Abstract
Key message
We report for the first time that expression of potato PR10a gene in faba bean causes enhanced tolerance to drought and salinity.
Abstract
Grain legumes such as soybean (Glycine max L. Merrill), pea (Pisum sativum L.) and faba bean (Vicia faba L.) are staple sources of protein for human and animal nutrition. Among grain legumes, faba bean is particularly sensitive to abiotic stress (in particular osmotic stress due to lack of water or enhanced soil salinity) and often suffers from severe yield losses. Many stress responsive genes have been reported with an effect on improving stress tolerance in model plants. Pathogenesis-related proteins are expressed by all plants in response to pathogen infection and, in many cases, in response to abiotic stresses as well. The PR10a gene isolated from the potato cultivar Desiree was selected for this study due to its role in enhancing salt and/or drought tolerance in potato, and transferred into faba bean cultivar Tattoo by Agrobacterium tumefaciens-mediated transformation system based upon direct shoot regeneration after transformation of meristematic cells derived from embryo axes. The transgene was under the control of the constitutive mannopine synthase promoter (p-MAS) in a dicistronic binary vector, which also contained luciferase (Luc) gene as scorable marker linked by internal ribosome entry site elements. Fertile transgenic faba bean plants were recovered. Inheritance and expression of the foreign genes were demonstrated by PCR, RT-PCR, Southern blot and monitoring of Luciferase activity. Under drought condition, after withholding water for 3 weeks, the leaves of transgenic plants were still green, while non-transgenic plants (WT) wilted and turned brown. Twenty-four hours after re-watering, the leaves of transgenic plants remained green, while WT plants did not recover. Moreover, the transgenic lines displayed higher tolerance to NaCl stress. Our results suggested that introducing a novel PR10a gene into faba bean could be a promising approach to improve its drought and salt tolerance ability, and that MAS promoter is not only constitutive, but also wound-, auxin/cytokinin- as well as stress-inducible.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Protein-rich grain legumes (pulses) are globally important crops; regions of production include drought-prone areas. However, environmental stress factors impose major limitations on pulse productivity. Abiotic stresses such as drought and salinity can reduce grain yields dramatically. Plants react to changes in their environment by large-scale regulation of their metabolism in order to cope with these challenges including stress-avoidance strategies (early maturing, adaptive growth habit and morphological changes). The metabolic changes are initiated by a differential expression of a subset of genes responding either specifically to a certain stress or unselectively to different stresses (Shinozaki et al. 2003). Identification of genes involved in tolerance to drought and/or salinity and transfer of these genes into commercial cultivars either through breeding or through genetic transformation could therefore significantly increase the agronomic potential of legumes and will thus contribute to improve food security for an expanding population mainly in developing countries (Pellegrineschi et al. 2005). Complex characters, such as abiotic stress tolerance, are difficult to select for and are scarce in the narrow gene pools of pulses such as faba bean (Bond 1987). One promising approach therefore is to integrate useful drought/salt-tolerant traits through genetic engineering of susceptible species. Several genes carrying these traits have been identified (Shinozaki and Yamaguchi-Shinozaki 2000; Xiong and Zhu 2002; Tran et al. 2004; Ma et al. 2006; OuYang et al. 2007; El-Banna et al. 2010; Xianjun et al. 2011). Applied together with traditional breeding, these genes may offer sustainable solutions for farming in water-limited conditions. The engineering of drought-tolerant crop plants has been a long-held and intensively sought objective. A wide range of genes encoding different structural and regulatory proteins have been employed over the past years, for developing abiotic stress-tolerant transgenic plants (see review, Bhatnagar-Mathur et al. 2008).
The Pathogenesis-related (PR) proteins comprise a large super-family of plant defense proteins that accumulate in response to various biotic and often also abiotic stresses (Liu et al. 2003; Srivastava et al. 2004; El-Banna et al. 2010). The major families of PR proteins have been grouped at least into 17 different classes, primarily based on their amino acid sequence similarity (Van Loon and Van Strien 1999). The PR10 proteins are elicitor- and osmotically inducible proteins, which exhibit ribonuclease activity (Somssich et al. 1986). The group of PR10 proteins was defined by Van Loon et al. (1994). This group included the PR10a protein (Marineau et al. 1987) from potato. The biotic and abiotic stress-inducible nature of the PR10 genes include pathogen infection (McGee et al. 2001; Tanaka et al. 2003), salt tolerance (El-Banna et al. 2010; Jain et al. 2012), UV irradiation (Rakwal et al. 1999), osmotic tolerance (Vaas et al. 2012) and ozone stress (Agrawal et al. 2002).
The first objective of the present study was to find out whether abiotic stress tolerance can be achieved in faba bean by expression of PR10a gene from potato without any visible effects on plant growth and development, compared to previous work of El-Banna et al. (2010) which studied the effect of PR10a gene at cellular level using well-established potato cell culture. The present study, however, demonstrates the effect of PR10a gene at multi-cellular level using whole plants of faba bean. The second objective was to characterize the transgenic events of faba bean under abiotic (drought and salt) stress. To achieve these objectives, a dicistronic transformation vector was used (El-Banna et al. 2010). In this vector, the PR10a gene is linked by an internal ribosome entry site (IRES) sequence to the Luc reporter gene. This construct leads to the formation of a unique mRNA for both genes, but the formation of two physically independent proteins, assuring full physiological functionality of both target and reporter genes (Dorokhov et al. 2002). The PR10a gene expression can simply be monitored by assaying the reporter gene expression. In the present study, faba bean plants have been transformed using Agrobacterium-mediated transformation method which was established by Hanafy et al. (2005) using bar gene as a selectable marker and PR10a gene from potato for enhancing its tolerance to drought and salinity.
Materials and methods
Plant material
Mature faba bean seeds (Vicia faba L.) cv. ‘Tattoo’ were surface sterilized in 70 % (v/v) ethanol for 1 min followed by 6 % sodium hypochlorite (v/v) for 7 min followed by washing thoroughly with sterile distilled water for 3–4 times. The seeds were then soaked overnight in sterile distilled water.
