Abstract
Dendrobium is a large genus in the family Orchidaceae that exhibits vast diversity in floral characteristics, which is of considerable importance to orchid breeders, biotechnologists and collectors. Native species have high value as a result of their medicinal properties, while their hybrids are important as ornamental commodities, either as cut flowers or potted plants and are thus veritable industrial crops. Thus, preservation of Dendrobium germplasm is valuable for species conservation, breeding programs and the floriculture industry. Cryopreservation represents the only safe, efficient and cost-effective long-term storage option to facilitate the conservation of genetic resources of plant species. This review highlights 16 years of literature related to the preservation of Dendrobium germplasm and comprises the most comprehensive assessment of thorough studies performed to date, which shows reliable and reproducible results. Air-drying, encapsulation–dehydration, encapsulation–vitrification, vitrification and droplet-vitrification are the current cryopreservation methodologies that have been used to cryopreserve Dendrobium germplasm. Mature seeds, pollen, protoplasts, shoot primordia, protocorms and somatic embryos or protocorm-like bodies (PLBs) have been cryopreserved with different levels of success. Encapsulation–vitrification and encapsulation–dehydration are the most used protocol, while PLBs represent the main explant explored.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Orchid cultivation is one of the most economically significant global nursery industries, constituting a multi-billion dollar market (Teixeira da Silva 2013a). Dendrobium is a large orchid genus represented by over 1,100 species distributed from India and Sri Lanka then eastward to Japan and southward through the Philippines, Malaysia, Indonesia, and New Guinea to Australia and through the Pacific Islands to New Zealand (Pridgeon and Morrison 2006; Xu et al. 2006). The name is derived from ‘Dendron’ which means tree and ‘bios’ means ‘life’ that is an epiphytic plant that exists by clinging to the branches and trunks of host trees (Pradhan et al. 2013). This genus represents one of the most important orchids either as pot plants or as cut flowers. Several Dendrobium hybrids are very important to the orchid cut-flower industry in a number of countries. As a result, the production of Dendrobium hybrids plants has spiked, with large-scale production occurring in many countries like the Netherlands, Germany, China, Taiwan, Thailand, Philippines, Unites States, and Japan (Puchooa 2004). In general, orchids rank second in potted flowering plants in the United States, with a wholesale value of US$ 200 million for the year 2011 (US Department of Agriculture 2012). However, the USDA Floriculture report of 2013 indicated an 11 % increase in the value of potted orchids in 2012 from the previous year, while all other crops in this category had their values reduced (US Department of Agriculture 2013). Among the main orchid genera produced in the US, Dendrobium ranks second as potted orchids, behind Phalaenopsis. The State of Hawaii is the main producer of Dendrobium in the US with a total sales value of US$ 5.25 million for 2011 (U.S. Department of Agriculture 2011, 2012).
In addition to their ornamental value, some native Dendrobium species have medicinal (Hou et al. 2012; Ng et al. 2012) and ethnopharmacological importance in many Asian countries. Flower extracts of the epiphytic species D. nobile possessed antimicrobial and antitumor properties in addition to antiperoxidative activity (Devi et al. 2009), and extracted polysaccharide fractions from its stem could be considered as effective natural antitumor agents (Wang et al. 2010). Several members of the Dendrobium genus have alkaloids, amino acids, trace elements, while others contain active ingredients that can enhance immunity, promote the secretion of digestive juice, inhibit platelet aggregation, lower blood pressure or hypoglycemia, or display a range of pharmacological effects such as antioxidant, anti-aging, antipyretic and analgesic (Ng et al. 2012).
There are several in vitro preservation methods available for the preservation of Dendrobium germplasm. The choice of method, however, usually depends on the storage duration required. For short-term and medium-term storage, the aim is to reduce growth and to increase the intervals between subcultures. For long-term storage, cryopreservation is the only current method available to achieve this goal (Engelmann 2011). In vitro slow growth storage techniques are routinely used for medium-term conservation of numerous species and growth reduction is generally achieved by modifying the environmental conditions and the culture medium (Kulus and Zalewska 2014). Changes in environment conditions include a reduction in temperature, while modifications of the culture medium can include dilution of mineral elements, reduction of sugar concentration, changes in the nature and/or concentration of plant growth regulators (for example, the use of sub-lethal levels of growth retardants) and addition of osmotically active compounds (Negash et al. 2001).
