Abstract
The function of root border cells (RBC) during aluminum (Al) stress and the involvement of oxalate oxidase, peroxidase and H2O2 generation in Al toxicity were studied in barley roots. Our results suggest that RBC effectively protect the barley root tip from Al relative to the situation in roots cultivated in hydroponics where RBC are not sustained in the area surrounding the root tip. The removal of RBC from Al-treated roots increased root growth inhibition, Al and Evans blue uptake, inhibition of RBC production, the level of dead RBC, peroxidase and oxalate oxidase activity and the production of H2O2. Our results suggest that even though RBC actively produce active oxygen species during Al stress, their role in the protection of root tips against Al toxicity is to chelate Al in their dead cell body.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Metals are serious environmental pollutants that can occur in the soil naturally or as industrial by-products. At plant physiological concentrations, several metals are essential micronutrient elements involved in a variety of metabolic processes. However, at elevated concentrations, these metals can be toxic to many plant species. Aluminum (Al) is one of the most abundant elements than can be found in the earth’s crust, but to date it has no known function in biological processes. It exists primarily in the form of insoluble aluminosilicates or oxides. These biologically inactive Al forms become highly toxic following solubilization (soluble Al3+), a process caused by acidification of the environment. Due to the rapidly increasing area of acid soils (40% of all arable land), Al is the major factor limiting crop productivity in several countries throughout the world. Consequently, in the past few years extensive efforts have been directed towards elucidating the mechanism of Al toxicity and tolerance in plants (for reviews: Kochian 1995; Matsumoto 2000; Samac and Tesfaye 2003).
The common feature of several metal toxicity symptoms is the enhanced production of active oxygen species (AOS) that result in oxidative stress. It has been recently supposed that although Al is a non-transition metal that cannot catalyze redox reactions, it probably has a pro-oxidant activity via the formation of the aluminum superoxide semi-reduced radical ion (Exley 2004). The results of studies of Al toxicity in plant roots suggest that AOS production is a determining factor of root-elongation inhibition by Al (Yamamoto et al. 2003; Kobayashi et al. 2004). In rice roots, Al was observed to intensify the production of AOS and lipid peroxidation and cause DNA damage (Meriga et al. 2004). Al-induced AOS activates signal transduction pathways that lead to cell death as a general symptom of Al-treated roots (Pan et al. 2001; Boscolo et al. 2003). The induction of oxidative stress genes by Al and of antioxidant elevation as a result of Al toxicity confirmed the important role of AOS during Al stress (Richards et al. 1998; Tamás et al. 2003; Kobayashi et al. 2004). Yamamoto et al. (2002) suggested that Al affects mitochondrial functions, which leads to AOS production causing the inhibition of cell growth. On the other hand, it has been recently confirmed that AOS production into the surrounding medium by germinated seeds or intact roots is an active developmentally controlled physiological process that is probably catalyzed by membrane-bound and extracellular enzymes (Schopfer et al. 2001; Liszkay et al. 2003). In a previous investigation, we described the elevated H2O2 production in intact germinating barley seeds during Al stress (Tamás et al. 2004a). We have recently partially purified the Al-induced cationic peroxidase (pI: approx. 9.2) which produces a significant amount of H2O2 (Šimonovičová et al. 2004). Nagy et al. (2004) have reported that the increase in several peroxidase isozymes is the characteristic feature of Al-treated spruce seedlings. Al also activates another H2O2-producing enzyme, oxalate oxidase, mainly at the lethal Al concentration inducing cell necrosis (Tamás et al. 2004b).
Several reports have described both the capacity of root caps to modulate the properties of the rhizosphere by releasing exudates or RBC and their role in plant growth and development (Hawes et al. 2003). Unfortunately, the majority of the experiments analyzing Al toxicity were performed under hydroponic conditions, and under these conditions normal physiological RBC development is destroyed as is probably their normal function during root growth. The most recent studies were directed to analyzing the possible role of RBC in determining Al and the involvement of RBC in Al-tolerance mechanism (Miyasaka and Hawes 2001; Zhu et al. 2003). RBC were found to play an important role in the protection of the root tip from Al stress. The production of RBC in barley roots was strongly inhibited by Al but was insensitive to temperature-induced root growth inhibition (Pan et al. 2004). Zhu et al. (2003) found that Al treatment also induced the death of RBC in vitro . These results suggest that RBC probably play a crucial role in the protection of the deeper root tissues during Al stress but that they are very sensitive to the presence of toxic Al levels.