Bacterial strains and vectors
The hypervirulent Agrobacterium tumefaciens strain EHA105 (Hood et al. 1993) harboring the pGreenII 0229 derivative binary vector containing a bar gene and the pSoup helper plasmid (Hellens et al. 2000) was used. The binary vector pGreenII 0229 has been modified to include IRES element leading to a cap-independent manner of translation (Kieft et al. 2001; Terenin et al. 2005). This vector contains the PR10a gene under MAS promoter as the first cistron linked by an IRES sequence to Luc as the second cistron.
Transformation of faba bean by Agrobacterium
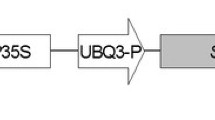
The dicistronic transformation vector pGII0229MAS-PR10a-cp148-Luc (Fig. 1) was used for transformation of faba bean. Agrobacterium-mediated transformation of faba bean was performed as previously described (Hanafy et al. 2005) with some minor modifications using bar gene as a selectable marker.
Schematic representation of the T-DNA region of the transformation vectors (not drawn to scale). Tnos 3′ terminator sequence of the nopaline synthase gene, bar bar gene, P-Nos promoter sequence derived from nopaline synthase, P-MAS manopine synthase promoter, PR10a pathogenesis-related proteins, formally sth2, IRES internal ribosome entry site, Luc luciferase gene, T-CAMV terminator sequence of the cauliflower mosaic virus, The right (RB) and left (LB) border sequences are represented by solid boxes
Briefly, mature embryos were dissected from surface-sterilized seeds and thinly sliced using a scalpel dipped in an Agrobacterium tumefaciens suspension culture. The sliced explants were incubated for 30 min in the suspension prior to being densely plated on co-cultivation medium for 72 h in the absence of selection in the dark. The explants were then placed on selection medium and transferred to fresh medium at 14- to 21-day intervals under 24 °C with a 16/8-h day/night photoperiod. Transgenic shoots were selected on medium supplemented with 2 mg/l phosphinothricin (PPT). After regenerated shoots had developed and produced more than two leaves, they were excised individually and micrografted on a non-transgenic rootstock (Pickardt et al. 1995; Hanafy et al. 2005). Individual shoots are often resistant to rooting and hence grafting is a viable alternative to establish plantlets. After substantial shoot and root growth, plants were hardened and directly transplanted to soil in a greenhouse and propagated via controlled self-fertilization. The T0 plants (original transgenic plants) and their progenies (T1 and T2 plants) were grown and allowed to produce seeds under 16/8 h day/night photoperiod and at a controlled temperature of 25 °C in the greenhouse. The presence or absence of PR10a, Luc and bar genes in transgenic plants of the T1 and T2 generations was determined by PCR analysis. T2 plants were analyzed for drought/salt stress tolerance.
PCR analysis of transgenic plants
Screening for transgenic plants among T0 regenerated plants resistant to PPT and following generations (T1, T2 and T3) was performed by PCR analysis. Total DNA was extracted from the leaves of regenerated plants according to the method previously described (Sul and Korban 1996) from young leaves with minor modifications. Standard PCR protocol was used. Cycling conditions were: 94 °C, 5 min; (1 min at 94 °C, 1 min at 57 °C, 1 min at 72 °C) 30 cycles, then a final step of 10 min at 72 °C. The following primers were used:
-
1.
PR10-For 5′-ATGGGTGTCACTAGCTATACACATG-3′ and PR10-Rev 5′-TTAAGCGTAGACAGAAGGATTGGC-3′ amplifying a 480-bp PR10a sequence;
-
2.
Luc 834F2-5′-CCTTCCGCATAGAACTGC CT-3′ and Luc 834R2 5′-TCC AAA ACAACAACGGCG-3′ amplifying a 834-bp Luciferase sequence;
-
3.
bar 447 F: 5′-GATTTCGGTGACGGGCAGGA-3′; bar 447 R: 5′-AACTTCCGTACCGAGCCGCA-3′ amplifying a 447-bp fragment of the bar gene. As an internal control, primers against the actin1 gene (accession no. J01298) were used which amplify a 831-bp fragment (Act1-931-For 5′-GACGCTGAGGATATTCAACC-3′ and Act1 931-Rev 5′-AGAAATCTGTGAGGTCACGA-3′).
Leaf paint test
The putative transgenic plants were tested in the greenhouse for the expression of bar gene by painting the leaflets with BASTA® (a commercial formulation of PPT containing 200 g/l ammonium glufosinate, Hoechst Ltd.) diluted to a concentration of 200–300 mg/l ammonium glufosinate. The opposite leaflet of each pair was marked and left untreated as a control. T1 and T2 generations were tested by the same method.
Luciferase activity detection
Luciferase activity was monitored using a cooled, back-illuminated, charge-coupled-device (CCD) camera system. Leaves were washed with detergent, rinsed with water and dried on filter paper. A 1-mM solution of d-Luciferin potassium salt (PJK GmbH, Germany) in water was sprayed over the leaves of transgenic and WT plants. After 5 min of incubation at room temperature, the chemiluminescence was detected.
Southern blot analysis of transformed plants
Total DNA for Southern blot analysis was isolated from young leaves of transgenic plants according to the DNeasy Kit (Qiagen, Valencia, CA). The DNA (20 μg) was digested with BamHI, and the resulting fragments were fractionated by electrophoresis on a 0.8 % agarose gel (w/v) and then transferred to a positively charged nylon membrane (Roche, Germany) as described by Sambrook et al. (1989). A fragment of PR10a amplified from the transformation binary vector with the corresponding primer set used for PCR analysis served as a hybridization probe. The fragment was labeled and detected with the use of PCR-DIG Mix (Roche, Germany) according to manufacturer’s instruction. The blots were exposed to X-ray film for 1 h.
Isolation of total RNA and RT-PCR analysis
Total RNA was isolated from young leaves of T2 plants with the use of RNAtidy G solution (A 2867, AppliChem, Germany). A portion of the RNA (5 μg) was incubated for 30 min at 37 °C with 3 U of RNase-free DNase (MBI Fermentas, St. Leon-Rot, Germany) in 10 μl of a solution containing 10 mM Tris–HCl (pH 7.5), 2.5 mM MgCl2, and 0.1 mM CaCl2. The RNA was then subjected to phenol–chloroform extraction and ethanol precipitation before reverse transcription (RT) with an oligo (dT)20 primer and a RevertAid™ H Minus First-Strand cDNA Synthesis Kit (MBI Fermentas, St. Leon-Rot, Germany). The resulting cDNA was subjected to PCR with PR10a primer and actin1 primer as internal control. The PCR conditions were the same as those used for PCR analysis of genomic DNA.
