salinity necessitate development of high yielding crop varieties for serving the rapidly growing human population (Martinez et al. 2016). Sugarcane (Saccahrum sp.) is an important commercial crop with multifarious usages grown mainly for sugar derived from cane juice and second-generation ethanol derived from its biomass (Boaretto et al. 2014). In India, about 3.0% (5.0 Mha) of area in tropical and subtropical zones is under sugarcane cultivation. Major obstacle for increasing ethanol and sugar production from sugarcane is found to be due to the irregular or inadequate rainfall in the country (Cheavegatti-Gianotto et al. 2011).
Plants undergo drought-stress when the rate of transpiration is higher or when the supply of water to roots becomes limiting. Sugarcane being a high-water requiring crop, water scarcity poses a serious problem to sugarcane production in India (Sarath et al. 2018). Water scarcity is known to affect the yield of sugarcane crop by about 70% (Gosal et al. 2009). Four growth phases of sugarcane which are sensitive to water deficit stress are germination, tillering, grand growth and maturity phase. Water scarcity can significantly decrease stomatal conductance and as a consequence leads to progressive decline in CO2 assimilation rate. It can also reduce the activity of enzymes involved in photosynthetic carbon reduction cycle such as ribulose-1,5-bisphosphate carboxylase/oxygenase (RuBisCo) (Reddy et al. 2004). Even though water deficit stress tolerance in plants are seen naturally, its extent is found to vary from species to species and as a function of severity of stress experienced. The biochemical and molecular basis of drought tolerance or drought susceptibility is still to be unraveled (Zhu 2002; Chaitanya et al. 2003; Chaves et al. 2003).
Methylglyoxal (MG), a cytotoxic compound, formed in surplus from glycolysis or tricarboxylic acid (TCA) cycle, is capable of complete cellular annihilation by inducing the biogenesis of advanced glycation end products (AGEs), oxidation of fatty acids and impairment of biomembrane structure or functions (Chaplen 1998; Gill and Tuteja, 2010; Ghosh et al. 2016). The presence and operational characteristics of MG is quite well established in diverse organisms, such as, bacteria, yeasts, animals as well as higher plants (Kaur et al. 2014). Tolerance to water deficit stress by involving highly intricate physiological and biochemical mechanisms is found to be in operation in higher plants. These findings demonstrate that plants are innately armed with defense mechanisms to scavenge excessively produced reactive oxygen species (ROS) and toxic metabolites, such as, MG. The catabolic process involved in the degradation and the consequent detoxification of MG in eukaryotes comprises the glyoxalase pathway, consisting of glyoxalase I (Gly I) and glyoxalase II (Gly II) which operates in the presence of glutathione as a coenzyme and glyoxalase III (Gly III) whose catalytic activity takes place without the involvement of the coenzyme. Gly I and Gly II displayed their metabolic function in response to several abiotic stresses as well as during different developmental stages (Ramaswamy et al. 1984; Sethi et al. 1988; Deswal et al. 1993). Several reports indicated that transgenic events with the overexpression of glyoxalase pathway genes conferred abiotic stress tolerance in several plants (Veena et al. 1999; Yadav et al. 2005; Singla-Pareek et al. 2003, 2006, 2008; Prashanth et al. 2008; Kim et al. 2008; Bhatnagar-Mathur et al. 2008; Ashraf 2009; Augustine et al. 2015a). Accordingly, it is inferred that the glyoxalase cycle serves as a prime route and confers defense against glycation end products (Bhowal et al. 2020). There are also a few reports indicating the elevated expression genes under abiotic stress conditions in wild sugarcane species which might play a role in conferring abiotic stress tolerance Augustine et al. (Augustine et al. 2015a, b, c; Narayan et al. 2019; Anunathini et al. 2019; Dharshini et al. 2020a, b; Peter et al. 2020).
Several studies reported during the past decades highlighted the mechanism underlying the strategies which plants adopt to maintain and tackle water status during water deficit conditions. Those strategies were shown to operate at physiological, biochemical and molecular levels with the prominence of tackling the oxidative stress to which plants are subjected to during water deficit stress. Relationship between root anatomy and water deficit stress tolerance has also been studied extensively (Jackson et al. 2000; Maggio et al. 2001; Dharshini et al. 2020a, b). Based on the results of the present study, we report for the first time the generation of sugarcane transgenics overexpressing Gly III and assessment of those transgenics based on morphological, physiological and biochemical parameters. The results revealed enhanced performance of the generated transgenics under water deficit stress condition.
Materials and methods
Development of plant transformation vector
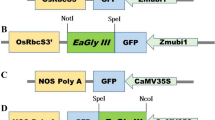
EaGly III gene (Acc. No.: MG701311) previously isolated from E. arundinaceus was used in this study (Manoj et al. 2019). pCAMBIA 1305.1 binary vector was restricted with SpeI and BstEII enzymes to clone EaGly III (1164 bp) gene in place of GUSPlus gene. Likewise, BamHI and NcoI restriction enzymes were used to replace CaMV35S promoter with Port Ubi 882 (Philip et al. 2013) promoter (Fig. S1). Primers used for cloning Port Ubi 882 promoter and EaGly III are given in Table 1. The generated plasmid construct was named pSBI::EaGlyIII and transformed to Escherichia coli DH5α cells by heat shock method.
Generation of sugarcane transgenic events
Embryogenic calli produced from meristematic leaf explants of Co 86032 were exposed to semi-solid Murashige and Skoog’s based osmotic media (MS + 50 mg/L Sorbitol and 50 mg/L Mannitol) for 3 h. pSBI::EaGlyIII plasmid (1 µg) was transformed into osmotic stress treated calli by particle bombardment method using Bio-Rad PDS 1000/He Biolistic System at 1100 psi of Helium. Those calli were bombarded two times initially at a distance of 4 cm and subsequently at a distance of 8 cm from stopping screen. Transformed calli were selected and regenerated (250–350 µmol m−2 s−1 light intensity, photoperiod of 16 h light and 8 h dark, temperature of 25 °C ± 1 °C and 50–60% relative humidity) on hygromycin containing (30 mg/L) MS media. Calli were sub-cultured every fifteen days and were subjected to eight rounds of selection on antibiotic containing medium. Regenerated plants of 5–6 cm length were transferred to White’s medium for rooting (Amin et al. 2017). Putative transgenic plantlets with well-established roots were hardened in pots containing a mixture of red soil, sand and farmyard manure (1:1:1 ratio) and maintained in transgenic glass house facility. Likewise, for control plants untransformed embryogenic calli produced from meristematic leaf explants of Co 86032 were regenerated and maintained in glass house conditions.