Conservation of Dendrobium germplasm
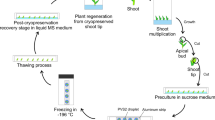
The reduction in wild orchid stocks by orchid collectors, over-exploitation for medicinal purposes, deforestation and destruction of habitats for urbanization, and unauthorized trade have led to a reduction in natural populations of many orchids (Swarts and Dixon 2009) leading many orchid species to become extinct and a large number of species to become rare or endangered (Yam et al. 2010). The entire Orchidaceae is included in the CITES (Convention on International Trade in Endangered Species) Appendix II (Shefferson et al. 2005). There are a number of techniques devised to conserve orchids: ex vitro, such as botanic garden collections or ex situ germplasm banks (Hou et al. 2012; Merritt et al. 2014), as well as in vitro, including seed bank development, slow growth conservation and cryopreservation of seed, meristem, tissue-cultured shoot primordia, somatic embryos, and pollen (Table 1). In Dendrobium, immature seeds have not yet been used as an explant for cryopreservation, although they have been used for Bletilla striata (Hirano et al. 2005a), Ponerorchis graminifolia (Hirano et al. 2005b), Dactylorhiza fuchsii (Nikishina et al. 2007), and Cyrtopodium hatschbachii (Surenciski et al. 2007). Cryopreservation, which can ensure the safe and cost-efficient long-term conservation of Dendrobium germplasm, has a wide applicability for orchids both from temperate and tropical origin in which seeds, shoot meristems, protocorms, somatic embryos (i.e., protocorm-like bodies, or PLBs) and cultured cells can be preserved in liquid nitrogen (LN; −196 °C) to create cryobanks for conserving rare and endangered species (Hirano et al. 2006; Hossain et al. 2013). Until 2006, three cryopreservation techniques had been applied to orchid species, namely desiccation (air-drying), vitrification and encapsulation–dehydration (Hirano et al. 2006). However, in dehydration, since naked PLBs that are very sensitive to dehydration are first dehydrated, and since vitrification uses chemicals that can be toxic (Teixeira da Silva 2013b), this list was broadened to include five methods: air-drying, encapsulation–dehydration, encapsulation–vitrification, vitrification and droplet-vitrification. Encapsulation–dehydration method may be the most suitable as it results in a high survival frequency after cryogenic storage. Table 1 indicates that from a total of 37 documented studies in the literature, 12 used encapsulation–vitrification while 9 used encapsulation–dehydration, 7 used vitrification alone, 3 reported air-drying methods and low temperature, and a single study used in vitro methods. The encapsulation of explants in alginate beads for cryopreservation has some benefits as compared to the use of non-encapsulated samples. The alginate beads provide enhanced protection of dried materials from mechanical and oxidative stress during storage and ease of handling of small samples during pre- and post-cryopreservation.
Many studies examined the cryopreservation of Dendrobium PLBs including D. ‘Walter Oumae’ (Lurswijidjarus and Thammasiri 2004) and D. candidum (Chen 2000; Chen et al. 2001; Bian et al. 2002; Lin et al. 2004; Yin and Hong 2009). Seeds of D. candidum (Wang et al. 1998, 1999), D. crystallinum and D. virgineum (Huehne and Bhinja 2012) were also successfully cryopreserved. Vendrame and Faria (2011) and Galdiano et al. (2012) showed that the cryopreservation of mature seeds was possible with vitrification, while droplet-vitrification was effective for protocorms, with phloroglucinol being an important aspect of the vitrification solution. Phloroglucinol has many biological effects in vitro and has shown to enhance a wide range of organogenic processes in plants (Teixeira da Silva et al. 2013). Orchid seeds are morphophysiologically different from other plants, to some extent limiting the application of cryopreservation and thus modification and proper adjustment of the techniques are required. Cryopreservation of vegetative tissue involves several stages, specifically the establishment of in vitro cultures, conditioning of these tissues, addition of an appropriate cryoprotectant, exposure of cultures to ultra-low temperature, re-warming and regeneration of plant cells and tissues (Kulus and Zalewska 2014). Each stage, which needs to be optimized, plays an important role in determining the survival of tissue upon re-warming.