Stress tolerance of plants grown in soil
Different sets of experiments were executed in order to assess potential drought/salt tolerance of PR10a-expressing plants. Transgenic and non-transgenic (WT) faba bean seeds were germinated in soil (peatmoss or perlite) in a greenhouse (16/8 h day/night photoperiod) at 25 °C. Transgenic (T2 generation) and WT plants were grown in pots (size 6 l) and watered every 2 days for 4 weeks before performing the drought/salt stress experiments. For drought stress, at week 5, the WT and transgenic plants were watered once and subjected to 3 weeks of drought followed by the previous watering schedule. For salt stress, at week 5, transgenic and WT plants were treated with 250 ml of 200 mM NaCl every third day for 6 weeks. All experiments were repeated at least twice with nine plants from each transgenic line and WT. The responses of the plants to stress were observed. The plants grown in perlite were removed from the substrate and the roots were soaked in 150 mM NaCl for 3 days. PR10a expression levels in leaves and roots of non-stressed and stressed transgenic and WT faba bean for 23 h and 3 days were studied by real-time PCR. For air drying stress, the plants were removed from the perlite and air dried for a maximum 20 h at room temperature (22 °C). Analysis of PR10a gene expression in leaves and roots in unstressed and stressed transgenic and WT faba bean plants for 4 and 20 h by air drying stress was performed by real-time PCR.
Proline determination
Free l-proline was determined according to Bates et al. (1973) with minor modifications. Leaf explants of 250 mg were ground in a pre-cooled mortar under liquid nitrogen. The homogenate was re-suspended in 10 ml of 3 % aqueous sulphosalicylic acid (w/v) in a 50-ml Erlenmeyer flask. The flask was shaken for 20 min and the cell debris was removed by filtering through filter paper (Whatman plc, Maidstone, UK). For proline determination, 2 ml of the extracts were mixed with 2 ml ninhydrin reagent [ninhydrin 1.25 g, glacial acetic acid 30 ml, phosphoric acid (6 M) 20 ml] and 2 ml glacial acetic and incubated for 1 h at 100 °C.
The reaction was stopped by placing the tubes in an ice bath. The reaction mixture was extracted with 4 ml toluene and mixed vigorously for 15–20 s. The chromophore containing toluene was aspirated from the aqueous phase, warmed to room temperature and the absorbance read at 520 nm using toluene as a blank. Proline content was calculated from a standard curve on a fresh weight basis. The experiment was repeated twice using T2 generation with three transgenic plants from line TL-3 and WT.
Real-time PCR analysis for the stressed plants
Quantitative real-time RT-PCR (qRT-PCR) was performed using SYBR Green PCR master mix kit (BioRad) in a Cycler CFX96™ Real-Time PCR Detection System (BioRad) using synthesized cDNA as template. For detecting expression profile of PR10a in WT and transgenic faba bean under stressed and unstressed conditions using qRT-PCR, 100 mg of leaves and roots were sampled and frozen in liquid nitrogen for RNA extraction and cDNA synthesis. Total RNA was extracted using the RNATidyG (AppliChem, Germany). 5 μg of RNA was treated with DNase I (MBI Fermentas, St. Leon-Rot, Germany) and subsequently tested for the presence of DNA residues by PCR as described by Li et al. (2007) using the primers Act1-931F and Act1-931R amplifying a 831-bp of faba bean actin sequence if the RNA was contaminated with DNA. The remaining RNA was reverse transcribed using oligo(dT)20 primers and the RevertAid™ H Minus First-Strand cDNA Synthesis Kit (MBI Fermentas, St. Leon-Rot, Germany) according to the manufacturer’s instruction. The success of reverse transcription was verified by PCR of 1 μl cDNA using primers Act1-931F and Act1-931R as described before.
Reactions for real-time PCR were prepared in a total volume of 15 μl containing: 1.0 μl cDNA, 0.3 μl of each 10 pmol/μl primer, 7.5 μl of iQ™ SYBR® Green Supermix (Bio-Rad) and 5.9 μl RNase/DNase-free sterile water (Qiagen). Triplicate quantitative assays and blank controls were performed for each master mix. The cycle conditions were set as follows: initial template denaturation at 95 °C for 3 min, followed by 39 cycles of denaturation at 95 °C for 10 s, annealing at 57 °C for 20 and 30 s elongation step at 72 °C followed by one step at 95 °C for 10 s. This cycle was followed by a melting curve analysis, ranging from 65 to 95 °C, with temperature increase by steps of 0.5 °C every 5 s. Baseline and threshold values were automatically determined for all plates using the CFX manager software 1.5 (Bio-Rad). The relative quantification method (Delta–Delta CT) was used to evaluate quantitative variation between the replicates examined.
The amplification of actin1 and the eukaryotic elongation factor 1-alpha (ELF1A) (Gutierrez et al. 2011) were used as internal control samples to normalize all data. Gene-specific primers for PR10a were rPR10-1F 5′-ATGGGTGTCACTAGCTATAC-3′ and rPR10-2R123 5′-CAACATTCTTAACATTTGGC-3′; for actin1 in faba bean, ACTI-120-For 5′-TATGGGCCAGAAAGATGCAT-3′ and ACTI-120-Rev. 5′-CAAATCTTTTCCATATCATCCCAGT-3′. For ELF1A, the following primers were used ELF1AF 5′-GTGAAGCCCGGTATGCTTGT-3′ and ELF1R 5′-CTTGAGATCCTTGACTGCAACATT-3′ (Gutierrez et al. 2011). Gene-specific amplification was evaluated by melting curve analysis and agarose gel electrophoresis. Single melt peaks were obtained for each gene. No primer dimers or other PCR artifacts were detected.