Confirmation of transgenic events by molecular analysis
Genomic DNA from putative transgenic events (V0—Regenerated plants) and untransformed control (wild type—WT) was isolated as described by Doyle and Doyle (1987) followed by RNase I (Thermo Fisher Scientific Company, USA) treatment to avoid any RNA contamination. Genomic DNA (50 ng) and plasmid DNA (10 ng) were used as template for polymerase chain reaction (PCR). Presence of transgene in putative transgenic lines was confirmed by PCR amplification using promoter specific primers (5′-GATCGGATCCACTATCACCCTCGAGGTG-3′ and 5′-GATCCCATGGTCTTTTTGTTT GTTGGT-3′) and hygromycin (hptII) primers (5′-GATCTCCAATCTGCGGGATC-3′ and 5′-ACTCACCGCGACGTCTGTCG-3′). PCR reaction consists of an initial denaturation at 95 °C for 4 min followed by 35 cycles of denaturation at 95 °C for 45 s, annealing at 60 °C for 30 s, 72 °C for 1 min and final extension at 72 °C for 7 min. Only plants with the expected size of amplicons for both promoter (882 bp) and hygromycin (416 bp) were selected for further analysis. The PCR amplified products were also cloned and confirmed through Sanger sequencing.
Sugarcane is usually propagated via stem cuttings or stalk sections called as setts. Eleven transgenic events (V0) that confirmed for the presence of EaGly III transgene and wild type (WT; untransformed control Co 86032) were further multiplied through planting of single bud cuttings to obtain V1 stage (V1—Vegetative generation). There were no significant morphological differences observed between the WT and the transgenic plants. Three biological replicates of each transgenic events and control were planted in 18″ pots containing sand, red soil and farmyard manure in the ratio 1:1:1 and grown under transgenic green house (1500–1800 µmol m−2 s−1 light intensity, photoperiod of 16 h light and 8 h dark, temperature of 30 °C ± 2 °C and ~ 75% relative humidity). Plants received regular watering served as control. Plants were exposed to stress by withholding watering during tillering stage (90 days post planting) for 15 days. On 0th day of stress soil moisture content was 25% and on 15th day of stress soil moisture content was 7.71%. Uniformity of plant water stress was monitored by gravimetrically weighing the pots twice a day following the procedure described by Geetha et al. (2009). Soil moisture content (%) was calculated through gravimetric method using soil moisture analyser (A & D Model Mx-50) by collecting soil from thee different depths (10, 20 and 30 cm) (Augustine et al. 2015c). Fully opened third leaves were collected at the end of stress period (15 days after stress), from both stressed and non-stressed plants.
Estimation of chlorophyll, carotenoid and gas exchange parameters
Leaf samples (50 mg) of 15th day of stressed along with control plantlets were excised into a test tube containing 10 mL of dimethyl sulfoxide (DMSO) and incubated at 65 °C for 4 h. The samples were cooled to room temperature (RT) and the absorbance was read at 470, 645 and 663 nm. The chlorophyll content was measured following Lorenzen (1967).
Gas exchange parameters were measured to find out the photosynthesis rate, transpiration rate and stomata conductance after 15th day of stress using a portable photosynthesis system (Li-6400, Li-COR Inc., Nebraska, USA). Photochemical efficiency (Fv/Fm), photosynthetically active radiation (PAR) and electron transport rate (ETR) were measured to determine the photosynthesis efficiency and plant activities after 15 days of stress using Plant Stress Kit (Opti-Sciences, USA).
Relative water content and cell membrane thermostability
Leaf hydration status on 15th day after inducing stress was evaluated by measuring the relative water content (RWC) as mentioned elsewhere (Augustine et al. 2015b). Leaf samples (200 mg) were excised from fully opened third leaves, measured (fresh weight; Fw) and soaked in deionized water for 4 h to record the turgid weight (Tw). Excess water from the tissue surface was removed by gently blotting with a tissue paper and dried in a hot air oven for 3 days to measure the dry weight (Dw). RWC of root after 15 days of stress was also estimated in the same way.
For the assessment of cell membrane thermostability, 200 mg of fully opened third leaves from control and stressed samples were weighed into two separate test tubes and washed thrice with deionized water. 20 mL of deionized water was added immediately to each tube. One set was treated at 60 °C by placing them in a water bath for 20 min. Both control and treated samples were kept at 10 °C for 12 h to allow the efflux of electrolytes to water. Initial conductance was measured at 25 °C using conductivity meter 4310 (Jenway, UK) and the tubes were heated at 100 °C for 20 min and cooled to measure the final conductance. Cell membrane injury (CMI) percentage was calculated using the formula: CMI % = 1 − [(1 − T1/T2)/(1 − C1/C2)] × 100, where T and C denotes the conductance of stress and control samples, subscripts 1 and 2 represents the initial and final conductance, respectively (Martineau et al. 1979).
Proline and total sugars
Plant samples (500 mg) of both leaf and root from control and stressed plants were ground using liquid nitrogen and homogenized with 3% sulfosalicylic acid. The mixture was then filtered through Whatmann No. 2 filter paper (Himedia, India) and the filtrate was used for proline (µmol/g FW) estimation as described by Bates et al. (1973). Total sugar content (mg/g FW) was estimated after boiling 200 mg of samples (control and stress) in 80% ethanol for two times followed by boiling in deionized water for 20 min, the supernatant was filtered through Whatmann No. 2 filter paper (Himedia, India) after cooling to RT. Filtrate (1 mL) was mixed with freshly prepared 5% phenol and concentrated sulfuric acid (H2SO4) and allowed to stand for 45 min to measure the absorbance at 490 nm as described by Buysse and Merckx (1993).
Estimation of enzyme activities
Leaf samples (1 g) from stressed and control plants were ground using liquid nitrogen and homogenized in 50 mM potassium phosphate buffer (PPB; pH 7.0) containing 1 mM ethylenediaminetetraacetic acid (EDTA), 3 mM dithiothreitol (DTT), and 5% (w/v) insoluble polyvinylpyrrolidone (PVP). The homogenate mixture was centrifuged at 10,000 g for 30 min at 4 °C and the supernatant was extracted (protein extract) for further assays. Same methodology was adopted to collect extract from root samples.
SOD activity (units/g FW) was assayed with a reaction mixture consisting of 50 mM sodium phosphate buffer (pH 7.8), 1 mM nitro blue tetrazolium (NBT), 50 mM methionine, 10 mM EDTA, 0.1 mM riboflavin and 25 µL of protein extract measured spectrophotometrically at 560 nm. The activity was measured by its ability to inhibit the photochemical reduction of NBT (Dhindsa et al. 1981). The reaction mixture for estimating total peroxidases activity (µmol/mg/min) consists of 50 µL of PPB (100 mM), 25 µL of guaicol (96 mM), 25 µL of H2O2 (12 mM), and 10 µL of sample extract. The absorbance was colorimetrically measured at 470 nm using a spectrophotometer following Castillo et al. (1984).
Plant samples (300 mg) of both leaf and root from stressed and control were ground using liquid nitrogen and homogenized in 0.1% trichloroacetic acid (TCA) buffer. Supernatant (TCA extract) was collected after centrifugation at 10,000 g for 10 min for estimating the lipid peroxidation by determining the presence of malondialdehyde (MDA). TCA extract (250 µL) was added to a solution containing 20% (w/v) TCA and 0.5% (w/v) thiobarbetureic acid (TBA) and incubated at 95 °C for 30 min. The reaction mixture was centrifuged at 4 °C for 5 min at 10,000 g and the supernatant was measured spectrophotometrically at 535 and 600 nm. MDA content (nmol/g FW) was determined by subtracting 600 nm value from 535 nm value.