Some orchid cryopreservation papers applied deep-freezing to seeds with a moisture content of 20 % or less (for vitrification protocols), while other studies dealt with direct freezing of orchid seeds. Seed moisture is a key factor because the presence of unbound water in seeds considerably reduces their germinability causing the embryo tissues to perish because of the formation of ice crystals in their cells during freezing in LN (Sakai et al. 1991; Sakai and Engelmann 2007). In the cryopreservation of terrestrial and epiphytic orchids with a moisture level below 11 %, seed germinability did not change after cryoconservation for most species examined (Pritchard 1984; Pritchard et al. 1999). In seeds of D. candidum, a high survival rate of 95 % was also obtained when desiccated seeds with 8–19 % water content with silica gel were directly plunged into LN (Wang et al. 1998). However, mature seeds of Dendrobium commercial hybrids with comparable seed moisture (9–18 %) did not survive after direct freezing (Vendrame et al. 2007; Galdiano et al. 2012, 2014), and the desirable dehydration with plant vitrification solution #2, PVS2 [30 % (w/v) glycerol, 15 % (w/v) ethylene glycol, 15 % (w/v) dimethyl sulfoxide (DMSO) and 0.4 M sucrose; Sakai et al. (1990)] for a period of time between 1–3 h prior to cryopreservation was essential to allow proper germination of cryopreserved seeds.
Conservation through storage should be supported by an appropriate explant viability test, since the success of any cryopreservant-based protocol is determined by the recovery of viable propagules. Viability tests such as the triphenyl tetrazolium chloride (TTC) reduction assay (Singh 1981) and the fluorescein diacetate (FDA) staining technique (Pritchard 1985) have been largely explored for seeds, protocorms and PLBs. The TTC assay, qualitative for large tissues and organs, is often used for orchid seeds and embryos because TTC reduction assay and regrowth observations used in the assessment of seedlings growth or plantlets survival were correlated (Lurswijidjarus and Thammasiri 2004; Vendrame et al. 2008; Tiau et al. 2009; Antony et al. 2010, 2011b; Subramaniam et al. 2011; Ching et al. 2012; Galdiano et al. 2012, 2014; Poobathy et al. 2013a). Dehydrogenases, through respiration in mitochondria, reduce colorless TTC to red triphenylformazan or reduced TTC (Verleysen et al. 2004). Figure 1 shows the assessment of survival using the TTC test in mature seeds and 45-day-old protocorms of Dendrobium hybrid ‘Uniwai Royale’ after cryopreservation by vitrification (unpublished data).
Stained seeds and protocorms of Dendrobium hybrid ‘Uniwai Royale’ with the 2,3,5-triphenyl tetrazolium chloride (TTC) reduction assay. Viable explants show insoluble scarlet formazan because the dehydrogenase enzyme in living tissues reduces soluble colorless TTC to insoluble scarlet formazan. a Mature seeds after cryopreservation by vitrification. b 45-days-old protocorms directly cryopreserved in liquid nitrogen (left Eppendorf tube) or PVS2-treated protocorms (right Eppendorf tube). ds dead seeds, ls living seeds, us unviable seed. Bars a: 1 mm, b: 1 cm. (Unpublished photos: Renato Fernandes Galdiano Jr.)
Cryopreservation of seeds, pollen, protocorms and PLBs of many Dendrobium orchids has been successfully attempted for long-term conservation. Vitrification and air-drying methods have resulted in both low and slow rates of regrowth of plantlets of D. candidum (Bian et al. 2002). Therefore, the cryopreservation protocol widely used in the literature is encapsulation–vitrification, followed by encapsulation–dehydration (Table 1). The encapsulation–vitrification method has been developed for apices of numerous species from tropical origin and involves the incubation of explants in a Na-alginate solution (2–5 %) and their subsequent release (immersed in a drop of alginate) into a complexation agent (50–100 mM CaCl2 solution), where bead hardening occurs within 20–30 min. Encapsulated explants are then precultured in liquid medium with a high sucrose concentration and partially desiccated before exposure to LN (Sakai et al. 2008). In encapsulation–dehydration, encapsulation protects explants against extreme treatments such as high sucrose concentrations, which allow the removal of most freezable water from the cells and thereby allowing internal solutes to enter a vitrified state when plunged into LN and subsequently preventing the formation of lethal intracellular ice (Engelmann et al. 2008).
