Abstract
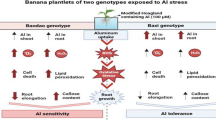
Aluminum is among the prominent restricting factor accountable for the growth of plants in acidic soils pertaining to its solubility. The absorption of aluminum (Al3+) by rice roots was examined in this work using seedlings from two cultivars (tolerant and sensitive) treated with AlCl3 (1 mM) in fresh sand culture. The progression of Al through the roots was seen using hematoxylin and Eriochrome cyanine R staining. When compared to the sensitive cultivar, the tolerant cultivar has a reduced Al absorption rate. It shows the presence of some extrinsic mechanisms that control the tolerant rice cultivar’s behavior. The increase in Evans blue absorption after Al3+ treatment indicated root cell injury, with more apparent uptake in sensitive cultivars than tolerant cultivars. Aluminum fluorescence intensity increased with Morin staining in sensitive than tolerant cultivars, according to confocal microscopy. The results presented here confirmed that the increased accumulation of Al3+ leads to reduced rice seedlings growth and increased invertase activity, callose accumulation, and cell death. It also indicates that metabolic procedures and the transduction of the signals contributing to increased invertase movement help the tolerant variety maintain higher root development in harmful Al3+ concentrations.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
One of the foremost contributors and constraints contributing to crop growth in acidic soils is aluminum (Al) phytotoxicity that accounts for 40 % of arable land (Sade et al. 2016). This phytotoxicity is found to be released in big quantities at low pH (pH 5.0 or less). This led to damage to overall root structure, growth, and thus yield. Al has a complex chemical structure as well as biological activities (Kochian et al. 2005). The pH scale of the soil determines the Al that is available in the soil. The structure is octahedral hexahydrate, Al (H2O)63+, at low pH (about 4.3), and is sometimes abbreviated as Al3. The problem of Al3+ toxicity is multifarious through soil acidification caused due to inconsistent usage of ammonia as well as fertilizers constituting amides. Nitrogen fixation in legumes, an imbalance between nitrogen and sulfur (Guo et al. 2010), and absorption of excess cations and anions also contribute to this acidification. Al is not just phytotoxic but inhibits plant growth too. Besides, it also harms human health because it is passed down the food chain from animals to people (Jaishankar et al. 2014). The key indication of toxicity caused by Al is suppression in the growth of the roots that usually happens as soon as 1 or 2 h after Al exposure (Horst et al. 2010). This root growth suppression results in a thin, thickened, diminished, and uneven root system (Ciamporova 2002). The variants that are Al sensitive tend to gather additional Al within the root tissue than Al tolerant. For example, Al-sensitive cultivars like maize, rice, wheat, and soybean displayed more Al contents in their root tips than the resistant version (Du et al. 2010; Kang et al. 2011; Matonyei et al. 2014; Garcia-Oliveira et al. 2016).
The progression and build-up of the Al in the root tissues could be measured using a variety of methods. The employment of Al-specific dyes is one of them. This build-up is required to be investigated for which staining techniques are often quick and easy. One of the most common methods is the comparison of the length and weight of the roots of the plants affected with or without Al. Nonetheless, this method is not ideal for the large population as there are significant variations in root length, and this process takes time also. Because of these drawbacks, a quick method, staining with hematoxylin , was discovered that utilizes the coloring formation when the Al-induced root with the stain (Polle et al. 1978). A few researchers have used this method to screen Al resistance phenotypes in various crop species, such as maize (Lidon et al. 2007), pea (Singh et al. 2009), and chickpea (Singh and Chaturvedi 2007). Another method, staining with Eriochrome cyanine R , enables the visualization of the Al across the root. In this, the roots damaged because of Al were deeply stained (pink) despite non-damaged roots remaining unstained (white). This method is suitable for assessing large numbers of plants at the same time. Evans blue staining is considered as an index of damages caused by plasma membranes and programmed cell death in plants (Yamamoto et al. 2001). Minute concentrations of Al could be identified using fluorophores like Morin using fluorescence microscopy (Eticha and Horst 2005). The detection limit has been identified as 2 × 10−9 M. (Lian et al. 2003).
Along with the inhibition of Al-induced growth of the root, the induction of callose formation was observed in several plant species as a physiological pointer to the Al sensitivity (Jones et al. 2006). Different stressors are identified as the triggering agent for callose synthesis whose in-depth methodology is unknown but compromises the plasma membrane’s integrity and function (Chen and Kim 2009). In biotic and abiotic stress conditions, an increase in cytosolic calcium (Ca2+) activity synthesizes the β-D-glucan in plants (Bhuja et al. 2004). Al also causes a transient upsurge in cytosolic Ca2+, according to Bhalerao and Prabhu (2013).