The aim of the investigation reported here was to analyze both the involvement of root border cells in the generation of H2O2 by peroxidase and oxalate oxidase during Al stress and its correlation with Al-induced cell death and root growth inhibition in barley.
Materials and methods
Plant material
Seeds of barley (Hordeum vulgare L.) cv. Jubilant were surface-sterilized and then incubated either in a 5 mM CaCl2 solution, pH 4.0 (control), or in a 3 mM CaCl2 containing a 2 mM AlCl3 solution (after binding of the Al to the filter paper, the Al concentration was reduced from millimolar concentrations to micromolar concentrations), pH 4.0 (Al-treated) for 4 h at 25°C in the dark. Following this short imbibition period, the seeds were germinated between two sheets of filter paper (Whatman No.1) fully moistened with the same solutions and under the same conditions as during the imbibition. At 20 h and a subsequent 24 h, the germinating seeds were transferred to another filter paper freshly moistened with same solutions; 24 h thereafter they were transferred to a filter paper moistened with the appropriate solutions with or without removal of the RBC (see figures). RBC from the root tips were removed by gentle shaking of the root tips (1 cm in length) in distilled water for 15 min. Root length was measured using a ruler, and excised root tips were used immediately for analysis. Each experiment was repeated at least five times with 100 replicates.
Aluminum uptake
Hematoxylin staining was used to determine Al uptake (Ownby 1993). Freshly harvested roots were washed in distilled water for 15 min and then stained with a 0.2% hematoxylin and 0.02% KIO3 solution for 15 min at room temperature. After washing with distilled water for 15 min, ten root tips (0.5 cm in length) were excised and soaked in 200 μl 1 M HCl for 1 h. Optical density of the released stain was measured at 490 nm.
Determination of cell death
The loss of plasma membrane (PM) integrity was evaluated using the spectrophotometric assay of Evans blue staining (Baker and Mock 1994). Roots were stained in 0.25% (v/v) aqueous solution of Evans blue for 15 min at room temperature. The stained roots were washed three times with distilled water, 10 min each time. Root tips (0.5 cm) were excised and soaked in N,N-dimethylformamide for 1 h at room temperature (RT). The optical density was measured spectrophotometrically at 600 nm.
Collection of the root border cells and the viability test
Root tips (1 cm) of 100 seedlings were immersed in 40 ml of distilled water under gentle agitation. After 15 min, RBC were collected by centrifugation at 1,500 g for 5 min and resuspended in 1 ml deionized water. The supernatant, following concentrating, was used for the analysis of extracellular enzymes. The number of released RBC was counted with the hemacytometer (Bürker chamber), while the viability of the RBC was detected with the Evans blue staining method: 20 μl of 0.01% Evans blue was added to 100 μl of cell suspension and the number of vital (unstained) and non-vital (blue) cells counted. The mortality index was obtained as the rate of non-vital and vital cells.
Enzyme assays
Enzyme activities were determined photometrically using a microplate reader. Specific enzyme activities were expressed in units of optical density as OD μg−1 protein or OD number of RBC−1 . Proteins were quantified using bovine serum albumin (BSA) as a standard according to the method of Bradford (1976). Changes in enzyme activities were expressed as a percentage of the control.
The activity of guaiacol peroxidase was determined by monitoring the formation of the guaiacol dehydrogenation product by following the increase of absorbance at 405 nm (Chance and Maehly 1955). The reaction mixture contained 0.04% guaiacol, 0.04 M Na-acetate buffer, pH 5.2, and 0.04% hydrogen peroxide.
The formation of H2O2 catalyzed by NADH-peroxidase was measured colorimetrically as described by Ishida et al. (1987). The reaction mixture consisted of 25 mM Tris-HCl buffer, pH 8.0, containing 1 mM 4-aminoantipyrine; 1 mM 2,4-dichlorophenol; 50 mM MnCl2; 0.2 mM NADH. The increase in absorbance was measured at 510 nm. To test the involvement of peroxidase or NADH oxidase in H2O2 production, we added the inhibitors KCN (1 mM), NaN3(1 mM), imidazole (10 mM) or DPI (1 μM) into the reaction mixture.