PLBs have been the main Dendrobium explant explored for the (cryo)preservation of Dendrobium germplasm (Table 1). They are clonal in nature (i.e., they are genetically identical), they have the potential to regenerate plants with an independent root and shoot system (hence their consideration as somatic embryos in the Orchidaceae), and represent thus a suitable and reliable source of material for germplasm conservation. PLBs behave as embryogenic cultures (similar to what was found in hybrid Cymbidium; Teixeira da Silva and Tanaka 2006) and are extremely susceptible to cryoinjuries due to the high water content within the cells (Renato Fernandes Galdiano Jr., personal observation). Consequently, additional steps such as extra preculture treatment (Antony et al. 2011b) or dehydration are required prior to preserving in LN (Yin and Hong 2009; Mohanty et al. 2012). During dehydration and freezing in LN, proteins and membranes are protected when soluble sugars such as sucrose accumulate in the cytoplasm (Sakai and Engelmann 2007; Yin and Hong 2009). Using protocorms as explants, vitrification and droplet-vitrification were explored by Vendrame and Faria (2011) and Galdiano et al. (2012) for the cryopreservation of Dendrobium germplasm. Droplet-vitrification provided limited effectiveness recovery and was influenced by sucrose pretreatment. For seeds, only vitrification has been used for an endangered species D. chrysanthum (He et al. 2010), as well as commercial hybrids D. ‘Sena Red’, D. ‘Mini WRL’, D. ‘Jaquelyn Thomas’, D. ‘BFC Pink’ and D. ‘Dong Yai’ (Vendrame et al. 2007; Galdiano et al. 2012, 2014).
In short-term conservation and cryopreservation, there may be some alterations to the genome, thus the determination of genetic integrity is necessary after (cryo)storage. Those processes can cause dehydration-, osmotic pressure- or freezing-related stresses that may result in genetic instability (Hazubska-Przbyl et al. 2010). Genetic instability and somaclonal variations may cause some differences in genotype and phenotype profiles of cryopreserved plants (Harding 2004). Some papers reported the use of randomly amplified polymorphic DNA (RAPD) for evaluating the genetic stability of Dendrobium PLBs after storage (Antony et al. 2012). Mohanty and Das (2013) had also reported one such protocol, but that study was subsequently retracted due to figure manipulation, casting doubts on the veracity, reliability or reproducibility of the results. RAPD results from selected primers indicated that the genetic stability of PLBs following storage (short-term and long-term) was maintained. However, molecular markers sometimes failed to detect somaclonal variations in ornamental plants, including in orchids (Park et al. 2009). Phenotypic characteristics of plantlets regenerated from D. candidum PLBs (Yin and Hong 2009) and of greenhouse-acclimatized seedlings associated with flow cytometry (FCM) for cryopreserved Dendrobium hybrid ‘Dong Yai’ seeds (Galdiano et al. 2014) were also used to assess genetic stability. In both studies, regenerated plantlets derived from cryopreserved PLBs or seeds were similar to controls (explants not cryopreserved). The discovery and development of new molecular markers to assess diversity are also fundamental for the study of genetic stability and to establish conservation programs for Dendrobium. Ding et al. (2008) analyzed D. officinale with sequence-related amplified polymorphism (SRAP) and obtained 96 polymorphic bands from a total of 109. Hou et al. (2012) developed 15 new and highly polymorphic microsatellite loci for D. officinale to evaluate the genetic diversity of wild and ex situ conserved populations.
There are no studies on the re-introduction of Dendrobium species back into the wild for in situ conservation, nor are there any studies that examine the storage of genetically modified material. These aspects need to be the focus of future projects.
Conclusions and future perspectives
Cryopreservation is a suitable means for preservation of Dendrobium germplasm. It requires minimal storage space and maintenance, while ensuring the stability of phenotypic or genotypic characteristics. Of the total of three cryopreservation techniques that have been applied to Dendrobium hybrids and species conservation and/or preservation, vitrification is the simplest technique because it does not require expensive equipment or laborious steps, and is thus particularly useful for Dendrobium seed and pollen. However, encapsulation–vitrification and encapsulation–dehydration are most likely the most suitable methods explored for this orchid genus because they resulted in a high survival frequency after cryogenic storage of PLBs, the major explant explored. Such techniques have been explored to a great extent and future perspectives may include optimization of existing cryopreservation protocols, as well as innovative approaches to cryopreservation.