Root anatomy
Paraffin wax method as described by Franklin et al. (2006) was followed to analyze the anatomical structure of roots under control and stress conditions. Root samples of regularly irrigated and drought stressed plants were fixed in FAA (formaldehyde: acetic acid: alcohol in the ratio 2:1:10) for 2 days followed by dehydrating in tertiary butyl alcohol. The root samples were then infiltrated and embedded in paraffin wax (Tm 56–58 °C) in a hot air oven kept at 59 °C (Dharshini et al. 2020a, b). Sections were made at 15 µm thickness using a microtome (Leica, Germany) and stained with safranin to view under a light microscope (Leica, Germany).
Statistical analysis
A complete randomized block design consisting of three biological and three technical replicates of each samples were followed. Results were expressed as mean values and the standard deviation was evaluated using OPSTAT program. P value below 0.05 was considered as significantly different.
Results
Cloning of Gly III, generation of transgenic events and molecular analysis
DJ-1/PfpI domain containing Gly III gene of 1164 bp isolated from E. arundinaceus (EaGly III) was cloned in pCAMBIA1305.1 vector driven by Port Ubi 882 (PD2), a constitutive ubiquitin promoter isolated at ICAR-Sugarcane Breeding Institute, Coimbatore, India. The resulted overexpression construct was used to generate transgenic sugarcane plants by particle bombardment following effective selection and regeneration protocol described elsewhere (Arvinth et al. 2010). Out of 21 V0 transgenic events screened, eleven transgenic events were confirmed by PCR amplification of promoter (PD2) and marker gene (hptII). Specific amplifications of 882 bp and 416 bp, respectively, were obtained for PD2 and hptII (Fig. S2) and further confirmed through sequence analysis of these amplicons. There were no morphological differences observed between V0 transgenic events and WT indicating that overexpression did not result in any phenotypic alterations.
Estimation of chlorophyll content and gas exchange parameters
Chlorophyll-a (Chl-a), chlorophyll-b (Chl-b) and carotenoid content measured using DMSO method was found to be significantly higher in transgenic events with respect to WT (Fig. 1). The photosynthetic parameters and photochemical efficiency measured after 15 days of water deficit stress including gas exchange parameters, such as, photosynthesis rate, stomatal conductance and transpiration rate were significantly higher in transgenic events compared to WT. Most of the transgenic events showed 2–3 times higher stomatal conductance subsequent to incubation under water deficit stress condition. Photosynthetic efficiency monitored as per Fv/Fm, PAR and ETR perceived enhanced readings in transgenic events compared to WT. Even though there were variations among transgenic events, higher photosynthetic parameters were noticed in all transgenic events compared to WT. Fv/Fm, PAR and ETR values in WT showed decline with the values as low as 0.39, 65 and 9.4, respectively, whereas transgenic lines maintained maximum of 0.68, 76 and 13.4 Fv/Fm, PAR and ETR values, respectively (Fig. 2).
Chlorophyll-a, Chlorophyll-b and Carotenoid content in control (a) and under stress (b); gas exchange parameters comprising photosynthesis rate (c), stomatal conductance (d) and transpiration rate (e) and changes in Fv/Fm (f), photosynthetically active radiation (PAR; g) and electron transport rate (ETR; h) in transgenic sugarcane events along with WT
Relative water content and cell membrane injury
There was a substantial conservation of leaf and root water content in all the transgenic events compared to WT upon drought stress (Fig. 3). A reduction of 22.04% to 25.91% was observed in transgenic events, while a drastic reduction of 48.17% was observed in WT leaf samples after 15 days of water deficit stress. The reduction percentage of root water status was observed to be between 11.36 and 16.82 in transgenic events apart from a reduction percentage of 38.34 in WT, which is more than twice the percentage of reduction compared to sugarcane transgenics overexpressing EaGly III.
Relative Water Content (RWC; a and b) and cell membrane thermostability (c and d) after 15 days of drought stress in leaf (a and c) and root (b and d) samples of sugarcane transgenic events along with WT. There is significant (P < 0.05) maintenance of water potential and cell membrane thermostability in transgenic events compared to WT
Cell membrane injury percentage in leaf and root samples was significantly lower in all transgenic events compared to WT upon water deficit stress representing the increased thermostability of the events (Fig. 3). Cellular injury of transgenic leaf samples ranged from 5.38% to 23.07% of decrease, while WT leaf samples had shown an increased membrane damage of 19.63% upon drought stress. Similarly, a reduction in membrane injury ranging within 5.76% to 41.54% was observed in transgenic sugarcane roots whereas membrane damage was found to increase to 33.21% in WT upon drought stress.
Proline content and total sugars
Highly increased free proline content was recorded in transgenic events compared to WT (Fig. 4). Proline content showed an increase to attain a maximum of 25.50 µmol g−1 FW in transgenic events, whereas only 1.06 µmol g−1 FW of proline was observed in WT leaves after 15 days of drought stress. Free proline content was also found to mark a highest value of 20.07 µmol g−1 FW proline in transgenic roots as compared to that of 1.02 µmol g−1 FW proline in WT.
Prolonged drought by withholding watering for 15 days increased total soluble sugars in both leaf and root samples of transgenic events as well as WT. The increase in total sugars was significantly higher in transgenic events compared to WT. Significant increase of total sugars was noted in transgenic roots as compared to the leaf tissues. Total sugars in transgenic events exhibited a maximum of 24.92 mg/g FW in the leaf and 28.21 mg/g FW in the root, whereas WT showed a slight increase in total sugar content from 1.10 mg/g FW to 6.89 mg/g FW in the leaf and from 4.71 mg/g FW to 8.20 mg/g FW in the root (Fig. 4).
Enzyme assays
No significant difference in SOD activity was noticed between transgenic lines and WT under irrigated conditions. The activity of SOD showed drastic increase due to water deficit stress in the transgenic lines as compared to WT. Transgenic events PG3 1, PG3 3 and PG3 7 displayed the highest SOD activity of 50.83, 50.85 and 51.09 units/g FW, respectively, in the leaf samples as compared to the other events. In contrast, the transgenic events PG3 1, PG3 3, PG3 7 and PG3 9 exhibited maximum SOD activity of 54.13, 55.06, 55.43 and 53.58 units/g FW, respectively, in the root samples.
Minimal activity of peroxidase was noticed under irrigated conditions and there was only insignificant variation observed between transgenic events as compared to WT. Peroxidase activity in transgenic events increased significantly after water deficit stress as compared to that of WT. It was observed that the transgenic lines- PG3 5, PG3 9, PG3 10 and PG3 11, had higher level of POD activity ranging between 39.23 to 46.03 µm/mg/min in the leaves and 45.56 to 50.45 µm/mg/min in the roots.