In abundance, metals such as Cd2+, Ni2+, Al3+, and As3+ induce toxicity in growing plants and also influence the activity behavior of various carbohydrate metabolism-related enzymes (Verma and Dubey 2001; Mishra and Dubey 2013; Jha and Dubey 2004; Mishra and Dubey 2008; Abbas et al. 2018). The main photosynthetic end products are starch and sugar, and their levels inside plants give important plant production information. Under diverse stress conditions, starch and sugar storage, translocation, and metabolism are all impacted. The metabolism of sucrose is critical for energy production and plant growth under stressful conditions (Harada et al. 2005). Invertases are found in enzymes that regulate the sucrose content in plants in acidic, alkaline, and neutral forms (Tauzin and Giardina 2014). Sucrose-aided root cell division, elongation, and expansion. As a result, Al3+-induced changes in invertase activity are probably part of a cell-wall-based toxicity resistance mechanism to aluminum. The most Al-resistant cereal crop is rice which uses several methods to achieve high Al resistance. Little is known on the mechanism of increase in Al tolerance in rice (Famoso et al. 2010). It promoted us to use rice as a model plant to examine the mechanism of Al tolerance.
To explore this phenomenon in detail, two rice cultivars have been used, namely, Malviya-36 as the Al-sensitive one and Vandana as the Al-tolerant one. It has been observed that distinct Al3+ concentration in roots generally leads to varying tolerance levels, and thus, invertase activity and callose accumulation have been examined as disclosed by Morin stain.
The selection of appropriate cultivars is essential for the study of tolerance mechanisms. For this, the sensitivity and tolerance mechanism of both cultivars was confirmed using Eriochrome cyanine R staining . Al uptake in roots is seen by hematoxylin staining in different cultivars to separate the degree of participation for exploring the tolerance mechanism at the external as well as internal levels.
2 Materials and Methods
2.1 Plant Material and Treatment Levels
Malviya-36 and Vandana are the two variants of Indica rice (Oryza sativa L.) cvs. seeds that are used for conducting the experimental work. After a 24-hour inhibition in water, seeds are wet with 0.5 mM CaCl2 solution and kept at 28±1°C with 80 % relative humidity in the BOD incubator cum humidity (York Scientific Industries, New Delhi, India) for 5 days. Seedlings subsequently were elevated in purified quartz sea sand, and Yoshida nutrient solution is used for the next 10 days for saturation (Yoshida et al. 1976). Further, the uprooted seedlings were treated with AlCl3 (1mM) for the next 2 days in the presence of fresh and sand cultures.
2.2 The Impact of Morin Staining in the Localization of Al in Roots
Morin staining as defined by Tice et al. (1992) has been utilized in the current work. The roots were initially washed for 10 min in the presence of a buffer, NH4OAc having a pH of 5.5, followed by staining by 100 μM for 1 hour in the same buffer. Till the elution of the stains, washing will be continued. The lateral segments of the roots were cut and then observed through a microscope coupled with fluorescence at an excitation and an emission wavelength of 420 and 510 nm, respectively, until it fluoresced green.
2.3 Hematoxylin Staining in the Detection of the Accumulated Al in Root Tips
Hematoxylin staining (Ownby 1993) has been utilized for visualizing the deposition of the Al in the roots of sensitive as well as tolerant variants of the cultivars. The roots were initially washed for 15 min followed by staining at room temperature. Staining is done using a solution of 0.2% hematoxylin (w/v) and 0.02% KIO3 (w/v). Further, distilled water is used for washing it for 15 min, and then photographs were taken. A spectrophotometer (Bausch and Lomb, Spectronic 20, USA) was utilized for quantifying the optical density of the stains at 490 nm on 15 root tips of 5 mm each after 1ml of 1 M HCL treatment for 1 hour.
2.4 Determination of Loss of Plasma Membrane Integrity
The loss of integrity of the plasma membranes has been determined using the modified Evans blue staining method as defined by Schützendübel et al. (2001). The staining of the seedlings was conducted for half an hour with a 0.25% (w/v) aqueous solution. The washed cross-sectional root tips with distilled water were utilized for viewing through the light microscope. To determined Evans Blue Uptake, stained cross-sections roots of 10 mm length were homogenized in 1 ml of 1% (w/v) aqueous sodium dodecyl sulfate (SDS) followed by centrifugation at 13,500 × g for 10 min at room temperature. Measure the optical density of the supernatant spectrophotometrically at 600 nm.
2.5 Eriochrome Cyanine R Staining
To confirm the sensitivity and tolerance of selected rice cultivars toward Al toxicity, Eriochrome cyanine R staining was used (Aniol 1995). Seedlings were removed and placed in distilled water for 30 min after 48 h of Al treatment. One-tenth of an aqueous solution of Eriochrome cyanine R was used to stain the roots for 10 min. The surplus dye was detached by several washes with distilled water, and then photographs were taken with a high-resolution camera.
2.6 Histochemical Detection of Callose in Root Tissues
The Al3+-induced callose accumulation detection has been conducted using aniline blue staining in the current work. The roots are secured for 1 h using 10% ethanol (FAA) and formaldehyde and 5 % glacial acetic acid. The top 1 cm root tip was cut and cleaned using de-ionized water and stained for 10 min (Kauss 1992). One-tenth of water-soluble aniline blue is applied to 50 mM glycine-NaOH buffer with a pH of 9.5 and then explored using a fluorescence microscope.