Abbreviations
- DMSO:
-
Dimethyl sulfoxide
- FDA:
-
Fluorescein diacetate
- LN:
-
Liquid nitrogen
- PLB:
-
Protocorm-like body
- TTC:
-
Triphenyl tetrazolium chloride
References
Antony JJJ, Keng CL, Rathinam X, Subramaniam S (2010) Preliminary study on cryopreservation of Dendrobium Bobby Messina protocorm-like bodies by vitrification technique. Afr J Biotechnol 9:7063–7070
Antony JJJ, Keng CL, Rathinam X, Marimuthu S, Subramaniam S (2011a) Effect of preculture and PVS2 incubation conditions followed by histological analysis in cryopreserved PLBs of Dendrobium Bobby Messina. Aus J Crop Sci 5:1557–1564
Antony JJJ, Sinniah UR, Keng CL, Pobathy R, Khoddamzadeh AA (2011b) Subramaniam S. Selected potential encapsulation–dehydration parameters on Dendrobium Bobby Messina protocorm-like bodies using TTC analysis. Aus J Crop Sci 5:1817–1822
Antony JJJ, Poobathy R, Danial M, Sinniah UR, Subramaniam S (2012) Polymorphism analysis of cryopreserved Dendrobium Bobby Messina protocorm-like bodies (PLBs) using RAPD markers. Plant Omics J 5:427–431
Antony JJJ, Keng CL, Mahmood M, Subramaniam S (2013) Effects of ascorbic acid on PVS2 cryopreservation of Dendrobium Bobby Messina’s PLBs supported with SEM analysis. Appl Biochem Biotechnol 171:315–329
Antony JJJ, Mubbarakh SA, Mahmood M, Subramaniam S (2014) Effect of plasmolysis on protocorm-like bodies of Dendrobium Bobby Messina orchid following cryopreservation with encapsulation–dehydration method. Appl Biochem Biotechnol 172:1433–1444
Bian H, Wang J, Lin W, Han M, Zhu M (2002) Accumulation of soluble sugars, heat-stable proteins and dehydrins in cryopreservation of protocorm-like bodies of Dendrobium candidum by air drying method. J Plant Physiol 153:1139–1145
Chen Y (2000) Protoplasm of Dendrobium candidum cryopreserved by vitrification. J Wenzhou Teach Coll (Nat Sci) 21:40–41 (in Chinese with English abstract)
Chen Y, Wang JH, Huang CL (2001) Germplasm cryopreservation of Dendrobium candidum by vitrification. J Zhejinag Uni (Agric Life Sci) 27:436–438 (in Chinese with English abstract)
Ching LP, Antony JJJ, Poobathy R, Subramaniam S (2012) Encapsulation–vitrification of Dendrobium sonia-28 supported by histology. Plant Omics J 5:345–350
Das MC, Kumaria S, Tandon P (2008) In vitro propagation and conservation of Dendrobium lituiflorum Lindl. through protocorm-like bodies. J Plant Biochem Biotechnol 17:177–180
Devi PU, Selvi S, Devipriya D, Murugan S, Suja S (2009) Antitumor and antimicrobial activities and inhibition of in vitro lipid peroxidation of Dendrobium nobile. Afr J Biotechnol 8:2289–2293
Ding G, Zhang D, Ding X, Zhou Q, Zhang W, Li X (2008) Genetic variation and conservation of the endangered Chinese endemic herb Dendrobium officinale based on SRAP analysis. Plant Syst Evol 276:149–156
Engelmann F (2011) Use of biotechnology for conservation of plant biodiversity. In Vitro Cell Dev Biol Plant 47:5–16
Engelmann F, Gonzalez-Arnao MT, Wu Y, Escobar R (2008) The development of encapsulation dehydration. In: Reed B (ed) Plant cryopreservation—a practical guide. Springer Science + Business Media LLC, New York, pp 59–76
Galdiano RF Jr, Lemos EGM, Faria RT, Vendrame WA (2012) Cryopreservation of Dendrobium hybrid seeds and protocorms as affected by phloroglucinol and Supercool X1000. Sci Hortic 148:154–160
Galdiano RF Jr, Lemos EGM, Faria RT, Vendrame WA (2014) Seedling development and evaluation of genetic stability of cryopreserved Dendrobium hybrid mature seeds. Appl Biochem Biotechnol 172:2521–2529
Harding K (2004) Genetic integrity of cryopreserved plant cells: a review. Cryo lett 25:3–22
Hazubska-Przbyl T, Chmielarz P, Michalak M, Bojarczuk K (2010) Cryopreservation of embryogenic tissues of Picea omorika (Serbian spruce). Plant Cell Tissue Organ Cult 102:35–44
He MG, Wang RX, Song XQ, Song SQ, Zhang RL (2010) Study on cryopreservation of Dendrobium chrysanthum (Orchidaceae) seeds. Acta Bot Yunnanica 32:334–338 (in Chinese with English abstract)