Membrane damage level was monitored by measuring the MDA content. There were not any significant difference in MDA content between transgenic events and WT under well irrigated conditions. Whereas, MDA content drastically increased upon water deficit stress in WT indicating more oxidative damage as compared to transgenic events. It was noted that events PG3 2, PG3 3, PG3 4 and PG3 6 had the minimal MDA content of 2.11, 1.87, 2.28 and 2.56 nmol g−1 FW, respectively. Very minimal MDA content was observed in the root samples ranging from 1.63 to 1.98 nmol g−1 FW in PG3 2, PG3 3, PG3 4, PG3 6, PG3 7 and PG3 8. Altogether, there were higher activity of SOD and POD and minimal MDA content in sugarcane transgenic events (Fig. 5).
Root anatomy
Comparison of features of root anatomy of transgenic events with that of WT was carried out using a light microscope. The transgenic events exhibited stable maintenance of root anatomy as compared to WT after 15 days of water deficit stress. Notably, WT plants showed remarkable structural changes as compared to transgenic events (Fig. 6).
Microscopic observation of root anatomy in transgenic events (a) and WT (b) during normally irrigated conditions (control) and upon drought stress. Root anatomy is marked as parenchymatous cortex (PAC), endodermis (ED), pericycle (PEC), protoxylem (PX), phloem (PH), metaxylem (MX), sclerenchyma cylinder (SC) and pith (PI). Transgenic events maintained better root anatomy than WT upon stress
Discussion
Plants get more often exposed to multiple stresses due to their sessile nature which in turn affects their productivity. There are several tolerance mechanisms being operated by plants in response to environmental stress factors, both biotic and abiotic (Govind et al. 2009). Genetically, stress tolerance traits are known to be mutagenic, which confer the trait by involving a complex network of metabolic pathways. Cytotoxic MG is known to be accumulated in excess under most of the abiotic stress conditions (Kaur et al. 2014). Accumulation of this α-oxoaldehyde is extremely toxic to the cell causing denaturation of macromolecules, such as, DNA, RNA and proteins. Glyoxalase pathway genes consisting of Gly I and Gly II were discovered more than 100 years ago which had given better insights towards cellular mechanism of detoxification of MG in plants. Detoxification of MG is being carried out by the sequential action of the enzymes- Gly I and Gly II in the presence of glutathione (GSH) that lead to the generation of non-toxic D-lactate. Thus, cellular level of GSH acts as the major cofactor for the detoxification of MG through the operation of glyoxalase pathway (Noctor et al. 2002). The glutathione concentration and its redox state are found to be highly dynamic due to complex interplay between biosynthesis, utilization, degradation, oxidation/reduction, and transport between intercellular and intracellular locations. It has also been reported that GSH could act as a feedback competitive inhibitor of Gly II enzyme (Ghosh et al. 2016). Recent studies pointed out the existence of Gly III in E. coli (Subedi et al. 2011), Schizosaccharomyces (Zhao et al. 2014) and eukaryotes (Ghosh et al. 2016; Li et al. 2019). Gly III from those sources was shown to possess DJ-1/Pfp domain which conferred its role in the unique MG detoxification pathway without having binding affinity for glutathione. Earlier studies in animal systems on DJ-1/Pfp domain containing proteins showed the important role of this group of proteins in cellular responses due to oxidative stress and mitochondrial dysfunction (Lee et al. 2012). Hence, in the present study, assessment of the performance of transgenic events of commercially cultivated sugarcane Co 86032 overexpressing EaGly III gene upon subjecting to drought stress was undertaken.
Eleven sugarcane transgenic events overexpressing EaGly III along with wild type (WT) were grown for 3 months under controlled conditions. All the events along with WT were exposed to drought stress by withholding watering for a period of 15 days. Transgenic and WT plants that were irrigated regularly served as control. There were notable morphometric differences observed between transgenic sugarcane events as compared to WT after 15 days of drought stress (Fig. 1).
Drought is a water availability related condition referred to as dry (water deficit) condition which can affect the growth and productivity of crop plants. Drought condition is known to prevail in certain geographical locations of the whole world. May and Milthrope (1962) defined plant drought resistance as “the ability of plants to grow and survive under water deficit conditions”. Plants have been bestowed with an array of highly advanced traits to grow and develop by encountering and overcoming drought stress. Several studies have reported the overexpression of glyoxalase pathway genes in higher plants to confer abiotic stress tolerance to factors such as, salinity, drought, etc. and their strong interactions with ROS and MG for conferring tolerance (Singla-Pareek et al. 2003; Yadav et al. 2005; Roy et al. 2008; Viveros et al. 2013; Mustafiz et al. 2014). The present study comprising the first report on the overexpression of EaGly III in sugarcane revealed the acquisition of relatively higher degree of tolerance in the transgenics to drought stress as compared to WT. Transgenic events in sugarcane demonstrated significantly higher photosynthesis rate, stomatal conductance and transcription rate under drought stress. In addition, transgenic events overexpressing EaGly III also displayed significantly higher Fv/Fm ratio, PAR and ETR compared to WT. Moreover, transgenics exhibited significantly higher content of Chl-a, Chl-b and carotenoid as compared to WT. Photosynthesis serves as the primary source of energy for the photoautotrophic plants and the protection of photosynthetic machinery aids in augmenting the capability of green plants to grow under hostile environmental conditions (Mackova et al. 2013). Several plant species have been shown to contain decreased chlorophyll content under stress regime depending upon the conditions of stress, such as, severity and duration of stress (Van Rensburg and Kruger, 1994; Kyparissis et al. 1995; Jagtap et al. 1998).
Transgenic sugarcane plants overexpressing EaGly III have shown significantly higher plant water status under drought stress compared to WT. It is evident from the previous studies that overexpression of LEA genes in rice results in higher RWC, turgor pressure and enhanced membrane stability together with higher growth rates under prolonged water deficit conditions (Babu et al. 2004). Results of the present study showed that relatively high degree of cell membrane thermostability was possessed by the transgenics upon drought stress as compared to WT. These observations indicate that the glyoxalase-overexpressing transgenic plants could tolerate damage triggered by oxidative stress. This is in line with the findings that tolerant genotypes with low level of MDA content perform well even under abiotic stress conditions (Molinari et al. 2007; Dharshini et al. 2020a, b).