Results
Generation of PR10a-transgenic faba bean lines
Faba bean embryo axes were transformed with a dicistronic transformation vector containing the potato PR10a, which was linked by an IRES sequence to the Luc reporter gene. Out of five transformation experiments using 1,500 explants of faba bean cv. Tattoo, 10 transgenic shoots representing 4 clones were recovered from all experiments under selection with 2 mg/l PPT. The shoots were selected over a period of 4–6 months and were grafted in vitro and finally transferred to the greenhouse to set seeds. Neither the putative transformants (4 clones) nor subsequent generations of transgenic plants (2 clones, i.e., TL-2 and TL-3) exhibited any phenotypical abnormalities compared with WT (non-transgenic) control plants. Transgenic plants (T0–T3) grown in the greenhouse were analyzed by testing the expression of bar gene by applying the leaf paint assay. Within 2 days, necrotic spots appeared on the non-transformed leaves. Ten days after BASTA® application, the treated leaflets of transgenic lines showed complete tolerance, in contrast to leaflets of non-transformed plants, which were completely necrotic (Fig. 2).
The stable integration of the T-DNA was confirmed in different clones and in different generations up to T3 by PCR using different primer sets. All clones tested showed the expected band of 480 bp for the PR10a gene, while no fragment was amplified in the non-transformed control DNA samples (WT). Inheritance of the transgenes was confirmed by PCR until T3. Figure 3 shows multiplex PCR results of different samples from different clones that were positive for the PR10a and Luc genes showing expected fragments of 480 and 834 bp, respectively. All of the PPT-resistant plants exhibited bands of the expected sizes of 447 bp corresponding to bar gene (data not shown).
PCR analysis of luciferase and PR10a genes in T1 transgenic plants. Lanes M 100 bp plus DNA molecular weight marker (MBI Fermentas, St. Leon-Rot, Germany), W water, C non-transformed control plants, P plasmid, lanes 1–3 transgenic plants from line TL-2, lanes 4–6 transgenic plants from line TL-3. The size of the amplified fragment is 834 and 480 bp for luciferase and PR10a genes, respectively
Southern blot analysis was performed to assess the independence of the transgenic plants on the basis of the pattern of transgene integration. Total genomic DNA was digested with BamHI, which cuts the vector only at one site (Fig. 1). The resulting DNA fragments were subjected to hybridization with a probe specific for PR10a. Two independent transgenic lines, TL-2 and TL-3, were thus identified (Fig. 4) and advanced for further investigations.
Southern blot analysis of transgenic plants of faba bean. 20 μg BamHI-digested genomic DNA, isolated from transgenic faba bean cv. Tattoo. Lanes 1–5 representing putative transgenic (T0) plants of line TL-2, lane 6 representing putative transgenic of line TL-3, lanes 7–9 representing stable transgenic plants of T1 of line TL-2 blotted on nylon membrane and probed with PR10a probe. WT control (non-transformed) plants, M Dig-labeled DNA molecular weight marker
Segregation for the presence of transgenes was observed in the T1 plants derived from the primary transgenic plants (T0). The two transgenic lines manifested stable integration of the transgenes in the T2 generation and different integration patterns, yielding two to four bands for each transgene. The same banding patterns were observed with the probes for Luc, indicating that both transgenes were integrated at the same positions (data not shown).
Luciferase activity was observed optically by a high sensitive CCD camera after application of d-Luciferin in all of the PPT-resistant plants with different levels (data not shown). Figure 5 shows the Luc expression in the leaves from one of the transgenic faba bean plants (TL-3), in contrast to the non-transformed plants, which did not show any Luc expression. In all generations analyzed so far, Luc activity was detected with varying luminescence intensities.
RT-PCR analysis indicated that PR10a was transcribed in the transgenic lines. The amplification of a 480-bp DNA fragment derived from PR10a cDNA is shown for two transgenic lines in Fig. 6a. No signal was obtained with cDNA from non-transgenic plants. The amplification of a 631-bp band from cDNA of actin1 gene (lacking the 200 bp intron) as shown in Fig. 6b proves the absence of gDNA contamination.
RT-PCR analysis of PR10a (a) and actin1 (b) transcription in the leaves of independent transgenic faba bean plants. PR10a transcript resulted in the amplification of 480 bp fragment; actin1 transcript resulted in the amplifications of 631 bp in all cDNA samples tested (lacking the intron sequence of 200 bp), while the amplification of gDNA was 831 bp. Lanes M 100 bp plus DNA molecular weight marker, 1 non-transformed control plant (WT), lanes 2–4 transgenic plants from clone TL-3, lanes 5–10 transgenic plants from clone TL-2, 11 pGIIPR10-cp-luc plasmid as positive control, 12 gDNA from transgenic plant, H2O water control
Characterization of drought/salt tolerance of transgenic faba bean plants
Four-week-old control and PR10a-expressing soil-grown plants were used to validate enhanced stress tolerance of the transgenic plants. Using nine plants under the conditions applied, drought stress by withholding water for 3 weeks caused a severe damage in control plants and most of the plants died. In contrast, most of the TL-3 plants still survived under drought stress although they grew relatively smaller as compared to the non-stressed transgenic controls (Fig. 7a, b). After re-watering, all the surviving TL-3 plants recovered and grew well, but the severely stressed control plants (WT) were unable to recover (Fig. 7c, d).
Wild-type (a) and PR10a transgenic faba bean plants cv. Tattoo (b) deprived of water. Wild-type plants and PR10a-expressing transgenic line TL-3 were grown on soil in a 16/8-h day/night photoperiod at 25 °C. Plants were watered every 2 days for 4 weeks. At week 5, the plants were watered once with no further addition of water. The photograph corresponds to plants at 3 weeks of water-deficit stress. Wild-type (c) and PR10a transgenic plants line TL-3 (d) after 3 weeks of re-watering
The plants were then treated with 200 mM NaCl, TL-2, TL-3; control plants exhibited clear growth retardation compared with unstressed plants. Leaves of control plants became yellow and 90 % died after 4 weeks of stress application, but more than 90 % of the leaves of lines TL-2 and TL-3 plants were still green (Fig. 8). For the T2 plants grown in perlite, 9 plants were removed from the soil and the roots were soaked in 150 mM NaCl solution; the control plants showed clear wilting compared with PR10a-expressing transgenic plants after 23 h of stress application (data not shown). The results indicate that expression of PR10a in faba bean plants can improve their tolerance to salt and drought stresses.