2.7 Measurement of Callose Content
The calculation of callose content has been done according to Bhuja et al. (2004). A total of 25 root segments were taken and washed in ethanol for 30 min followed by homogenization in 1M NaOH. The samples were incubated at 80 °C for 20 min and further centrifuged at 12,000 × g for 5 min. The resultant was further treated to the following combination.
-
50 μl of supernatant along with 100 μl of 0.1% aniline blue
-
50 μl of 1M HCl
-
150 μl of glycine-NaOH buffer pH 9.5
Afterward, incubation for 20 min at 50 °Cand 30 min at room temperature fluorescence signal recorded using excitation at 380 nm (filter 530/20) and detection of fluorescence at 485 nm (filter 485/20).
2.8 Analysis of Invertase Activity
A total of six seedlings of each Al3+ exposed cultivar is incubated for 3 h to detect invertase activity. These seedlings were treated to a neutral reaction medium buffer at room temperature; after which, photographs were captured using Olympus light microscope. The buffers (Doehlert and Felker 1987; Zrenner et al. 1995) consisted of the following solutions:
-
0.38 mM sodium phosphate with pH of 7.5
-
0.024% tetrazolium blue
-
0.014% phenazine metosulfate
-
30 U of glucose oxidase
-
30 mM of sucrose
-
For control, incubation medium without sucrose and Glucose oxidase
2.9 Statistical Analysis
The experimentations were repeated thrice, and the data was used as the mean of the standard deviation of the obtained replicates. Analysis of variance (ANOVA) is used for obtaining the mean difference of the control and treatment groups. The significance of this difference is represented as * for p≤0.05 and ** for p≤0.01. Further, Tukey’s multiple range test has been used.
3 Results
3.1 The Impact of Morin Staining in the Localization of Al in Roots
Morin (3,5,7,2′,4′-pentahydroxyflavone) is an Al-specific fluorescent dye that constitutes a fluorescent complex when combined with Al. The greater the Al accumulation, the brighter the fluorescent light. An intense green Morin fluorescence was seen in the root section, whereas there was almost little fluorescence in control (–Al) plants (Fig. 4.1). It was observed that low fluorescence was found in roots stained with Morin that was unexposed to Al and whatever fluorescence was seen was confined to the epidermal surface. When compared to sensitive roots, tolerant cultivar root cross-sections revealed significantly less Morin staining.
(a) Fluorescence detection of Al in rice root tips after Morin (100 μM) staining in roots of Malviya-36 and Vandana seedlings. The Seedlings were grown for 10 days and then uprooted followed by treatment either in Yoshida nutrient solution (control, without Al3+, denoted as A, C) or in nutrient solutions containing 1mM Al3+ (B, D) for 48 h. (b) The lateral roots of the seedlings obtained from matured segments of the primary roots
3.2 Determination of Aluminum Uptake
A hematoxylin stain was utilized to investigate the uptake of Al in the seedlings’ roots. When the roots of cvs. Malviya-36 and Vandana seedlings are stained with the Al-specific dye hematoxylin, it is detected that the roots of Al-treated seedlings of Malviya-36 take up a greater amount of stain than tolerant cv. Vandana (Fig. 4.2). Though there was no reaction with Al3+, the hematoxylin stain was absorbed by control roots, and thus dull redness is visible. Spectrophotometric analyses revealed that dye uptake was increased by four- and twofold in roots of 1mM Al3+-treated seedlings in cv. Malviya-36 and cv. Vandana, respectively, as compared to controls.
(a) Microscopic view of hematoxylin-stained roots of Malviya-36 and Vandana seedlings. The seedlings were grown in sand cultures for 10 days followed by its treatment in either Yoshida nutrient solution (control, without Al3+, denoted as A) or in nutrient solutions containing 1mM Al3+ (B) for 48 h. (b) The exposure of hematoxylin staining resulted in the accumulation of Al in root tips (5 mm). The resultant value after three independent values is mean ± SD (standard deviation depicted by bars). As per Turkey’s test, * and ** depict the differences as per controls at p ≤ 0.05 and p ≤ 0.01, respectively
3.3 The Damage in a Membrane Formed Due to Al in Root Tips
Evans blue has been utilized as a marker for cell death as well as for identifying the loss in the integrity of the plasma membrane. An increase of Evans blue absorption was detected in comparison to controls for Al-treated rice seedlings. Evans blue stain uptake increased significantly (p ≤ 0.01) in the roots of both rice cultivars as the concentration of Al3+ treatment increased, with notably higher uptake in the Al sensitive cultivar than the tolerant cultivar (Fig. 4.3). Even in controls, the stain taken by roots of cv. Malviya-36 seedlings were higher than cv. Vandana. The findings make a strong point regarding the increased permeability of the plasma membranes after treating them with Al.