Proline accumulation is interpreted to be the distinguishing plant stress response which is known to be involved in mitigating cytosolic acidosis in response to various abiotic stresses, such as, drought and salinity (Heuer 1994; Kurkdjian and Guern, 1989). Proline is known to be a strong water holding, compatible and key osmoregulatory amino acid in plants capable of conserving healthy cell structure, and provide tolerance to hostile environmental conditions (Hayat et al. 2012; Sun et al. 2017). Free proline content was found to be higher in sugarcane transgenic events as compared to WT upon imposition of drought stress demonstrating the possible occurence of proline mediated tolerance mechanism in the transgenic events. Similarly, total sugar content of sugarcane transgenic events showed an increase due to drought stress. It has also been suggested that the metabolic regulations that leads to the accumulation of organic solutes, such as, sugars are also one of the adaptation mechanisms seen in plants grown under various stresses (Gill et al. 2002). In higher plants, soluble sugars are recognized for their osmoregulatory role upon osmotic stress (Gandonou et al. 2011). Soluble sugars are known to contribute up to 50% towards the total osmotic potential in crops such as sugarcane (Cram 1976). An earlier report on Populus sp. and sunflower stated that tolerant crops accumulate soluble sugars upon stress (Watanabe et al. 2000; Ashraf and Tufail, 1995). It has been shown that expression of Grifola frondosa trehalose synthase (TSase) gene in sugarcane resulted in the accumulation of trehalose sugar that in turn improved drought tolerance (Zhang et al. 2006).
Oxidative damages triggered by excessively produced ROS are known to result in serious metabolic disorders in plants. Antioxidant enzymes are involved in scavenging the ROS in the cells during stress conditions. SOD plays an important role in detoxification of superoxide (O2−) radicals in different compartments of a cell (Alscher et al. 2002). In the present study, drought stress incubation considerably increased the activity of SOD in leaves as well as roots after 15 days of stress incubation. This observation indicated the possibility of the involvement of SOD via the scavenging of ROS during stress alleviation in the transgenic events as compared to WT. Likewise, considerable increase in the activity of POD was detected in the transgenic events as compared to WT after 15 days of drought stress. SOD and POD alleviated the adverse environmental distress in transgenic events by acting together as protective enzymes have been reported earlier (Sun et al. 2017). Antioxidant capacity of plants is known to play a crucial role in conferring resistance to abiotic stresses (Monk et al. 1989; Dharshini et al. 2020a, b). Lipid peroxidation is generally an indication of oxidative damage due to generation of free radicals and is quantified by detecting the MDA content (Sun et al. 2017; Gratao et al. 2005). Insignificant level of MDA was found in roots as compared to the leaves of transgenic events.
Predominantly thicker and circular roots are found in Saccharum sp. (da Cruz Maciel et al. 2015). Plants grown under drought conditions need to retain specific root architecture to thrive under poor water availability. Protruding cell wall thickening, suberized exodermis, cortical lysigenous aerenchyma, and endodermis with U-thickening have been reported in Poaceae members including sugarcane in response to drought stress (Vasellati et al. 2001; Pereira et al. 2008; da Cruz Maciel et al. 2015). Results of the present study showed the expression of adaptive traits related to physiological and molecular parameters which could have facilitated transgenic plants to maintain stable root microstructures. It is pertinent to indicate that no notable changes were recorded in anatomical features of the roots of the transgenics even after prolonged exposure to drought stress.
Conclusion
In a nutshell, sugarcane transgenic events overexpressing EaGly III maintained higher photosynthesis efficiency and induced expression of stress responsive biochemical parameters such as chlorophyll content, RWC, cell membrane thermostability, proline content, antioxidant enzyme activities and unaffected root microstructures signifying enhanced drought stress tolerance. This study opens up new avenues in generating abiotic stress tolerant varieties for sustainable agriculture. In addition, the tolerance level conferred by EaGly III under drought stress conditions make the glyoxalase pathway genes as the choice for generating transgenics to evolve superior biotypes with desirable stress tolerance traits. More studies are needed to determine the tolerance level and yield potential of the crop species under field conditions.
References
Alscher RG, Erturk N, Heath LS (2002) Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J Exp Bot 53:1331–1341. https://doi.org/10.1093/jexbot/53.372.1331
Anunathini P, Manoj VM, Padmanabhan TS, Dhivya S, Narayan JA, Appunu C, Sathishkumar R (2019) In silico characterisation and functional validation of chilling tolerant divergence 1 (COLD1) gene in monocots during abiotic stress. Funct Plant Biol 46:524–532. https://doi.org/10.1071/FP18189
Amin S, Kaloo ZA, Singh S (2017) Effect of cytokinins on multiple shoot regeneration from leaf derived callus of inula royleana DC. Plant Tissue Cult Biotechnol 27:189–194. https://doi.org/10.3329/ptcb.v27i2.35024
Arvinth S, Arun S, Selvakesavan RK, Srikanth J, Mukunthan N, Kumar PA, Premachandran MN, Subramonian N (2010) Genetic transformation and pyramiding of aprotinin-expressing sugarcane with cry1Ab for shoot borer (Chilo infuscatellus) resistance. Plant Cell Rep 29:383–395. https://doi.org/10.1007/s00299-010-0829-5
Ashraf M, Tufail M (1995) Variation in salinity tolerance in sunflower (Helianthus annum L.). J Agron Crop Sci 174:351–362. https://doi.org/10.1111/j.1439-037X.1995.tb01122.x
Ashraf M (2009) Biotechnological approach of improving plant salt tolerance using antioxidants as markers. Biotechnol Adv 27:84–93. https://doi.org/10.1016/j.biotechadv.2008.09.003
Augustine SM, Narayan JA, Syamaladevi DP, Appunu C, Chakravarthi M, Ravichandran V, Tuteja N, Subramonian N (2015a) Overexpression of EaDREB2 and pyramiding of EaDREB2 with the pea DNA helicase gene (PDH45) enhance drought and salinity tolerance in sugarcane (Saccharum spp. hybrid). Plant Cell Rep 34:247–263. https://doi.org/10.1007/s00299-014-1704-6