Oxalate oxidase activity was determined using the method of Zhang et al. (1996). The reaction mixture contained 40 mM succinic acid/NaOH buffer, pH 4.0, 60% ethanol (v/v), 2 mM oxalic acid, 20 μl/100 ml N,N-dimethylaniline, 8 mg/100 ml 4-aminoantipyrine and 5 U ml −1 of horseradish peroxidase. The mixture was incubated at 37°C for 15 min, and activity was measured at 555 nm against the control reaction without oxalic acid.
Results
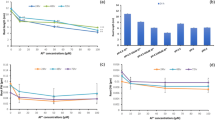
Due to the high binding capacity of the filter paper, the millimolar Al concentration was required to inhibit barley root growth. At a concentration of 2 mM Al did not affect germination of the barley seeds between the two layers of moistened filter paper (data not shown). Root growth was not inhibited following the transfer of the control seedlings to 2 mM Al for 24 h when RBC were not removed from the area surrounding the root tip (Fig. 1a), although a slight Al uptake (Fig. 1b) and Evans blue uptake (Fig. 1c) were detected. Removal of the RBC before the transfer of the seedlings to Al caused a slight inhibition of root growth (Fig. 1a) and an increase in both Al and Evans blue uptake (Fig. 1b,c). Root growth in seedlings germinated and grown 72 h in the presence of Al without removal of the RBC from the area surrounding the root tip was about 80% of that of the control plants (Fig. 1a); both hematoxylin uptake and Evans blue uptake were considerably increased (Fig. 1b,c). Each of the parameters analyzed increased when the RBC were removed; in this state, inhibition of the root growth was 69% of that of the control (Fig. 1a), and both hematoxylin and Evans blue uptake (Fig. 1b and c) were twofold higher than in the control plants.
Root growth inhibition (a), aluminum uptake (b) and Evans blue uptake (c) of barley roots. −Al Control plants growing 72 h without Al, −Al48h +Al24h plants growing an initial 48 h without Al and the subsequent 24 h in the presence of Al, −Al48h –RBC +Al24h plants growing an initial 48 h without Al and, after the removal of RBC, a subsequent 24 h in the presence of Al, +Al72h plants growing 72 h in the presence of Al, +Al48h –RBC +Al24h plants growing 72 h in the presence of Al with removal of the RBC after 48 h. Data represent the means ± standard deviation (n=5). Values followed by the same letter are not significantly different (P<0.05) according to the one-way anova test
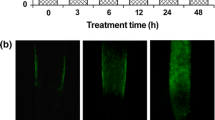
In all of the experiments the RBC showed a high viability (above 95%) and were spherical or elongated with a length between 40 μm and 250 μm. In the control roots almost all of the RBC could be easily removed from the root surface by a 15-min washing in distilled water, while in all of the other treatments the cells clumped onto the root surface (data not shown). RBC release was the highest in the control roots and was only insignificantly changed when the roots were transferred to Al without removal of the RBC (Fig. 2a). However, the amount of released dead RBC was significantly increased relative to the control plants (Fig. 2b,c). Removal of the RBC before the plants were transferred to Al significantly reduced the amount of RBC released into the solution (Fig. 2a), and a twofold higher mortality index was observed (Fig. 2c). Evans blue accumulation was detected in several RBC clumped to the root surface (data not shown). RBC release was a little higher in the seedlings germinated and grown 72 h in the presence of Al without removal of the RBC than in those transferred to the Al for 24 h after the RBC had been removed (Fig. 2a). On the other hand, the highest number of dead cells was detected in this experiment (Fig. 2b). The removal of RBC from the plants growing in Al further inhibited the release of RBC during the next 24 h (Fig. 2a). This much lower release of the RBC and their simultaneously lower viability enhanced the mortality index of the RBC in this experiment (Fig. 2c).