(a) Representation of loss of plasma membrane in root tips of Malviya-36 and Vandana seedlings through histochemical detection. The seedlings were grown in sand cultures for 10 days followed by its treatment in either Yoshida nutrient solution (control, without Al3+, denoted as A) or in nutrient solutions containing 1mM Al3+ (B) > for 48 h. (b) The loss of plasma membrane integrity after exposure of dye Evans’s blue is depicted. The resultant value after three independent values is mean ± SD (standard deviation depicted by bars). As per Turkey’s test, * and ** depict the differences as per controls at p ≤ 0.05 and p ≤ 0.01, respectively
3.4 Eriochrome Cyanine R Staining
When cultivars were kept for 12 days with or without nutrient solution constituting 1mM AlCl3 in the growth medium followed by staining with Eriochrome cyanine R; depicted that the stain taken up by roots of treated seedlings of Malviya-36 was substantially greater than that of Vandana (Fig. 4.4). Control grown seedlings of any cultivar show no stain in their roots.
Pictures showing (a) tip regions and (b) middle regions of main roots after Eriochrome cyanine R staining in Malviya-36 and Vandana seedlings. The seedlings were grown in sand cultures for 10 days followed by its treatment in either Yoshida nutrient solution (control, without Al3+, denoted as A, C, E, G) or in nutrient solutions containing 1mM Al3+ (B, D, F, H). > for 48 h
3.5 Callose Accumulation
The production of callose has been regarded as a measure for determining the level of Al damage to the treated plants. Aniline blue staining and fluorescence microscopy are used to visualize callose formation. Callose exhibited bright green-yellowish spots under the fluorescence microscope, whereas the initial fluorescence observed was vivid blue (Fig. 4.5). The sensitive type root tips that had not been treated fluoresced faintly, and those exposed to Al fluoresced brightly, indicating callose accumulation. In the presence of Al, callose accumulation in the tolerance cultivar decreased considerably to levels somewhat higher than those reported in the untreated controls, compared to the sensitive variety. When dye uptake for callose accumulation was compared to the control in roots of 1mM, Al3+-treated seedlings, dye uptake for callose accumulation was increased by 5.7- and 4 fold in cv. Malviya-36 and cv. Vandana, respectively, implying callose induction to be an early and robust Al stress indicator.
(a) The representation of callose localization on exposure of aniline blue staining in root tips of Malviya-36 and Vandana seedlings. Seedlings were grown in sand cultures for 10 days followed by its treatment in either Yoshida nutrient solution (control, without Al3+, denoted as A) or nutrient solutions containing 1mM Al3+ (B) for 48 h. (b) The quantification of the root tips grown after control and the seedlings after treatment with Aniline blue stained was recoded. The resultant value after three independent values is mean ± SD (standard deviation depicted by bars). As per Turkey’s test, * and ** depict the differences as per controls at p ≤ 0.05 and p ≤ 0.01, respectively
3.6 Invertase Activity Assay
Tolerant roots (Vandana) exhibited strong activity (cellular divisions in the basal part and more noticeable in the apex) and invertase expression after its exposure to 1mM AlCl3 for 2 days (Fig. 4.6). When compared to tolerant roots (Malviya-36), invertase activity was lower in sensitive roots (Malviya-36) (Fig. 4.6).
(a) Tip regions and (b) middle regions of primary roots showing invertase activity staining patterns in Malviya-36 and Vandana seedlings. The seedlings were grown in sand cultures for 10 days followed by its treatment in either Yoshida nutrient solution (control, without Al3+, denoted as A, C, E, G) or in nutrient solutions containing 1mM Al3+ (B, D, F, H). > for 48 h
4 Discussion
For a long time, aluminum has been regarded as one of the key problems restricting agricultural output around the world. A major portion of the arable land across the world is acidic with significant Al toxicity. A vast area of such land lies in developing countries. Al was, therefore, a potential threat to these countries’ development. It is imperative to choose and breed crops for Al resistance to a fast, reliable screening system to discriminate between sensitive and tolerant genotypes. Plants have built up a few techniques to deal with Al toxicity. Organic acid exudation was first detected as helping to protect snap beans against Al toxicity (Miyasaka et al. 1991). It was found that the cultivars that are Al resistant emit almost eightfold citrate than the sensitive ones in a long run. Despite several attempts in past, most of the works could not justify the tolerance of rice to Al toxicity. Another notion of rice resistance was cell wall polysaccharides (Yang et al. 2008). As a result, it’s critical to understand its molecular and genetic mechanisms for Al tolerance.