Salt treated wild-type (WT) and transgenic faba bean lines (TL-3 and TL-2). Plants were grown on soil in a 16/8-h day/night photoperiod at 25 °C. The plants were watered every 2 days for 4 weeks. At week 5, the plants were treated with 250 ml of 200 mM NaCl every third day. The photograph corresponds to plants at 6 weeks of salt treatment
Biochemical characterization of transgenic cells under salt stress
The PR10a-expressing transgenic plants showed significantly different responses concerning proline accumulation. This was demonstrated by growing three plants of transgenic and wild-type plants under salt stress conditions (200 mM NaCl) with the experiments repeated three times. The proline content in the transgenic plant was about 4.6 μM/g fresh weight compared to 1.6 μM/g in WT under stress condition (Fig. 9), indicating a link between expression of PR10a and proline accumulation.
Analysis of PR10a expression profiles
The transcripts of PR10a (Fig. 10a) were detected in the leaves of transgenic lines TL-2 and TL-3. The expression level of PR10a gene was higher in transgenic plants under stress than in non-stressed transgenic plants, indicating that the MAS promoter is stress-inducible. No PR10a expression was observed in non-transgenic control samples, while comparable actin1 expression was detected in all cDNA samples (Fig. 10a). Figure 10b presents the normalized fold expression of PR10a gene of the transgenic lines under salt and non-stressed conditions. The expression levels of PR10a were found to be significantly (at 5 % level) higher with a maximum of 31-fold in the stressed transgenic plants (TL-3) than that in the non-stressed transgenic plants. No expression was detected in either stressed or non-stressed WT.
Analysis of PR10a gene expression in two transgenic faba bean lines (TL-2 and TL-3) by real-time PCR. a Gel electrophoresis of real-time PCR products using actin1 as a reference gene and PR10a specific primers under 200 mM NaCl stress (St lanes) and non-stressed condition, b expression levels in unstressed and stressed (St) faba bean with 200 mM NaCl, c expression analysis in leaves (L) and roots (R) in unstressed (USt) and stressed (St) faba bean for 23 h with 150 mM NaCl solution. Expression levels were normalized using actin1 gene as the reference gene. Normalized fold expression data (mean ± SE) represented the mean of three replicates
The expression levels of the PR10a gene in different plant organs (leaves and roots) under stress were also investigated. For this purpose, the expression pattern of PR10a under various stress treatments in leaves and roots was investigated (Fig. 10b). Under salt stress, the expression level increased at maximum of 24-fold in roots and 4-fold in the leaves of transgenic plants after 23 h of application and further increased with prolonged stress time course (3 days) with 2,144- and 47-fold increase in the roots and leaves of line TL-3, respectively. In general, the expression levels in the roots under salt stress were found to be much higher than in the leaves (Fig. 10c).
Under air drying treatment, the expression of PR10a was also increased after 4 h of treatment in leaves and roots. PR10a expression in the leaves of line TL-3 was increased 2- and 62-fold after 4 and 20 h of air drying, respectively. However, the expression in the roots of TL-3 was increased 1.3-fold after 4 h air drying treatment. PR10a expression in line TL-2 was increased 13- and 23-fold in the leaves after 4 and 20 h of air drying treatment, respectively. While the expression of PR10a in the roots of line TL-2 was increased 45-fold after 4 h of air drying treatment. Due to complete drying of the roots, we could not study the PR10a expression in the roots after 20 h of air drying.
Discussion
Generation and characterization of transgenic faba bean plants
Although several stable transformants of grain legumes have been described (Popelka et al. 2004; Eapen 2008; Solleti et al. 2008; Ishimoto et al. 2010), the production of transformed faba bean remains difficult. Faba bean is an important grain legume, e.g., in West Asia and North Africa (WANA) Region. Limited research groups are working on the use of gene transfer for faba bean improvement (Böttinger et al. 2001; Hanafy et al. 2005; Hanafy et al. 2008). We introduced a PR10a gene from potato into faba bean in order to improve the drought/salt tolerance of this important grain legume. The Agrobacterium-mediated transformation protocol used (Hanafy et al. 2005) has an advantage in reducing the time for recovering transgenic plants to 9 months compared to 16–24 months using de novo transformation protocol (Böttinger et al. 2001).
To our knowledge, we report here for the first time the recovering of transgenic faba bean plants expressing a PR10a gene from potato. We also demonstrated that the PR10a gene is expressed ubiquitously in the transgenic faba bean leaves and roots under non-stressed condition (Fig. 6). Different transgenic faba bean lines of the variety Tattoo were recovered and produced seeds. All of the transgenic faba bean lines expressing PR10a were fertile and advanced to the T3 generation without any visible detrimental effect on plant growth. Stable integration and inheritance of the introduced genes to subsequent generations were proved by PCR, leaf paint test, Luc activity test and Southern blot analysis. Based on the analysis of PR10a transgenic lines in this work and the studies published previously (Hashimoto et al. 2004; Srivastava et al. 2004; El-Banna et al. 2010; Vaas et al. 2012; Jain et al. 2012), it is clear that the PR10a expression level was enhanced by drought/salt conditions and improved the tolerance to osmotic stress.
Most of PR10 proteins are known to be induced by various biotic and abiotic stresses, and constitutive expression of these proteins was also reported (Awade et al. 1991; Jellouli et al. 2010), suggesting their role in plant defense and development. Many of the proteins of the PR10 family have first been isolated from cell cultures, such as PR10 proteins from parsley (Somssich et al. 1986), peanut (Jain et al. 2006) or soybean (Crowell et al. 1992). PR10a protein was previously detected after osmotic and salt stress as the most prominent de novo induced protein spot in the proteome of cell cultures established from potato plants cv. Desiree (El-Banna et al. 2010). The transgenic potato cell line overexpressing PR10a gene showed better survival under salt and osmotic challenge than the non-transgenic (El-Banna et al. 2010) as well as during cryopreservation process of potato cell culture (Vaas et al. 2012).