Relative amount of RBC (a), relative amount of dead RBC (b) released from barley root tips and the mortality index (c). See caption of Fig. 1 for definitions
Oxalate oxidase and guaiacol peroxidase activities and the production of H2O2 in the RBC increased in all of the Al-treated root tips relative to those of the control plants. Following the removal of the original RBC, new ones developed in the presence of Al which had a higher activity of these enzymes and also an elevated production of H2O2 relative to those observed in the original RBC that had not been removed (Fig. 3). With respect to peroxidase activity, similar results were obtained in experiments in which the washing fluid (extracellular enzymes) was analyzed after removal of the RBC by centrifugation (Fig. 4b); however no difference between the control plants and Al-treated plants was detected with respect to oxalate oxidase activity (Fig. 4a). A significant increase in the H2O2 content of the washing fluid was detected only in experiments in which the plants grew 72 h in the presence of Al with the removal of RBC after 48 h (Fig. 4c). Using NADH oxidase and peroxidase inhibitors, we demonstrated that in addition to oxalate oxidase, peroxidase—but not NADH oxidase—was responsible for the Al-induced H2O2 production that occurred in the RBC and in the rhisosphere (Fig. 5).
Oxalate oxidase (a) and guaiacol peroxidase (b) activities and the production of H2O2 (c) by RBC of barley root tips. See caption of Fig. 1 for definitions
Oxalate oxidase (a) and guaiacol peroxidase (b) activities and the production of H2O2 (c) by extracellular enzymes. See caption of Fig. 1 for definitions
Discussion
The root cap covered by mucilage has long been considered to function as a shield protecting the root tip from extracellular biotic and abiotic stresses. RBC, previously denoted sloughed root cap cells, were initially regarded only as a by-product of the root cap. It is now well known that, following their segregation from the root cap, RBC have a specific function in sensing Al, secreting metabolites and enzymes and modifying the surroundings of the growing root (Hawes et al. 2003). In order to prevent the disruption of RBC from the area surrounding the root tip, we germinated and cultivated the barley seedlings between two layers of filter paper. However, due to the high binding capacity of the filter paper to Al, a millimolar concentration of Al was needed to attain a range of root growth inhibition similar to the micromolar concentration of Al found under hydroponic conditions (Tamás et al. 2004b). The actual Al concentration affecting the roots when 2 mM Al was applied to the filter paper was approximately 85 μM (Šimonovičová et al. 2004).
It is this concentration of Al that is used to inhibit root growth in hydroponics; however, it is still very toxic for barley and causes a nearly complete inhibition of root growth under hydroponic conditions (Tamás et al. 2004b). Our results suggest that the presence of RBC around the root tip effectively protects the barley root tip from Al compared to cultivation in hydroponics. The removal of RBC from Al-treated roots increased the inhibition of root growth, Al and Evans blue uptake, the inhibition of RBC production, the amount of dead RBC, peroxidase and oxalate oxidase activities and the production of H2O2 compared to Al-treated roots with RBC. In cereals and legumes, the removal of RBC induces the generation of several hundred new RBC together with cap-secreted mucilage within 1 h, and within 24 h there is a new RBC/mucilage capsule (Hawes et al. 2003). Consequently, no differences were detected between experiments using control roots with or without removal of the RBC after 24 h (data not shown). Zhu et al. (2003) also observed that the removal of RBC from root tips enhanced Al-induced root growth inhibition and also Al accumulation in an Al-sensitive or Al-resistant wheat cultivar.
Detached RBC surrounding the root cap are the first living layer of cells that come into the contact at first with toxic Al during root growth. Our results show that short-term (24 h) exposure to Al did not inhibit root growth, although it did increase the number of dead RBC several fold. It was probably during this short time that RBC effectively detoxified Al by its rapid uptake, which subsequently activated the production of AOS and death of the RBC. We also observed that RBC were very sensitive in vitro to Al and that they were killed by the 20-h treatment in the presence of 25 μM Al in solution (Zhu et al. 2003). A similar suicidal process of Al tolerance was described by Delisle et al. (2001) based on an accelerated turnover of root epidermal cells. This epidermal cell death was specific only for an Al-tolerant wheat cultivar and probably correlated with an elevated production of AOS through the activation of extracellular oxalate oxidase.