The toxic symptoms of Al3+-treated roots in our research were essentially the same as those previously discovered (Alvarez et al. 2012). Sivaguru and Horst (1998) have reported the distal region to be more Al sensitive in the root apex. As a result, root length is the most reliable metric for determining Al3+ toxicity tolerance and sensitivity. In the present study, Al3+ inhibited root growth in both rice cultivars, with Malviya-36 being significantly more pronounced than Vandana. The use of a fluorochrome such as Morin (2,3,4,5,7-pentahydroxyflavone), which forms a fluorescent complex with aluminum (Al) (Eticha et al. 2005) and thus used for the detection of Al at the outside of roots with significant differences among cultivars, is the simplest and widely used method for Al localization. At low pH, it is found to be selective to Al and has a detection limit of 2 nM in vitro (Lian et al. 2003). Al3+ works as a mordant in hematoxylin staining, binding to oxidized hematoxylin (hematein) and forming a colorful complex between hematoxylin and root-bound Al (Polle et al. 1978). In comparison to cv. Malviya-36, hematoxylin staining revealed decreased uptake of the Al by roots than that of Vandana seedlings. It was also found that Al content is more in the roots of Malviya-36 than that of Vandana. It was also observed that the Al content amplified with rise in the concentration of Al treatment as well as its exposure. Vandana’s roots absorb less Al, indicating that this cultivar benefits from an external resistance mechanism. Alike outcomes were conveyed for hematoxylin staining in rice (Pandey et al. 2015; Rosello et al. 2015), wheat (Shao et al. 2015), and chickpea (Sharma et al. 2015). The damage in the Al-induced membrane as observed using Evans’s blue could be attributed to prolonged exposure that resulted in mechanical disturbance of cells in the elongation zone. More exposure of these stains by roots of Al3+-treated saplings of sensitive cv. Malviya-36 suggests more damage and cell death in this cultivar than similarly stressed seedlings of tolerant cv. Vandana. In the roots of cv. Malviya-36, cell death paralleled with high Al-induced ROS production and severe oxidative stress which indicates that cell demise happened because of the damage caused by overproduced ROS in cells by Al3+ stress. According to Pan et al. (2001), increased ROS production could result in cell death, and higher Al concentrations could cause root cell necrosis. Even with modest Al toxicity treatment, a considerable increase in Evans blue dye absorption was observed in the prior study (Pandey et al. 2015). Similar findings have been found in maize (Wang et al. 2015), tobacco (Sivaguru et al. 2005), Melaleuca tree (Tahara et al. 2008), pea (Motoda et al. 2011), and wheat (Motoda et al. 2011). (Aggarwala et al. 2015). Evans blue uptake by roots of barley seedlings was also seen by Zelinová et al. (2011), even at low Al concentrations that did not hinder root growth. Al absorption and cell death in root tips are not only a substantial result of Al-induced oxidative stress, but they also serve as one of the Al tolerance mechanisms (Giannakoula et al. 2010). Current work incorporates Eriochrome cyanine R for classifying Al sensitivity and rice cultivar tolerance by showing a much higher stain uptake in roots of Al3+-treated seedlings of cv. Malviya-36 than cv. Vandana. The root section formed following Al treatment was white (unstained) when Al treatment did not harm the root apical meristem, whereas roots impacted by Al treatment were intensely pink stained (Aniol 1995). This method takes only 10 min to stain and was appropriate for assessing large numbers of plants at once. This rapid staining method has been used to screen Al tolerance in wheat, barley, and rye cultivars (Wang et al. 2006; Ma et al. 2004; de Sousa et al. 2016).
Aluminum-induced callose seems to be a physiological marker for Al-induced injury. Callose builds up in the cell wall around plasmodesmata in response to the Al damage within the roots. Increased Al concentration from 0 to 100 mM could result in an enlarged accumulation of callose (Larsen et al. 1996). The lack of callose deposition suggests that there are more primary mechanisms of Al3+ resistance that could activate sooner than callose synthesis.
Thus, callose deposition could not be attributed as a primary resource for averting Al3+ penetration. Though studies reported (Sivaguru et al. 2000; Wissemeier and Horst 1995) callose to be a cell-to-cell inhibitor and Al-induced callose formation in some cultivars root tips, the current study has found callose formation in newly root tips of higher Al concentration. Increased Al tolerance is linked to lower callose formation and lower oxidative stress in transgenic plants. Callose deposition and accumulation especially in aluminum-sensitive cultivars reflect physiological stress and the degree of cumulative cell damage.
The generation of hexoses is maximized by cell wall and vacuolar invertases, which enhance cell respiration, division, and growth (Koch 2004). When compared to Malviya-36, Vandana roots have more invertase activity, which could indicate alterations in primary metabolism and root cell proliferation, as well as a resistance mechanism.
Past studies revealed that increased sucrose activity in rice could lead to hexose accumulation under Al toxicity. Simon et al. (1994) observed reduced invertase acid activity under Al3+. Similar observations were made where increase invertase activity has been observed under arsenic, Al, and Cd toxicity (Verma and Dubey 2001; Jha and Dubey 2004; Shahnawaz et al. 2017) and salinity and cold stress (Mishra and Dubey 2008; Livingston and Hensen 1998).
The current results demonstrated better and improved discriminating Al susceptibility in variants of cultivars using several staining techniques. Thus, the hypothesis taken has confirmed that high Al concentration results in reduced growth and callose accumulation, associated with invertase activity and cell deaths. It is clear that improvised root growth at toxic Al3+ concentrations is accompanied by increased invertase activity, and thus better understanding of expression and activities in root regions could be made at apoplastic and symplastic root regions.