Hirano T, Godo T, Mii M, Ishikawa K (2005a) Cryopreservation of immature seeds of Bletilla striata by vitrification. Plant Cell Rep 23:534–539
Hirano T, Ishikawa K, Mii M (2005b) Cryopreservation of immature seeds of Ponerorchis graminifolia by vitrification. Cryo Lett 26:139–146
Hirano T, Ishikawa K, Mii M (2006) Advances in orchid cryopreservation. In: Teixeira da Silva JA (ed) Floriculture, ornamental and plant biotechnology: advances and topical issues, vol II Global Science Books, Ltd., Isleworth, UK, pp 410–414
Hossain MM, Kant R, Van PT, Winarto B, Zeng SJ, Teixeira da Silva JA (2013) The application of biotechnology to orchids. Crit Rev Plant Sci 32:69–139
Hou B, Tian M, Luo J, Ji Y, Xue Q, Ding X (2012) Genetic diversity assessment and ex situ conservation strategy of the endangered Dendrobium officinale (Orchidaceae) using new trinucleotide microsatellite markers. Plant Syst Evol 298:1483–1491
Huehne PS, Bhinja K (2012) Application of cryoprotectants to improve low temperature storage survival of orchid seeds. Sci Hortic 135:186–193
Kulus D, Zalewska M (2014) Cryopreservation as a tool used in long-term storage of ornamental species—a review. Sci Hortic 168:88–107
Lin WQ, Bian HW, Wang JH, Zhu MY (2004) Analysis of dehydrins in cryopreservation of protocorm-like-bodies of Dendrobium candidum by the air-drying method. Acta Hor Sin 31:64–68 (in Chinese with English abstract)
Liu XD, Li XD, Wu YL, Shen XH (2013) Physiological and biochemical characteristic of Dendrobium wardianum protocorms during cryopreservation. J Northeast For Uni 41:79–84 (in Chinese with English abstract)
Lurswijidjarus W, Thammasiri K (2004) Cryopreservation of shoot tips of Dendrobium Walter Oumae by encapsulation/dehydration. Sci Asia 30:293–299
Merritt DJ, Hay FR, Swarts ND, Sommerville KD, Dixon KW (2014) Ex situ conservation and cryopreservation of orchid germplasm. Int J Plant Sci 175:46–58
Mohanty P, Das J (2013) Synthetic seed technology for short term conservation of medicinal orchid Dendrobium densiflorum Lindl. Ex Wall and assessment of genetic fidelity of regenerants. Plant Growth Reg 70(297–303):305 (erratum; retracted)
Mohanty P, Das MC, Kumaria S, Tandon P (2012) High-efficiency cryopreservation of the medicinal orchid Dendrobium nobile Lindl. Plant Cell Tissue Organ Cult 109:297–305
Mohanty P, Das MC, Kumaria S, Tandon P (2013a) Cryopreservation of pharmaceutically important orchid Dendrobium chrysanthum Wall. ex Lindl. using vitrification based method. Acta Physiol Plant 35:1373–1379
Mohanty P, Nongkling P, Das MC, Kumaria S, Tandon P (2013b) Short-term storage of alginate-encapsulated protocorm-like bodies of Dendrobium nobile Lindl.: an endangered medicinal orchid from North-east India. 3. Biotech 3:235–239
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:493–497
Negash A, Krens F, Schaart J, Visser B (2001) In vitro conservation of enset under slow-growth conditions. Plant Cell Tissue Organ Cult 66:107–111
Ng TB, Liu JY, Wong JH, Ye XJ, Sze SCW, Tong Y, Zhang KYB (2012) Review of research on Dendrobium, a prized folk medicine. Appl Microbiol Biotechnol 93:1795–1803
Nikishina TV, Popova EV, Vakhrameeva MG, Varlygina TI, Kolomeitseva GL, Burov AV, Popovich EA, Shirokov AI, Shumilov VYu, Popov AS (2007) Cryopreservation of seeds and protocorms of rare temperate orchids. Russ J Plant Physiol 54:121–127
Park SY, Murthy HN, Chakrabarthy D, Paek KY (2009) Detection of epigenetic variation in tissue-culture-derived plants of Doritaenopsis by methylation-sensitive amplification polymorphism (MSAP) analysis. In Vitro Cell Dev Biol Plant 45:104–108
Poobathy R, Izwa N, Julkifle AL, Subramaniam S (2013a) Cryopreservation of Dendrobium sonia-28 using an alternative method of PVS2 droplet freezing. Emir J Food Agric 25:531–538