Augustine SM, Narayan JA, Syamaladevi DP, Appunu C, Chakravarthi M, Ravichandran V, Tuteja N, Subramonian N (2015b) Introduction of pea DNA helicase 45 into sugarcane (Saccharum spp. hybrid) enhances cell membrane thermostability and upregulation of stress-responsive genes leads to abiotic stress tolerance. Mol Biotechnol 57:475–488. https://doi.org/10.1007/s12033-015-9841-x
Augustine SM, Narayan AJ, Divya PS, Appunu C, Chakravarthi M, Ravichandran V, Tuteja N, Subramonian N (2015c) Erianthus arundinaceus HSP70 (EaHSP70) overexpression increases drought and salinity tolerance in sugarcane. Plant Sci 232:23–34. https://doi.org/10.1016/j.plantsci.2014.12.012
Babu RC, Zhang J, Blum A, Ho THD, Wu R, Nguyen HT (2004) HVA1, a LEA gene from barley confers dehydration tolerance in transgenic rice (Oryza sativa L.) via cell membrane protection. Plant Sci 166:855–862. https://doi.org/10.1016/j.plantsci.2003.11.023
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207. https://doi.org/10.1007/BF00018060
Bhatnagar-Mathur P, Vadez V, Sharma KK (2008) Transgenic approaches for abiotic stress tolerance in plants: retrospect and prospects. Plant Cell Rep 27:411–424. https://doi.org/10.1007/s00299-007-0474-9
Bhowal B, Singla-Pareek SL, Sopory SK, Kaur C (2020) From methylglyoxal to pyruvate: a genome-wide study for the identification of glyoxalases and d-lactate dehydrogenases in Sorghum bicolor. BMC Genomics 21:145. https://doi.org/10.1186/s12864-020-6547-7
Boaretto LF, Carvalho G, Borgo L, Creste S, Landell MG, Mazzafera P, Azevedo RA (2014) Water stress reveals differential antioxidant responses of tolerant and non-tolerant sugarcane genotypes. Plant Physiol Biochem 74:165–175. https://doi.org/10.1016/j.plaphy.2013.11.016
Buysse JAN, Merckx R (1993) An improved colorimetric method to quantify sugar content of plant tissue. J Exp Bot 44:1627–1629. https://doi.org/10.1093/jxb/44.10.1627
Castillo FJ, Celardin F, Greppin H (1984) Peroxidase assay in plants: Interference by ascorbic acid and endogenous inhibitors in sedum and pelargonium enzyme extracts. Plant Growth Regul 2:69–75. https://doi.org/10.1007/BF00024855
Chaitanya KV, Jutur PP, Sundar D, Reddy AR (2003) Water stress effects on photosynthesis in different mulberry cultivars. Plant Growth Regul 40:75–80. https://doi.org/10.1023/A:1023064328384
Chaplen FW (1998) Incidence and potential implications of the toxic metabolite methylglyoxal in cell culture: a review. Cytotechnology 26:173–183. https://doi.org/10.1023/A:1007953628840
Chaves MM, Maroco JP, Pereira JS (2003) Understanding plant responses to drought-from genes to the whole plant. Funct Plant Biol 30:239–264. https://doi.org/10.1071/FP02076
Cheavegatti-Gianotto A, de Abreu HMC, Arruda P, Bespalhok Filho JC, Burnquist WL, Creste S, di Ciero L, Ferro JA, de Oliveira Figueira AV, de Sousa FT, de Fátima Grossi-de-Sá M (2011) Sugarcane (Saccharum officinarum): a reference study for the regulation of genetically modified cultivars in Brazil. Tropi Plant Biol 4:62–89. https://doi.org/10.1007/s12042-011-9068-3
Cram WJ (1976) Negative feedback regulation of transport in cells. The maintenance of turgor, volume and nutrient supply. Transp Plants II 2:284–316. https://doi.org/10.1007/978-3-642-66227-0_11
da Cruz Maciel JR, de Oliveira D, Fadin DA, das Graças Sajo M, Pedroso-de-Moraes C (2015) Morpho-anatomical characteristics conferring drought tolerance in roots of sugar cane genotypes (Saccharum L., Poaceae). Braz J Bot 38:951–960. https://doi.org/10.1007/s40415-015-0191-5
Deswal R, Chakaravarty TN, Sopory SK (1993) The glyoxalase system in higher plants: regulation in growth and differentiation. Biochem Soc Trans 21:527–530
Dharshini S, Hoang NV, Mahadevaiah C, Padmanabhan TS, Alagarasan G, Suresha GS, Kumar R, Meena MR, Ram B, Appunu C (2020a) Root transcriptome analysis of Saccharum spontaneum uncovers key genes and pathways in response to low-temperature stress. Environ Exp Bot 171:103935. https://doi.org/10.1016/j.envexpbot.2019.103935
Dharshini S, Manoj VM, Suresha GS, Ashwin Narayan J, Sarath Padmanabhan TS, Kumar R, Meena MR, Manickavasagam M, Ram B, Appunu C (2020b) Isolation and characterization of nuclear localized abiotic stress responsive cold regulated gene 413 (SsCor413) from Saccharum spontaneum. Plant Mol Biol Repor (Appeared online). https://doi.org/10.1007/s11105-020-01224-z
Dhindsa RS, Plumb-Dhindsa P, Thorpe TA (1981) Leaf senescence: correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J Exp Bot 32:93–101. https://doi.org/10.1093/jxb/32.1.93
Doyle JJ, Doyle JL (1987) A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem Bull 19(1):11–15
Franklin G, Arvinth S, Sheeba CJ, Kanchana M, Subramonian N (2006) Auxin pretreatment promotes regeneration of sugarcane (Saccharum spp. hybrids) midrib segment explants. Plant Growth Regul 50:111–119. https://doi.org/10.1007/s10725-006-9108-4
Gandonou CB, Bada F, Abrini J, Skali-Senhaji N (2011) Free proline, soluble sugars and soluble proteins concentration as affected by salt stress in two sugarcane (Saccharum sp.) cultivars differing in their salt tolerance. Int J Biol Chem Sci 5:2441–2453. https://doi.org/10.4314/ijbcs.v5i6.23
Ghosh A, Kushwaha HR, Hasan MR, Pareek A, Sopory SK, Singla-Pareek SL (2016) Presence of unique glyoxalase III proteins in plants indicates the existence of shorter route for methylglyoxal detoxification. Sci Rep 6:1–15. https://doi.org/10.1038/srep18358
Gill PK, Sharma AD, Singh P, Bhullar SS (2002) Osmotic stress-induced changes in germination, growth and soluble sugar content of Sorghum bicolor (L.) moench seeds. Bulg J Plant Physiol 28:12–25
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930. https://doi.org/10.1016/j.plaphy.2010.08.016
Gosal SS, Wani SH, Kang MS (2009) Biotechnology and drought tolerance. J Crop Improv 23:19–54. https://doi.org/10.1080/15427520802418251
Govind G, Thamme Gowda HV, Kalaiarasi PJ, Ramchandra Iyer D, Muthappa SK, Nese S, Makarla UK (2009) Identification and functional validation of a unique set of drought induced genes preferentially expressed in response to gradual water stress in peanut. Mol Genet Genomics 281:591–605. https://doi.org/10.1007/s00438-009-0432-z
Gratao PL, Polle A, Lea PJ, Azevedo RA (2005) Making the life of heavy metal-stressed plants a little easier. Funct Plant Biol 32:481–494. https://doi.org/10.1071/FP05016
Hayat S, Hayat Q, Alyemeni MN, Wani AS, Pichtel J, Ahmad A (2012) Role of proline under changing environments: a review. Plant Signal Behav 7:1456–1466. https://doi.org/10.4161/psb.21949
Heuer B (1994) Osmoregulatory role of proline in water-and salt-stressed plants. Handbook of plant and crop stress 19:363–481. Marcel Dekker, New York.