References
Abbas G, Murtaza B, Bibi I, Shahid M, NiaziN, Khan M, Amjad M, Hussain M (2018) Arsenic uptake, toxicity, detoxification, and speciation in plants: physiological, biochemical, and molecular aspects. Int J Environ Res Public Health. 15(1):59.
Aggarwala A, Ezakib B, Tripathi BN (2015) Two detoxification mechanisms by external malate detoxification and anti-peroxidation enzymes cooperatively confer aluminum tolerance in the roots of wheat (Triticum aestivum L.). Environ Exp Bot 120:43–54
Alvarez I, Sam O, Reynaldo I, Testillano P, Risueno MC and Arias M (2012) Morphological and cellular changes in rice roots (Oryza sativa L.) caused by Al stress. Bot. Studies 53”67–73
Aniol AM (1995) Physiological aspects of aluminium tolerance associated with the long arm of chromosome 2D of the wheat (Triticum aestivum L.) genome, Theoretical and Applied Genetics 91:510–516
Bhalerao SA, Prabhu DV (2013) Aluminium toxicity in plants: a review. J Applicable Chem 2(3):447–474
Bhuja P, McLachlan K, Stephens J, Taylor G (2004) Accumulation of 1,3-beta-Dglucans, in response to aluminum and cytosolic calcium in Triticum aestivum. Plant Cell Physiol 45:543–549
Chen XY, Kim JY (2009) Callose synthesis in higher plants. Plant Signal Behav 4(6):489–492. https://doi.org/10.4161/psb.4.6.8359
Ciamporova M (2002) Morphological and structural responses of roots to aluminium at organ, tissue and cellular levels. Biol Plant 45:161–171
de Sousa A, AbdElgawad H, Han A, Teixeira J, Matos M, Fidalgo F (2016) Oxidative metabolism of rye (Secale cereale L.) after short term exposure to aluminum: uncovering the glutathione–ascorbate redox network. Frontiers in plant science 7:685
Doehlert DC, Felker FC (1987) Characterization and distribution of invertase activity in developing maize (Zea mays) kernels, Physiol Plant 70:51–57
Du B, Nian H, Zhang Z (2010) Effects of aluminum on superoxide dismutase and peroxidase activities, and lipid peroxidation in the roots and calluses of soybeans differing in aluminum tolerance. Acta Physiol Plant 32: 883. https://doi.org/https://doi.org/10.1007/s11738-010-0476-z
Eticha D, Stass A, Horst WJ (2005) Localization of aluminium in the maize root apex: can Morin detect cell wall-bound aluminium? J Exp Bot 56:1351–1357
Famoso A, Clark R, Shaff J, Craft E, McCouch SR,Kochian LV (2010) Development of a novel Aluminum tolerance phenotyping platform used for comparisons of cereal Aluminum tolerance and investigations into rice Aluminum tolerance mechanisms, Plant Phy 153:1678
Garcia-Oliveira A, Martins-Lopes P, Tolrà R, PoschenriederC, Guedes-Pinto H, Benito C (2016) Differential physiological responses of Portuguese bread wheat (Triticum aestivum L.) genotypes under aluminium stress. Diversity 8(4):26
Giannakoula A, Moustakas M, Syros T, Yupsanis T (2010) Aluminum stress induces up-regulation of an efficient antioxidant system in the Al-tolerant maize line but not in the Al-sensitive line, Environ Exp Bot 67:487–494
Guo JH, Liu XJ, Zhang Y, Shen JL, Han WX, Zhang WF, Zhang FS (2010) Significant acidification in major Chinese croplands. Science 327(5968):1008–1010
Harada T, Satoh S, Yoshioka T, Ishizawa K (2005) Expression of sucrose synthase genes involved in enhanced elongation of pondweed (Potamogeton distinctus) turions under anoxia, Annalsof Bot 96:683–692
Horst WJ, Wang YX, Eticha D (2010) The role of the root apoplast in aluminium-induced inhibition of root elongation and in aluminum resistance of plants: a review. Annals of Bot 106:185–197
Jaishankar M, Tseten T, Anbalagan N, Mathew BB, Beeregowda KN (2014) Toxicity, mechanism and health effects of some heavy metals. Interdiscip Toxicol 7(2):60–72. https://doi.org/10.2478/intox-2014-0009
Jha AB, Dubey RS (2004) Carbohydrate metabolism in growing rice seedlings under arsenic toxicity, J Plant Physiol 161:867–872
Jones DL, Blancaflor EB, Kochian LV, Gilroy S (2006) Spatial coordination of aluminium uptake, production of reactive oxygen species, callose production and wall rigidification in maize roots. Plant, cell & environment 29(7):1309–1318
Kang DJ, Seo YJ, Futakuchi K, Vijarnsorn P, Ishii R (2011) Effect of aluminum toxicity on flowering time and grain yield on rice genotypes differing in Al-tolerance. J Crop Science and Biotech 14(4): 305–309