Poobathy R, Sinniah UR, Mahmood M, Subramaniam S (2013b) Refinement of a vitrification protocol for protocorm-like bodies of Dendrobium sonia-28. Turk J Bot 37:940–949
Poobathy R, Sinniah UR, Xavier R, Subramaniam S (2013c) Catalase and superoxide dismutase activities and the total protein content of protocorm-like bodies of Dendrobium Sonia-28 subjected to vitrification. Appl Biochem Biotechnol 170:1066–1079
Pradhan S, Paudel YP, Pant B (2013) Efficient regeneration of plants from shoot tip explants of Dendrobium densiflorum LindL., a medicinal orchid. Afr J Biotechnol 12:1378–1383
Pridgeon A, Morrison A (2006) The illustrated encyclopedia of orchids–over 1100 species illustrated and identified. Timber Press, Ltd., Portland pp 87–103
Pritchard HW (1984) Liquid nitrogen preservation of terrestrial and epiphytic orchid seed. Cryo Lett 5:295–300
Pritchard HW (1985) Determination of orchid seed viability using fluorescein diacetate. Plant Cell Environ 8:727–730
Pritchard HW, Poyner ALC, Seaton PT (1999) Interspecific variation in orchid seed longevity in relation to ultra-dry storage and cryopreservation. Lindleyana 14:92–101
Puchooa D (2004) Comparison of different culture media for the in vitro culture of Dendrobium (Orchidaceae). Int J Agric Biol 6:884–888
Sakai A, Engelmann F (2007) Vitrification, encapsulation–vitrification and droplet-vitrification: a review. Cryo Lett 28:151–172
Sakai A, Kobayashi S, Oiyama I (1990) Cryopreservation of nucellar cells of navel orange (Citrus sinensis var. brasiliensis Tanaka) by vitrification. Plant Cell Rep 9:30–33
Sakai A, Kobayashi S, Oiyama I (1991) Cryopreservation of nucellar cells of navel orange (Citrus sinensis Osb. var. brasiliensis Tanaka) cooled to −196 °C. J Plant Physiol 137:465–470
Sakai A, Hiraii D, Niino T (2008) Development of PVS-based vitrification and encapsulation–vitrification protocols. In: Reed B (ed) Plant cryopreservation—a practical guide. Springer Science Business Media, LLC, New York, pp 33–58
Shefferson RP, Weiss M, Kull T, Taylor DL (2005) High specificity generally characterizes mycorrhizal association in rare lady’s slipper orchids, genus Cypripedium. Mol Ecol 14:613–626
Shi YZ, Pan RH, Wang XJ, Ye QS, Guo LR (1999) In vitro conservation of germplasm at room temperature in Dendrobium officinale. J South China Normal Uni (Nat Sci) 4:73–77 (in Chinese with English abstract)
Shi YZ, Pan RH, Wang XJ, Ye QS, Guo LR (2000) In vitro conservation of Dendrobium officinale at low temperature. Chin J Appl Environ Biol 6:326–330 (in Chinese with English abstract)
Singh F (1981) Differential staining of orchid seeds for viability testing. Am Orchid Soc Bull 50:416–418
Subramaniam S, Sinniah UR, Khoddamzadeh AA, Periasamy S, James JJ (2011) Fundamental concept of cryopreservation using Dendrobium sonia-17 protocorm-like bodies by encapsulation–dehydration technique. Afr J Biotechnol 10:3902–3907
Surenciski MR, Dematteis M, Flachsland EA (2007) Chromosome stability in cryopreserved germplasm of Cyrtopodium hatschbachii (Orchidaceae). Ann Bot Fennici 44:287–292
Swarts ND, Dixon KW (2009) Terrestrial orchid conservation in the age of extinction. Ann Bot 104:543–556
Teixeira da Silva JA (2012) Is BA (6-benzyladenine) BAP (6-benzylaminopurine)? Asian Australasian J Plant Sci Biotech 6(Special Issue 1):121–124
Teixeira da Silva JA (2013a) Orchids: advances in tissue culture, genetics, phytochemistry and transgenic biotechnology. Floric Ornam Biotech 7:1–52
Teixeira da Silva JA (2013b) Cryopreservation of hybrid Cymbidium protocorm-like bodies by encapsulation–dehydration and vitrification. In Vitro Cell Dev Biol Plant 49:690–698
Teixeira da Silva JA, Tanaka M (2006) Embryogenic callus, PLB and TCL paths to regeneration in hybrid Cymbidium (Orchidaceae). J Plant Growth Reg 25:203–210
Teixeira da Silva JA, Dobránszki J, Ross S (2013) Phloroglucinol in plant tissue culture. In Vitro Cell Dev Biol Plant 49:1–16