The removal of the RBC before Al was applied to the root tip or a longer exposure to Al inhibited the production and release of RBC from the root tip. The production and release of new RBC were disrupted by the Al-induced inhibition of pectin methylesterase activity (Pan et al. 2004). This was accompanied by a higher production of AOS and by appearance of clumps of dead RBC on the surface of the root tip. This high AOS production may affect deeper cell layers and activate their cell death. As a result of these processes Al can penetrate deeper into the root tissue, thereby causing death of whole root tip by induction of oxidative stress. On the other hand—at least at the beginning and at low Al concentrations—this elevated production of H2O2 by RBC around the root tip can serve as a signal molecule and activate the antioxidant root defense system. It is well documented that H2O2 as moderately reactive and relatively long-lived molecule is involved in several signal transduction processes activated by both biotic and abiotic stresses and recognized by plants as a signal for triggering the defense response (Vranová et al. 2002). In tobacco cell culture, intracellular Al tolerance is associated with an elevated antioxidant system (Devi et al. 2003).
Both oxalate oxidase and peroxidase have been described as enzymes involved in the generation of H2O2 in extracellular spaces during the oxidative burst (Bolwell and Wojtaszek 1997). Our results demonstrate the involvement of both enzymes in the generation of H2O2 in the rhizosphere of barley root tips by RBC. In addition, peroxidase is also secreted from RBC during Al treatment. The presence of great numbers of dead RBC during Al stress indicates the role of H2O2 in the signal transduction pathway leading to the oxidative burst. Our results suggest that even though RBC actively produce AOS during Al stress, they are involved in the protection of root tips against toxic Al simply by chelating Al in their dead cell body.
Abbreviations
- AOS:
-
Active oxygen species
- DPI:
-
Diphenyleneiodonium chloride
- RBC:
-
Root border cells
References
Baker CJ, Mock NM (1994) An improved method for monitoring cell death in cell suspension and leaf disc assays using Evans blue. Plant Cell Tissue Organ Cult 39:7–12
Bolwell GP, Wojtaszek P (1997) Mechanisms for the generation of reactive oxygen species in plant defence—a broad perspective. Physiol Mol Plant Pathol 51:347–366
Boscolo PRS, Menossi M, Jorge RA (2003) Aluminum-induced oxidative stress in maize. Phytochemistry 62:181–189
Bradford M (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Chance B, Maehly AC (1955) Assay of catalases and peroxidases. In: Colowick SP, Kaplan NO (eds) Methods in enzymology, vol 2. Academic Press, New York, pp 764–775
Delisle G, Champoux M, Houde M (2001) Characterization of oxalate oxidase and cell death in Al-sensitive and tolerant wheat roots. Plant Cell Physiol 42:324–333
Devi SR, Yamamoto Y, Matsumoto H (2003) An intracellular mechanism of aluminum tolerance associated with high antioxidant status in cultured tobacco cells. J Inorg Biochem 97:59–68
Exley C (2004) The pro-oxidant activity of aluminum. Free Radic Biol Med 36:380–387
Hawes MC, Bengough G, Cassab G, Ponce G (2003) Root caps and rhizosphere. J Plant Growth Regul 21:352–367
Ishida A, Ookubo K, Ono K (1987) Formation of hydrogen peroxide by NAD(P)H oxidation with isolated cell wall-associated peroxidase from cultured liverwort cells, Marchantia polymorpha L. Plant Cell Physiol 28:723–726
Kobayashi Y, Yamamoto Y, Matsumoto H (2004) Studies on the mechanism of aluminum tolerance in pea (Pisum sativum L.) using aluminum-tolerant cultivar ‘Alaska’ and aluminum-sensitive cultivar ‘Hyogo’. Soil Sci Plant Nutr 50:197–204
Kochian LV (1995) Cellular mechanisms of aluminum toxicity and resistance in plants. Annu Rev Plant Physiol Plant Mol Biol 46:237–260