Kauss H (1992) Callose and callose synthase; in Molecular plant morphology: A practical approach, In: (Eds) Gurr, SJ, McPherson, MJ and Bowles, DJ, (Oxford University Press) 1–8
Koch K (2004) Sucrose metabolism: regulatory mechanisms and pivotal roles in sugar sensing and plant development. Curr Op Plant Biol 7:235–246
Kochian LV, Pineros MA, Hoekenga OA (2005) The physiology, genetics and molecular biology of plant aluminum resistance and toxicity, Plant Soil 274:175–195
Larsen PB, Tai CY, Kochian LV, Howell SH (1996) Arabidopsis mutants with increased sensitivity to aluminum. Plant Physiol 110:743–751
Lian H-Z, Kang Y-F, Bi S, Yasin A, Shao D-L, Chen Y-J, Dai L-M, Tian L-C (2003) Morin applied in speciation of aluminium in natural waters and biological samples by reversed-phase high performance liquid chromatography with fluorescence detection. Analytical and Bioanalytical Chem 376:542–548
Lidon FC, Azinheira HG, Barreiro MG (2007) Aluminum toxicity in maize: Biomass production and nutrient uptake and translocation. J Plant Nutr 23:361–368
Livingston DP, Henson CA (1998) Apoplastic sugars, fructans, fructan exohydrolase, and invertase in winter oat: responses to seconf phse cold hardening. Plant Physiology 116: 403–408
Ma JF, Shen R, Nagao S, Tanimoto E (2004) Aluminum targets elongating cells by reducing cell wall extensibility in wheat roots. Plant and Cell Physiol 45(5):583–589
Matonyei TK, Cheprot RK, Liu J, Piñeros MA, Shaff JE, Gudu S, Were B, Magalhaes JV,Kochian LV (2014) Physiological and molecular analysis of aluminum tolerance in selected Kenyan maize lines. Plant and soil 377(1–2):357–367
Mishra, P. & Dubey, R.S. Biometals (2013) 26: 97. https://doi.org/10.1007/s10534-012-9597-8
Mishra S, Dubey RS (2008) Effect of aluminium on metabolism of starch and sugars in growing rice seedlings, ActaPhysiologiaePlantarum 30(3):265–275
Miyasaka S, Bute J, Howell R, Foy C (1991) Mechanisms of aluminium tolerance in snapbeans, Root exudation of citric acid, Plant Physiol 96: 737–743
Motoda H, Kano Y, Hiragami F, Kawamura K, Matsumoto H (2011) Changes in rupture formation and zonary region stained with Evans blue during the recovery process from aluminum toxicity in the pea root apex. Plant signal Behav 6: 98–100. https://doi.org/10.4161/psb.6.1.14148
Ownby JD (1993) Mechanisms of reaction of hematoxylin with aluminium-treated wheat roots. Physiol Plant 87: 371–380
Pan JW, Zhu MY, Chen H (2001) Aluminum-induced cell death in root-tip cells of barley. Environ Exp Bot 46:71–79.
Pandey P, Srivastava RK, Rajpoot R, Rani A, Pandey AK, Dubey RS (2015) Water deficit and aluminum interactive effects on generation of reactive oxygen species and responses of antioxidative enzymes in the seedlings of two rice cultivars differing in stress tolerance. Environ Sci Poll Res 23(2):1516–1528
Polle E, Konzac CF, Kittrick JA (1978) Visual detection of aluminum tolerance levels in wheat by hematoxylin staining of seedling roots, Crop Science 18:823–827
Rosello M, Poschenrieder C, Gunsé B, Barceló J, Llugany M (2015) Differential activation of genes related to aluminium tolerance in two contrasting rice cultivars. J InorgBiochem 152:160–166. https://doi.org/10.1016/j.jinorgbio.2015.08.021
Sade H, Meriga B, Surapu V, Gadi J, Sunita MSL, Suravajhala P, KaviKishor PB (2016) Toxicity and tolerance of aluminum in plants: tailoring plants to suit to acid soils. Biometals 29:187–210
Schützendübel A, SchwanzP, Teichmann T, Gross K, Langenfeld-Heyser R, Godbold DL, Polle A (2001) Cadmium-induced changes in antioxidative systems, hydrogen peroxide content and differentiation in scot pine (Pinus sylvestris) roots. Plant Physiol 127: 887–892,
Shahnawaz MD, Chouhan R,Sanadhya D(2017) Impact of aluminum toxicity on physiological aspects of barley (Hordeum vulgare L.) cultivars and its alleviation through ascorbic acid and salicylic acid seed priming. Int J CurrMicrobiolAppl Sci 6(5): 875–891
Shao JF, Che J, Chen RF, Ma JF, Shen RF (2015) Effect of in planta phosphorus on aluminum-induced inhibition of root elongation in wheat. Plant Soil 395(1–2):307–315
Sharma M, Trofimova M, Sharma V, Tripathi BN (2015) Genotypic variation to aluminium sensitivity in chickpea depends on its ability to efficiently accumulate nitrate. Advances in Agro and Plant Sci 1:01–12