Tiau TK, Rathinam X, Keng CL, Subramaniam S (2009) An assessment of early factors influencing the PVS2 vitrification method using protocorm-like bodies of Dendrobium Sonia 28. Am Eurasian J Sustain Agric 3:280–289
US Department of Agriculture (2011) Floriculture crops 2010 summary. National Agricultural Statistics Service. Washington, DC. Online: http://usda.mannlib.cornell.edu/usda/nass/FlorCrop//2010s/2011/FlorCrop-04-21-2011_revision.pdf. Accessed 14 May 2014
US Department of Agriculture (2012) Floriculture crops 2011 summary. National Agricultural Statistics Service. Washington, DC. Online: http://usda.mannlib.cornell.edu/usda/nass/FlorCrop//2010s/2012/FlorCrop-05-31-2012.pdf. Accessed 14 May 2014
US Department of Agriculture (2013) Floriculture crops 2012 summary. National Agricultural Statistics Service. Washington, DC. Online: http://usda.mannlib.cornell.edu/usda/current/FlorCrop/FlorCrop-04-25-2013.pdf. Accessed 14 May 2014
Vacin E, Went FW (1949) Some pH changes in nutrient solutions. Bot Gaz 110:605–613
Vendrame WA, Faria RT (2011) Phloroglucinol enhances recovery and survival of cryopreserved Dendrobium nobile protocorms. Sci Hortic 128:131–135
Vendrame WA, Carvalho VS, Dias JMM (2007) In vitro germination and seedling development of cryopreserved Dendrobium hybrid mature seeds. Sci Hortic 114:188–193
Vendrame WA, Carvalho VS, Dias JMM, Maguire I (2008) Pollination of Dendrobium hybrids using cryopreserved pollen. HortSci 43:264–267
Verleysen H, Samyn G, Van Bockstaele E, Debergh P (2004) Evaluation of analytical techniques to predict viability after cryopreservation. Plant Cell Tissue Organ Cult 77:11–21
Wang JH, Ge J-G, Liu F, Bian H-W, Huang CN (1998) Cryopreservation of seeds and protocorms of Dendrobium candidum. Cryo Lett 19:123–128
Wang JH, Zhang YQ, Liu F, Huang CL, Ge JG (1999) Cryopreservation of seeds, protocorm and protocorm-like bodies of Dendrobium candidum. Acta Hort Sin 26:59–61 (in Chinese with English abstract)
Wang JH, Luo JP, Zha XQ, Feng BJ (2010) Comparison of antitumor activities of different polysaccharide fractions from the stems of Dendrobium nobile Lindl. Carbohydr Polym 79:114–118
Wu YL, Shen XH (2011) Cryopreservation of Dendrobium wardianum Warner protocorms by vitrification. Chin J Cell Bio 33:279–287 (in Chinese with English abstract)
Xu H, Wang ZT, Ding XY, Zhou KY, Xu LS (2006) Differentiation of Dendrobium species used as “Huangcao Shihu” by rDNA ITS sequence analysis. Planta Med 72:89–92
Yam TW, Chua J, Tay F, Ang P (2010) Conservation of the native orchids through seedling culture and reintroduction—a Singapore experience. Bot Rev 76:263–274
Yin MH, Hong SR (2009) Cryopreservation of Dendrobium candidum wall. ex Lindl. protocorm-like bodies by encapsulation vitrification. Plant Cell Tissue Organ Cult 98:179–185
Zainuddin M, Julkifle AL, Pobathy R, Sinniah UR, Antony JJJK, Pavallekoodi, Subramaniam S (2011) Preliminary analysis of cryopreservation of Dendrobium Bobby Messina orchid using an encapsulation–dehydration technique with Evans blue. Afr J Biotechnol 10:11870–11878
Zhang ZG, Liu H, Xia ZJ, Wang JH (1997) Cryopreservation of seeds of Dendrobium candidum. J Anhui Coll 6:40–42 (in Chinese with English abstract)
Zhang M, Wei XY, Huang HR (2001) A study on the solid encapsulating system of the artificial seed of Dendrobium candidum. Acta Hort Sin 28:435–439 (in Chinese with English abstract)
Conflict of interest
The authors declare no conflicts of interest, financial, or other.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Communicated by N. Stewart.
Rights and permissions
About this article
Cite this article
Teixeira da Silva, J.A., Zeng, S., Galdiano, R.F. et al. In vitro conservation of Dendrobium germplasm. Plant Cell Rep 33, 1413–1423 (2014). https://doi.org/10.1007/s00299-014-1631-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-014-1631-6