Our results showed that transgenic plants expressing PR10a confer enhanced tolerance against salt and drought stresses. Generally, all the transgenic plants tested showed better growth than the WT control plants under induced salt stress of 200 mM NaCl. The leaves were much greener in the transgenics, whereas the leaves of the WT plants were brown and wilted-dry under salt stress (Fig. 8). Previous studies demonstrated that constitutive expression of a pea PR10 protein enhanced the Brassica napus germination under saline conditions (Srivastava et al. 2004). Also, transgenic tobacco plants overexpressing AhSIPR10 gene from peanut showed higher tolerance to salt, mannitol and heavy metal (Jain et al. 2012). Moreover, through this study, we found that the potato PR10a gene enhanced the drought tolerance ability of transgenic faba bean, showing that PR10a gene may influence abiotic stress tolerance (Fig. 7). Generally, WT faba bean displayed growth retardation and lethality when treated with high salt concentration or water deprivation, whereas the transgenic plants survived and continued a normal development after stress removal.
In response to environmental stresses, some plants accumulate soluble osmotic molecules, such as proline (Liu and Zhu 1997; Armengaud et al. 2004) as osmoprotectants. Proline is known to be the most widely distributed osmoprotectant, with the highest water solubility among amino acids, and under stress conditions, it functions to adjust osmotic balance, to maintain stability of proteins, membranes and subcellular structures, and to remove reactive oxygen species (ROS) (Zhou et al. 2009). Several studies have demonstrated proline accumulation is positively related to plant tolerance to stresses (Hong et al. 2000). Banu et al. (2009) have reported that proline and betaine protect plants from NaCl-induced cell death by decreasing ROS accumulation. In the present study, it was found that PR10a transgenic faba bean plants produced significantly more proline (about 3-fold more than WT control) which presumably protected the transgenic plants from damage as a consequence to the salt stress treatments (Fig. 9). On the other hand, the WT control plants could not tolerate the same degree of stress. These results are in accordance with those of El-Banna et al. (2010), Agarwal and Pandey (2004) and Dash and Panda (2001).
Differential expression of potato PR10a in response to abiotic stress
We noticed that line TL-3 exhibited higher expression levels of PR10a than line TL-2 in the leaves under non-stressed conditions. MAS promoter was considered to be constitutive promoter, but it was mentioned that it is also induced by wounding and auxin/cytokinin treatment (Langridge et al. 1989; Guevara-García et al. 1993). Our study showed that PR10a under the MAS promoter is enhanced by drought and salt stresses in the leaves and roots of the transgenic plants. However, different transgenic events may have differences in the expression level and stress tolerance levels as seen between lines TL-3 (highest expression level of PR10a with higher stress tolerance) compared to line TL-2 (low expression level of PR10a). Our findings are in accordance with the previous report of EL-Banna et al. (2010) using the same promoter. They reported that overexpressing PR10a in transgenic potato tissues led to enhanced salt and osmotic tolerance up to 500 mM sorbitol and 200 mM NaCl without growth reduction.
In the present study, when transgenic faba bean plants were subjected to salt stress, the expression level of PR10a in the roots was much higher than the expression levels in the leaves (Fig. 10c). In contrast, when the transgenic plants were subjected to air drying, the expression level of PR10a in the leaves was significantly higher than the levels detected in the roots. Similarly, Przymusinski et al. (2004) observed increased levels of the RzPR10 protein in lupine roots exposed to 500 mM NaCl for 48 h. Comparable results on increased levels of PR10 protein were reported in peanut callus cells obtained from leaves and subjected to a 15-day salt stress (Jain et al. 2006), while significant changes in abundance of PR10 in pea roots occurred after 6 weeks of treatment with 150 mM NaCl (Kav et al. 2004).
Our observation that potato PR10a expression is induced upon salinity and drought stress supporting the hypothesis that this protein may have a significant role in protecting plant cellular components from the deleterious effects of salinity and drought (Kav et al. 2004). Our findings suggest that transformation with this gene can be a feasible strategy to improve drought/salt stress tolerance of legume plants. However, the precise roles of the PR10a protein in abiotic stress responses are not fully clear. Further investigations are needed to clarify the molecular mechanisms of stress tolerance involving the PR10a protein.
Abbreviations
- bar :
-
Bialaphos resistance gene
- PPT:
-
Phosphinothricin
- BAP:
-
6-Benzylaminopurine
- IBA:
-
Indole-3-butyric acid
- CTAB:
-
Cetyltrimethylammoniumbromide
- PR:
-
Pathogenesis-related
References
Agarwal S, Pandey V (2004) Antioxidant enzyme responses to NaCl stress in Cassia angustifolia. Biol Plant 48:555–560
Agrawal GK, Rakwal R, Tamogami S, Yonekura M, Kubo A, Saji H (2002) Chitosan activates defense/stress response(s) in the leaves of Oryza sativa seedlings. Plant Physiol Biochem 40:1061–1069
Armengaud P, Thiery L, Buhot N, Grenier-De MG, Savoure A (2004) Transcriptional regulation of proline biosynthesis in Medicago truncatula reveals developmental and environmental specific features. Physiol Plant 120:442–450