Simon L, Kieger M, Sung SS and Smalley TJ (1994) Aluminum toxicity in tomato. Part 2. Leaf gas exchange, chlorophyll content, and invertase activity. J Plant Nut 17:307–317
Singh D, Chaturvedi SK (2007) Rapid and effective screening technique for aluminum tolerance in chickpea at seedling stage. Paper presented in the national symposium on legumes for ecologocal sustainability: emerging challenges and opportunities, IIPR, Kanpur, 3–5 November 2007
Singh D, Rai AK, Panyang O (2009) Hematoxylin staining as a potential screening technique for aluminum tolerance in pea (Pisum sativum L.). Curr Sci 96:1029–1030
Sivaguru M, Horst W (1998) The distal part of the transition zone is the most aluminum-sensitive apical root zone of maize. Plant Physiol 116:155–163
Sivaguru M, Fujiwara T, Samaj J, Baluska F, Yang ZM, Osawa H, Maeda T, Mori T, Volkmann D and Matsumoto H (2000) Aluminum-induced 1→3, β-D-glucan inhibits cell-to-cell trafficking of molecules through plasmodesmata. Anew mechanism of aluminum toxicity in plants. Plant Physiol124:991–1005
Sivaguru M, Yamamoto Y, Rengel Z, Ahn SJ, Matsumoto H (2005) Early events responsible for aluminum toxicity symptoms in suspension-cultured tobacco cells. New Phytologist 165:99–109
Tahara K, Yamanoshita T, Norisada M, Hasegawa I, Kashima H, Sasaki S, Kojima K(2008) Aluminum distribution and reactive oxygen species accumulation in root tips of two Melaleuca trees differing in aluminum resistance. Plant and Soil 307(1–2):167–178
Tauzin AS, Giardina T (2014) Sucrose and invertases, a part of the plant defense response to the biotic stresses. Frontiers in plant science 5:293.
Tice KR, Parker DR, DeMason DA (1992) Operationally defined apoplastic and symplastic aluminum fractions in root tips of aluminum-intoxicated wheat, Plant Phy 100:309–318
Verma S, Dubey RS (2001) Effect of cadmium on soluble sugars and enzymes of their metabolism in rice, Biol Plant 44: 117–123
Wang JP, Raman H, Read B, Zhou MX, Mendham NJ, Venkatanagappa S (2006) Validation of an Alt locus for aluminium tolerance scored with eriochrome cyanine R staining method in barley cultivar Honen (Hordeum vulgare L.) Aust J Agric Res57(1):113–118 doi: https://doi.org/10.1071/AR05202.
Wang I, Fan XW, Pan JL, Huang ZB, Li YZ (2015) Physiological characterization of maize tolerance to low dose of aluminum, highlighted by promoted leaf growth. Planta 242(6):1391–1403. https://doi.org/10.1007/s00425-015-2376-3
Wissemeier AH, Horst WJ (1995) Effect of calcium supply on aluminium induced callose formation, its distribution and persistence in roots of soybean (Glycine max (L.) Merr.). JPlant Physiol 145:470–476.
Yamamoto Y, Kobayashi Y, Matsumoto H (2001) Lipid Peroxidation Is an Early Symptom Triggered by Aluminum, But Not the Primary Cause of Elongation Inhibition in Pea Roots. Plant Physiol 125:199–208
Yang JL, Li YY, Zhang YJ, Wu YR, Wu P, Zheng SJ (2008) Cell wall polysaccharides are specifically involved in the exclusion of aluminum from the rice root apex. Plant Physiol 146:602–611
Yoshida S, Forno DA, Cock JH, Gomez KA (1976) Laboratory manual for physiological studies of rice. Philippines IRRI.
Zelinová V, Halušková Ľ, Huttová J, Illéš P, Mistrík I, Valentovičová K (2011) Short-term aluminium induced changes in barley root tips, Protoplasma 248:523–530
Zrenner R, Salanoubat M, WillmitzerL SonnnewaldU (1995) Evidence of the crucial role of sucrose synthase for sink strength using transgenic potato plants (Solanum tuberosum L.), Plant J 7:97–107
Acknowledgments
The first author is obliged to the University Grants Commission (UGC) for providing Fellowship to conduct this work.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Pandey, P., Dubey, R.S. (2022). Aluminum Uptake, Callose Accumulation, and Invertase Activity in Lowland and Upland Rice Genotypes in Relation to Aluminum Stress Tolerance. In: Kumar, A., Kumar, P., Singh, S.S., Trisasongko, B.H., Rani, M. (eds) Agriculture, Livestock Production and Aquaculture. Springer, Cham. https://doi.org/10.1007/978-3-030-93258-9_4
Download citation
DOI: https://doi.org/10.1007/978-3-030-93258-9_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-93257-2
Online ISBN: 978-3-030-93258-9
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)