Jackson RB, Sperry JS, Dawson TE (2000) Root water uptake and transport: using physiological processes in global predictions. Trends Plant Sci 5:482–488. https://doi.org/10.1016/S1360-1385(00)01766-0
Jagtap V, Bhargava S, Streb P, Feierabend J (1998) Comparative effect of water, heat and light stresses on photosynthetic reactions in Sorghum bicolor (L.) Moench. J Exp Bot 49:1715–1721. https://doi.org/10.1093/jxb/49.327.1715
Kaur C, Singla-Pareek SL, Sopory SK (2014) Glyoxalase and methylglyoxal as biomarkers for plant stress tolerance. Crit Rev Plant Sci 33:429–456. https://doi.org/10.1080/07352689.2014.904147
Kim YH, Kim CY, Song WK, Park DS, Kwon SY, Lee HS, Bang JW, Kwak SS (2008) Overexpression of sweetpotato swpa4 peroxidase results in increased hydrogen peroxide production and enhances stress tolerance in tobacco. Planta 227:867–881. https://doi.org/10.1007/s00425-007-0663-3
Kurkdjian A, Guern J (1989) Intracellular pH: measurement and importance in cell activity. Annu Rev Plant Biol 40:271–303
Kyparissis A, Petropoulou Y, Manetas Y (1995) Summer survival of leaves in a soft-leaved shrub (Phlomis fruticosa L., Labiatae) under Mediterranean field conditions: avoidance of photoinhibitory damage through decreased chlorophyll contents. J Exp Bot 46:1825–1831. https://doi.org/10.1093/jxb/46.12.1825
Lee J, Giordano S, Zhang J (2012) Autophagy, mitochondria and oxidative stress: cross-talk and redox signalling. Biochem J 441:523–540. https://doi.org/10.1042/BJ20111451
Li T, Cheng X, Wang Y, Yin X, Li Z, Liu R, Liu G, Wang Y, Xu Y (2019) Genome-wide analysis of glyoxalase-like gene families in grape (Vitis vinifera L.) and their expression profiling in response to downy mildew infection. BMC Genomics 20:362. https://doi.org/10.1186/s12864-019-5733-y
Lorenzen CJ (1967) Determination of chlorophyll and pheo-pigments: spectrophotometric equations 1. Limnol Oceanogr 12:343–346. https://doi.org/10.4319/lo.1967.12.2.0343
Mackova H, Hronková M, Dobrá J, Turečková V, Novák O, Lubovská Z, Motyka V, Haisel D, Hájek T, Prášil IT, Gaudinová A (2013) Enhanced drought and heat stress tolerance of tobacco plants with ectopically enhanced cytokinin oxidase/dehydrogenase gene expression. J Exp Bot 64:2805–2815. https://doi.org/10.1093/jxb/ert131
Maggio A, Hasegawa PM, Bressan RA, Consiglio MF, Joly RJ (2001) Unravelling the functional relationship between root anatomy and stress tolerance. Funct Plant Biol 28:999–1004. https://doi.org/10.1071/PP01099
Manoj VM, Anunanthini P, Swathik PC, Dharshini S, Narayan JA, Manickavasagam M, Sathishkumar R, Suresha GS, Hemaprabha G, Ram B, Appunu C (2019) Comparative analysis of glyoxalase pathway genes in Erianthus arundinaceus and commercial sugarcane hybrid under salinity and drought conditions. BMC Genomics 19:986. https://doi.org/10.1186/s12864-018-5349-7
Martineau JR, Specht JE, Williams JH, Sullivan CY (1979) Temperature tolerance in soybeans. I. Evaluation of a technique for assessing cellular membrane thermostability 1. Crop Sci 19:75–78. https://doi.org/10.2135/cropsci1979.0011183X001900010017x
Martinez V, Mestre TC, Rubio F, Girones-Vilaplana A, Moreno DA, Mittler R, Rivero RM (2016) Accumulation of flavonols over hydroxycinnamic acids favors oxidative damage protection under abiotic stress. Front Plant Sci 7:838. https://doi.org/10.3389/fpls.2016.00838
May LH, Milthorpe FL (1962) Drought resistance of crop plants. Field Crop Abstracts 15:171–179
Molinari HBC, Marur CJ, Daros E, De Campos MKF, De Carvalho JFRP, Filho JCB, Pereira LFP, Vieira LGE (2007) Evaluation of the stress-inducible production of proline in transgenic sugarcane (Saccharum spp.): osmotic adjustment, chlorophyll fluorescence and oxidative stress. Physiol Plant 130:218–229. https://doi.org/10.1111/j.1399-3054.2007.00909.x
Monk LS, Fagerstedt KV, Crawford RM (1989) Oxygen toxicity and superoxide dismutase as an antioxidant in physiological stress. Physiol Plant 76:456–459. https://doi.org/10.1111/j.1399-3054.1989.tb06219.x
Mustafiz A, Ghosh A, Tripathi AK, Kaur C, Ganguly AK, Bhavesh NS, Tripathi JK, Pareek A, Sopory SK, Singla-Pareek SL (2014) A unique Ni2+-dependent and methylglyoxal-inducible rice glyoxalase I possesses a single active site and functions in abiotic stress response. Plant J 78:951–963. https://doi.org/10.1111/tpj.12521
Narayan JA, Dharshini S, Manoj VM, Padmanabhan TS, Kadirvelu K, Suresha GS, Subramonian N, Ram B, Premachandran MN, Appunu C (2019) Isolation and characterization of water-deficit stress-responsive α-expansin 1 (EXPA1) gene from Saccharum complex. 3 Biotech 9:186. https://doi.org/10.1007/s13205-019-1719-3
Noctor G, Gomez L, Vanacker H, Foyer CH (2002) Interactions between biosynthesis, compartmentation and transport in the control of glutathione homeostasis and signalling. J Exp Bot 53:1283–1304. https://doi.org/10.1093/jexbot/53.372.1283
Pereira FJ, Castro EMD, Souza TCD, Magalhães PC (2008) Evolution of the root anatomy of ‘Saracura’ corn in successive selection cycles. Braz Agric Res 43:1649–1656. https://doi.org/10.1590/S0100-204X2008001200002
Peter SC, Murugan N, Mohanan MV, Sasikumar SPT, Selvarajan D, Jayanarayanan AN, Shivalingamurthy SG, Chennappa M, Ramanathan V, Govindakurup H, Ram B (2020) Isolation, characterization and expression analysis of stress responsive plant nuclear transcriptional factor subunit (NF-YB2) from commercial Saccharum hybrid and wild relative Erianthus arundinaceus. 3 Biotech 10:1–14. https://doi.org/10.1007/s13205-020-02295-1
Philip A, Syamaladevi DP, Chakravarthi M, Gopinath K, Subramonian N (2013) 5′ regulatory region of ubiquitin 2 gene from Porteresia coarctata makes efficient promoters for transgene expression in monocots and dicots. Plant Cell Rep 32:1199–1210. https://doi.org/10.1007/s00299-013-1416-3
Prashanth SR, Sadhasivam V, Parida A (2008) Over expression of cytosolic copper/zinc superoxide dismutase from a mangrove plant Avicennia marina in indica rice var Pusa Basmati-1 confers abiotic stress tolerance. Transgenic Res 17:281–291. https://doi.org/10.1007/s11248-007-9099-6
Ramaswamy O, Pal S, Guha-Mukherjee S, Sopory SK (1984) Correlation of glyoxalase I activity with cell proliferation in Datura callus culture. Plant Cell Rep 3:121–124. https://doi.org/10.1007/BF02441015
Reddy AR, Chaitanya KV, Vivekanandan M (2004) Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. J Plant Physiol 161:1189–1202. https://doi.org/10.1016/j.jplph.2004.01.013