Awade A, Metz-Boutigue MH, Le Ret M, Genot G, Amiri I, Burkard G (1991) The complete amino acid sequence of the pathogenesis-related (PR2) protein induced in chemically stressed bean leaves. Biochim Biophys Acta 1077:241–244
Banu MNA, Hoque MA, Watanabe-Sugimoto M, Matsuoka K, Nakamura Y, Shimoishi Y, Murata Y (2009) Proline and glycinebetaine induce antioxidant defense gene expression and suppress cell death in cultured tobacco cells under salt stress. J Plant Physiol 166:146–156
Bates LS, Waldren RP, Teare LD (1973) Rapid determination of free proline for water stress studies. Plant Soil 39:205–207
Bhatnagar-Mathur P, Vadez V, Sharma KK (2008) Transgenic approaches for abiotic stress tolerance in plants: retrospect and prospects. Plant Cell Rep 27:411–424
Bond DA (1987) Recent developments in breeding of field beans (Vicia faba L.). Plant Breeding 99:1–26
Böttinger P, Steinmetz A, Schieder O, Pickardt T (2001) Agrobacterium-mediated transformation of Vicia faba. Mol Breeding 8(3):243–254
Crowell DN, John ME, Russel D, Amasona R (1992) Characterization of a stress induced, developmentally regulated gene family from soybean. Plant Mol Biol 18:459–466
Dash M, Panda SK (2001) Salt stress induced changes in growth and enzyme activities in germinating Phaseolus mungo seeds. Biol Plant 44:587–589
Dorokhov YL, Skulachev MV, Ivanov PA, Zvereva SD, Tjulkina LG, Merits A, Gleba YY, Hohn T, Atabekov JG (2002) Polypurine (A)-rich sequences promote cross-kingdom conservation of internal ribosome entry. Proc Natl Acad Sci USA 99:5301–5306
Eapen S (2008) Advances in development of transgenic pulse crops. Biotechnol Adv 26:162–168
El-Banna A, Hajirezaei MR, Wissing J, Ali Z, Vaas L, Heine-Dobbernack E, Jacobsen HJ, Schumacher H, Kiesecker H (2010) Over-expression of PR10a leads to increased salt and osmotic tolerance in potato cell cultures. J Biotechnol 150:277–287
Guevara-García A, Mosqueda-Cano G, Argüello-Astorga G, Simpson J, Herrera-Estrella L (1993) Tissue-specific and wound-inducible pattern of expression of the mannopine synthase promoter is determined by the interaction between positive and negative cis-regulatory elements. Plant J 4(3):495–505
Gutierrez N, Giménez MJ, Palomino C, Avila CM (2011) Assessment of candidate reference genes for expression studies in Vicia faba L. by real-time quantitative PCR. Mol Breeding 28:13–24
Hanafy MS, Pickardt T, Kiesecker H, Jacobsen HJ (2005) Agrobacterium-mediated transformation of faba bean (Vicia faba L.) using embryo axes. Euphytica 142:227–236
Hanafy MS, Jacobsen HJ, Böttinger P, Pickardt T (2008) Agrobacterium-mediated genetic transformation of faba bean, Vicia faba L. In: Kirti PB (ed) Handbook of new technologies for genetic improvement of legumes. The Haworth Press, Taylor & Francis Group, New York, pp 287–300
Hashimoto M, Kisseleva L, Sawa S, Furukawa T, Komatsu S, Koshiba T (2004) A novel rice PR10 Protein, RSOsPR10, specifically induced in roots by biotic and abiotic stresses, possibly via the jasmonic acid signaling pathway. Plant Cell Physiol 45:550–559
Hellens RP, Edwards A, Leyland NR, Bean S, Mullineaux PM (2000) pGreen: a versatile and flexible binary Ti vector for Agrobacterium-mediated plant transformation. Plant Mol Biol 42:819–832
Hong ZL, Lakkineni K, Zhang ZM, Verma DPS (2000) Removal of feedback inhibition of Δ1-pyrroline-5-carboxylate synthetase results in increased proline accumulation and protection of plants from osmotic stress. Plant Physiol 122:1129–1136
Hood EE, Gelvin SB, Melchers LS, Hoekema A (1993) New Agrobacterium helper plasmids for gene transfer to plants. Transgenic Res 2:208–218
Ishimoto M, Rahman S, Hanafy MS, Khalafalla MM, El-Shemy HA, Nakamoto Y, Kita Y, Takanashi K, Matsuda F, Murano Y, Funabashi T, Miyagawa H, Wakasa K (2010) Evaluation of amino acid content and nutritional quality of transgenic soybean seeds with high-level tryptophan accumulation. Mol Breeding 25:313–326
Jain S, Srivastava S, Sarin NB, Kav NNV (2006) Proteomics reveals elevated levels of PR 10 proteins in saline-tolerant peanut (Arachis hypogaea) calli. Plant Physiol Biochem 44:253–259
Jain S, Kumar D, Jain M, Chaudhary P, Deswal R, Sarin NB (2012) Ectopic overexpression of a salt stress-induced pathogenesis-related class 10 protein (PR10) gene from peanut (Arachis hypogaea L.) affords broad spectrum abiotic stress tolerance in transgenic tobacco. Plant Cell Tiss Organ Cult 109:19–31
Jellouli N, Ben Jouira H, Daldoul S, Chenennaoui S, Ghorbel A, Ben Salem A, Gargouri A (2010) Proteomic and transcriptomic analysis of grapevine PR10 expression during salt stress and functional characterization in yeast. Plant Mol Biol Rep 28:1–8
Kav NNV, Srivastava S, Goonewardene L, Blade SF (2004) Proteome-level changes in the roots of Pisum sativum L. in response to salinity. Ann Appl Biol 145:217–230
Kieft JS, Zhou K, Jubin R, Doudna JA (2001) Mechanism of ribosome recruitment by hepatitis C IRES RNA. RNA 7:194–206
Langridge WHR, Fitzgerald KJ, Koncz C, Schell J, Szalay AA (1989) Dual promoter of Agrobacterium tumefaciens mannopine synthase genes is regulated by plant growth hormones. Proc Natl Acad Sci USA 86(9):3219–3223 PMCID: PMC287101
Li H, Flachowsky H, Fischer TC, Hanke V, Forkmann G, Treutter D, Schwab W, HoVmann T, Szankowski I (2007) Maize Lc transcription factor causes induction of anthocyanins, distinct proanthocyanidins and phenylpropanoids in apple Malus domestica Borkh.). Planta 226:1243–1254
Liu J, Zhu J-K (1997) Proline accumulation and salt-stress-induced gene expression in a salt-hyper sensitive mutant of Arabidopsis. Plant Physiol 114:591–596
Liu JJ, Ekramoddoullah AKM, Yu X (2003) Differential expression of multiple PR10 proteins in western white pine following wounding, fungal infection and cold-hardening. Physiol Plant 119:544–553
Ma SS, Gong QQ, Bohnert HJ (2006) Dissecting salt stress pathways. J Exp Bot 57:1097–1107