Liszkay A, Kenk B, Schopfer P (2003) Evidence for the involvement of cell wall peroxidase in the generation of hydroxyl radicals mediating extension growth. Planta 217:658–667
Matsumoto H (2000) Cell biology of aluminum toxicity and tolerance in higher plants. Int Rev Cytol 200:1–46
Meriga B, Reddy BK, Rao KR, Kishor PBK (2004) Aluminium-induced production of oxygen radicals, lipid peroxidation and DNA damage in seedlings of rice (Oryza sativa). J Plant Physiol 161:63–68
Miyasaka SC, Hawes MC (2001) Possible role of root border cells in detection and avoidance of aluminum toxicity. Plant Physiol 125:1978–1987
Nagy NE, Dalen LS, Jones DL, Swensen B, Fossdal CG, Eldhuset TD (2004) Cytological and enzymatic responses to aluminum stress in root tips of Norway spruce seedlings. New Phytol 163:595–607
Ownby JD (1993) Mechanism of reaction of hematoxylin with aluminium-treated wheat roots. Physiol Plant 87:371–380
Pan J, Zhu M, Chen H (2001) Aluminum-induced cell death in root-tip cells of barley. Environ Exp Bot 46:71–79
Pan JW, Ye D, Wang LL, Zhao GF, Pan WH, Han N, Zhu MY (2004) Root border cell development is a temperature-insensitive and Al-sensitive process in barley. Plant Cell Physiol 45:751–760
Richards KD, Schott EJ, Sharma YK, Davis KR, Gardner RC (1998) Aluminum induces oxidative stress genes in Arabidopsis thaliana. Plant Physiol 116:409–418
Samac DA, Tesfaye M (2003) Plant improvement for tolerance to aluminum in acid soils—a review. Plant Cell Tissue Organ Cult 75:189–207
Schopfer P, Plachy C, Frahry G (2001) Release of active oxygen intermediates (superoxide radicals, hydrogen peroxide, and hydroxyl radicals) and peroxidase in germinating radish seeds controlled by light, gibberillin, and abscisic acid. Plant Physiol 125:1591–1602
Šimonovičová M, Huttová J, Mistrík I, Široká B, Tamás L (2004) Root growth inhibition by aluminum is probably caused by cell death due to peroxidase-mediated hydrogen peroxide production. Protoplasma 224:91–98
Tamás L, Huttová J, Mistrík I (2003) Inhibition of Al-induced root elongation and enhancement of Al-induced peroxidase in Al-sensitive and Al-resistant barley cultivars are positively correlated. Plant Soil 250:193–200
Tamás L, Šimonovičová M, Huttová J, Mistrík I (2004a) Aluminum stimulated hydrogen peroxide production of germinating barley seeds. Environ Exp Bot 51:281–288
Tamás L, Šimonovičová M, Huttová J, Mistrík I (2004b) Elevated oxalate oxidase activity is correlated with Al-induced plasma membrane injury and root growth inhibition in young barley roots. Acta Physiol Plant 26:85–93
Vranová E, Inzé D, Van Breusegem F (2002) Signal transduction during oxidative stress. J Exp Bot 53:1227–1236
Yamamoto Y, Kobayashi Y, Devi SR, Rikiishi S, Matsumoto H (2002) Aluminum toxicity is associated with mitochondrial dysfunction and the production of reactive oxygen species in plant cells. Plant Physiol 128:63–72
Yamamoto Y, Kobayashi Y, Devi SR, Rikiishi S, Matsumoto H (2003) Oxidative stress triggered by aluminum in plant roots. Plant Soil 255:239–243
Zhang Z, Yang J, Collinge DB, Thordal-Christensen H (1996) Ethanol increases sensitivity of oxalate oxidase assays and facilitates direct activity staining in SDS gels. Plant Mol Biol Rep 14:266–272
Zhu M, Ahn S, Matsumoto H (2003) Inhibition of growth and development of root border cells in wheat by Al. Physiol Plant 117:359–367
Acknowledgements
This investigation was supported by the Science and Technology Assistance Agency under the contract No. APVT-51-001002
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by L. Peña
Rights and permissions
About this article
Cite this article
Tamás, L., Budíková, S., Huttová, J. et al. Aluminum-induced cell death of barley-root border cells is correlated with peroxidase- and oxalate oxidase-mediated hydrogen peroxide production. Plant Cell Rep 24, 189–194 (2005). https://doi.org/10.1007/s00299-005-0939-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-005-0939-7