Roy SD, Saxena M, Bhomkar PS, Pooggin M, Hohn T, Bhalla-Sarin N (2008) Generation of marker free salt tolerant transgenic plants of Arabidopsis thaliana using the gly I gene and cre gene under inducible promoters. Plant Cell Tissue Organ Cult 95:1–11. https://doi.org/10.1007/s11240-008-9402-0
Sarath PTS, Dharshini S, Manoj VM, Ashwin NJ, Appunu C, Hemaprabha G (2018) Isolation, characterization and expression analysis of Hordeum vulgare 22 (HVA 22) gene from a drought tolerant sugarcane variety. J Sugarcane Res 8:114–126
Sethi U, Basu A, Guha-Mukherjee S (1988) Control of cell proliferation and differentiation by regulating polyamine biosynthesis in cultures of Brassica and its correlation with glyoxalase-I activity. Plant Sci 56:167–175. https://doi.org/10.1016/0168-9452(88)90031-3
Singla-Pareek SL, Reddy MK, Sopory SK (2003) Genetic engineering of the glyoxalase pathway in tobacco leads to enhanced salinity tolerance. Proc Natl Acad Sci 100:14672–14677. https://doi.org/10.1073/pnas.2034667100
Singla-Pareek SL, Yadav SK, Pareek A, Reddy MK, Sopory SK (2006) Transgenic tobacco overexpressing glyoxalase pathway enzymes grow and set viable seeds in zinc-spiked soils. Plant Physiol 140:613–623. https://doi.org/10.1104/pp.105.073734
Singla-Pareek SL, Yadav SK, Pareek A, Reddy MK, Sopory SK (2008) Enhancing salt tolerance in a crop plant by overexpression of glyoxalase II. Transgenic Res 17:171–180. https://doi.org/10.1007/s11248-007-9082-2
Subedi KP, Choi D, Kim I, Min B, Park C (2011) Hsp31 of Escherichia coli K-12 is glyoxalase III. Mol Microbiol 81:926–936. https://doi.org/10.1111/j.1365-2958.2011.07736.x
Sun B, Liu GL, Phan TT, Yang LT, Li YR, Xing YX (2017) Effects of cold stress on root growth and physiological metabolisms in seedlings of different sugarcane varieties. Sugar Tech 19:165–175. https://doi.org/10.1007/s12355-016-0452-z
Van Rensburg L, Krüger GHJ (1994) Evaluation of components of oxidative stress metabolism for use in selection of drought tolerant cultivars of Nicotiana tabacum L. J Plant Physiol 143:730–737. https://doi.org/10.1016/S0176-1617(11)81166-1
Vasellati V, Oesterheld M, Medan D, Loreti J (2001) Effects of flooding and drought on the anatomy of Paspalum dilatatum. Ann Bot 88:355–360. https://doi.org/10.1006/anbo.2001.1469
Veena Reddy VS, Sopory SK (1999) Glyoxalase I from Brassica juncea : molecular cloning, regulation and its over-expression confer tolerance in transgenic tobacco under stress. Plant J 17:385–395. https://doi.org/10.1046/j.1365-313X.1999.00390.x
Viveros MFÁ, Inostroza-Blancheteau C, Timmermann T, González M, Arce-Johnson P (2013) Overexpression of GlyI and GlyII genes in transgenic tomato (Solanum lycopersicum Mill.) plants confers salt tolerance by decreasing oxidative stress. Mol Biol Rep 40:3281–3290. https://doi.org/10.1007/s11033-012-2403-4
Watanabe S, Kojima K, Ide Y, Sasaki S (2000) Effects of saline and osmotic stress on proline and sugar accumulation in Populus euphratica in vitro. Plant Cell Tissue Organ Cult 63:199. https://doi.org/10.1023/A:1010619503680
Yadav SK, Singla-Pareek SL, Reddy MK, Sopory SK (2005) Transgenic tobacco plants overexpressing glyoxalase enzymes resist an increase in methylglyoxal and maintain higher reduced glutathione levels under salinity stress. FEBS Lett 579:6265–6271. https://doi.org/10.1016/j.febslet.2005.10.006
Zhang SZ, Yang BP, Feng CL, Chen RK, Luo JP, Cai WW, Liu FH (2006) Expression of the Grifola frondosa trehalose synthase gene and improvement of drought-tolerance in sugarcane (Saccharum officinarum L.). J Integr Plant Biol 48:453–459. https://doi.org/10.1111/j.1744-7909.2006.00246.x
Zhao Q, Su Y, Wang Z, Chen C, Wu T, Huang Y (2014) Identification of glutathione (GSH)-independent glyoxalase III from Schizosaccharomyces pombe. BMC Evol Biol 14:86. https://doi.org/10.1186/1471-2148-14-86
Zhu JK (2002) Salt and drought stress signal transduction in plants. Ann Rev Plant Biol 53:247–273. https://doi.org/10.1146/annurev.arplant.53.091401.143329
Acknowledgements
We gratefully acknowledge The Director, ICAR-Sugarcane Breeding Institute, Coimbatore, Tamil Nadu, India for providing the infrastructure and facilities. ICAR-Sugarcane Breeding Institute is affiliated to Bharathidasan University, Tiruchirappalli, Tamil Nadu for pursuing the doctoral programme. One of the authors, Manoj V M, thanks Council of Scientific and Industrial Research (CSIR), New Delhi, India, for the award of Senior Research Fellowship (SRF; CSIR-Direct SRF) grant (09/706/0004/2019-EMR-I). This work was supported by the Department of Biotechnology (DBT) (Grant no. F. No. BT/PR12949/AGII/106/986/2015), Government of India, New Delhi. The authors would also thank Mr. K. Selvamuthu for his assistance during the course of research for the maintenance of plants.
Author information
Authors and Affiliations
Contributions
MVM and AC designed the work plan. MVM carried out major works and wrote the manuscript. AP helped in isolating the gene of interest. SPTS carried out physiology experiments. DS helped in physiology and root anatomy studies. ANJ helped in some of the physiological experiments and interpretation of results. AKR, SNK and RS helped in analyzing the photosynthetic parameters and interpretation of data. AC conceptualized the work design and evaluated all the data interpretations. AC, SR and BR reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Ethics approval
Not applicable. This is to confirm that no specific permits were needed for the described experiments, and this study did not involve any endangered or protected species.
Availability of data and material
All data generated or analyzed during this study are included in this article (and its additional files). The gene sequence was deposited in NCBI database under the accession number MG701311. Materials are available with corresponding author.
Additional information
Communicated by Neal Stewart.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mohanan, M.V., Pushpanathan, A., Sasikumar, S.P.T. et al. Ectopic expression of DJ-1/PfpI domain containing Erianthus arundinaceus Glyoxalase III (EaGly III) enhances drought tolerance in sugarcane. Plant Cell Rep 39, 1581–1594 (2020). https://doi.org/10.1007/s00299-020-02585-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-020-02585-1