Marineau C, Matton DP, Brisson N (1987) Differential accumulation of potato tuber mRNAs during the hypersensitive response induced by arachidonic acid elicitor. Plant Mol Biol 9:335–342
McGee JD, Hamer JE, Hodges TK (2001) Characterization of a PR-10 pathogenesis-related gene family induced in rice during infection with Magnaporthe grisea. Mol Plant Microbe Interact 14:877–886
OuYang B, Yang T, Li H, Zhang L, Zhang Y, Zhang J, Fei Z, Ye Z (2007) Identification of early salt stress response genes in tomato root by suppression subtractive hybridization and microarray analysis. J Exp Bot 58:507–520
Pellegrineschi A, Pulleman M, Sullivan S, Trethowan R, Reynolds M (2005) Using transgenic plants as a source of genetic diversity for breeding greater drought tolerance into wheat. ISB news report, July
Pickardt T, Saalbach I, Waddell D, Meixner M, Müntz K, Schieder O (1995) Seed specific expression of the 2S albumin gene from Brazil nut (Bertholletia excelsa) in transgenic Vicia narbonensis. Mol Breeding 1:295–301
Popelka JC, Terryn N, Higgins TJV (2004) Gene technology for grain legumes: can it contribute to the food challenge in developing countries? Plant Sci 167:195–206
Przymusinski R, Rucinska R, Gwozdz EA (2004) Increased accumulation of pathogenesis-related proteins in response of lupine roots to various abiotic stresses. Environ Exp Bot 52:53–61
Rakwal R, Agrawal GK, Yonekura M (1999) Separation of proteins from stressed rice Oryza sativa L. leaf tissues by two dimensional polyacrylamide gel electrophoresis, induction of pathogenesis-related and cellular protectant proteins by jasmonic acid, UV irradiation and copper chloride. Electrophoresis 20:3472–3478
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Shinozaki K, Yamaguchi-Shinozaki K (2000) Molecular responses to dehydration and low temperature: differences and cross-talk between two stress signaling pathways. Curr Opin Plant Biol 3(3):217–223
Shinozaki K, Yamaguchi-Shinozaki K, Seki M (2003) Regulatory network of gene expression in the drought and cold stress responses. Curr Opin Plant Biol 6:410–417
Solleti SK, Bakshi S, Purkayastha J, Panda SK, Sahoo L (2008) Transgenic cowpea (Vigna unguiculata) seeds expressing a bean alpha-amylase inhibitor 1 confer resistance to storage pests, bruchid beetles. Plant Cell Rep 27:1841–1850
Somssich IE, Schmelzer E, Bollmann J, Hahlbrock K (1986) Rapid activation by fungal elicitor of genes encoding “pathogenesis-related” proteins in cultured parsley cells (two-dimensional gels/doned cDNAs/RNA blot hybridization/nuclear run-off transcription). Proc Nat Acad Sci USA 83:2427–2430
Srivastava S, Fristensky B, Kav NNK (2004) Constitutive expression of a PR-10 protein enhances the germination of Brassica napus under saline conditions. Plant Cell Physiol 45:1320–1324
Sul IW, Korban SS (1996) A highly efficient method for isolating genomic DNA from plant tissues. Plant Tissue Culture Biotechnol 2:113–116
Tanaka N, Che FS, Watanabe N, Fujiwara S, Takayama S, Isogai A (2003) Flagellin from an incompatible strain of Acidovorax avenae mediates H2O2 generation accompanying hypersensitive cell death and expression of PAL, Cht-1, and PBZ1, but not of LOX in rice. Mol Plant Microbe Interact 16(5):422–428
Terenin IM, Dmitriev SE, Andreev DE, Royall E, Belsham GJ, Roberts LO, Shatsky IN (2005) A cross-kingdom internal ribosome entry site reveals a simplified mode of internal ribosome entry. Mol Cell Biol 25:7879–7888
Tran LSP, Nakashima K, Sakuma Y, Simpson SD, Fujita Y, Maruyama K, Fujita M, Seki M, Shinozaki K, Yamaguchi-Shinozaki K (2004) Isolation and functional analysis of Arabidopsis stress-inducible NAC transcription factors that bind to a drought-responsive cis-element in the early responsive to dehydration stress 1 promoter. Plant Cell 16:2481–2498
Vaas L, Marheine M, Seufert S, Schumacher HM, Kiesecker H, Heine-Dobbernack E (2012) Impact of pr-10a overexpression on the cryopreservation success of Solanum tuberosum suspension cultures. Plant Cell Rep 31(6):1061–1071. doi:10.1007/s00299-011-1225-5
Van Loon LC, Van Strien EA (1999) The families of pathogenesis-related proteins, their activities and comparative analysis of PR-1 type proteins. Physiol Mol Plant Pathol 55:85–97
Van Loon LC, Pierpoint WC, Boller Th, Conejero V (1994) Recommendations for naming plant pathogenesis-related proteins. Plant Mol Biol Rep 12:245–264
Xianjun P, Xingyong M, Weihong F, Man S, Liqin C, Alam I, Lee B, Dongmei Q, Shihua S, Gongshe L (2011) Improved drought and salt tolerance of Arabidopsis thaliana by transgenic expression of a novel DREB gene from Leymus chinensis. Plant Cell Rep 30:1493–1502
Xiong L, Zhu J-K (2002) Molecular and genetic aspects of plant responses to osmotic stress. Plant, Cell Environ 25:131–139
Zhou W, Li Y, Zhao BC, Ge RC, Shen YZ, Wang G, Huang ZJ (2009) Overexpression of TaSTRG gene improves salt and drought tolerance in rice. J Plant Physiol 166:1660–1671
Acknowledgments
This study was supported by Alexander von Humboldt foundation (AvH), Germany. M.S. Hanafy was holder of a fellowship for experienced researcher from AvH (Georg Forster program). The authors thank Carolyn Lee-Parsons (Northeastern University) for language editing.
Conflict of interest
The authors have declared no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by P. Kumar.
Rights and permissions
About this article
Cite this article
Hanafy, M.S., El-Banna, A., Schumacher, H.M. et al. Enhanced tolerance to drought and salt stresses in transgenic faba bean (Vicia faba L.) plants by heterologous expression of the PR10a gene from potato. Plant Cell Rep 32, 663–674 (2013). https://doi.org/10.1007/s00299-013-1401-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-013-1401-x