Abstract
Aluminium-induced oxidative damage caused by excessive ROS production was evaluated in black gram pulse crop. Black gram plants were treated with different aluminium (Al3+) concentrations (10, 50 and 100 μM with pH 4.7) and further the effects of Al3+ were characterised by means of root growth inhibition, histochemical assay, ROS content analysis, protein carbonylation quantification and 1H-NMR analysis. The results showed that aluminium induces excessive ROS production which leads to cellular damage, root injury, stunt root growth and other metabolic shifts. In black gram, Al3+ induces cellular damage at the earliest stage of stress which was characterised from histochemical analysis. From this study, it was observed that prolonged stress can activate certain aluminium detoxification defence mechanism. Probably excessive ROS triggers such defence mechanism in black gram. Al3+ can induce excessive ROS initially in the root region then transported to other parts of the plant. As much as the Al3+ concentration increases, the rate of cellular injury and ROS production also increases. But after 72 h of stress, plants showed a lowered ROS level and cellular damage which indicates the upregulation of defensive mechanisms. Metabolic shift analysis also showed that the black gram plant under stress has less metabolic content after 24 h of treatment, but gradually, it was increased after 72 h of treatment. It was assumed that ROS played the most important role as a signalling molecule for aluminium stress in black gram.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Aluminium toxicity and acidic soil are becoming the major constraints in the field of agricultural productivity worldwide (Panda et al. 2009). Acid soil causes 25–80 % yield losses in various crop plants (Singh et al. 2011). Von Uexkull and Mutret (1995) found that the acid soil level globally expands up to 3950 million ha of total ice-free land in the world. Forests and woodlands covered 66.3 % acid soil globally; on the other hand, 17.7 % of acid soils is covered by savanna, prairie and steppe vegetation. 5.4 % of acid soil is used to cultivate crop plants. In soil, clay particles help to maintain soil acidity, because they can hold alkaline cations to maintain the soil pH. Due to high rainfall, alkaline cations like Ca2+, Mg2+ and K+ leach from soil, and instead it deposits certain acidic cations to soil such as Al3+ and H+. Field application of lime with gypsum can reduce the acidity of soil and increases crop productivity (Carvalho and van Raij 1997). However, it is an expensive approach for local farmers.
Aluminium is non-toxic to plants until and unless soil becomes acidic (pH < 5). In normal soil, aluminium is present as silicates, phosphate sulphides and oxides. But under an acidic soil condition, it becomes toxic to plants and occurs as a toxic Al3+ form. High rainfall, Earth crust mining and other human activities are the major sources of acidic soil contamination. Any kind of stress induces oxidative stress in plants, but generation of excessive reactive oxygen species (ROS) indicates stress conditions in plants. Different abiotic and biotic stress conditions can induce ROS (O2 ·−, H2O2, −OH and 1O2) production in plants, which is a major toxic substance for plant system (Elstner et al. 1988). In normal conditions, ROS production also occurs in plants via different metabolic pathways (Hippeli et al. 1999) but its production increases in response to various stress conditions in plants (Shao et al. 2008) and that leads to severe oxidative damages in plants. Boscolo et al. 2003 (in maize), Achary et al. 2008 (in Allium) and Panda and Matsumoto 2010 (in pea) found that Al3+ can induce dose- and time-dependent ROS accumulation in cells. Several research groups found significant ROS production associated with oxidative damage and antioxidative activity in response to Al3+ stress in plants (Yamamoto 2001; Boscolo et al. 2003; Ikegawa et al. 2000; Meriga et al. 2004; Wang et al. 2004; Panda et al. 2009).
Black gram is an important pulse crop which is used as food in many parts of the world, mostly in the southern part of India. Moreover, black gram plants can maintain the nitrogen requirement in the soil environment because they have a root nodule bacterial association to fix atmospheric nitrogen. But unfortunately, acidic soil is a major constraint for crop productivity in its native place. So far, from previous reports, it has been confirmed that Al3+ induces root growth inhibition and modifies root morphology (Panda et al. 2003; Sharma et al. 2015; Wang et al. 2015). From this point of view, we tried to understand the morphological behaviour on black gram in response to Al3+ stress. This impact is entirely related to the soil environment. Very less information is known about the stress responses and loss of productivity of this crop under an acid soil situation where Al3+ toxicity is the major constraint. Understanding the Al3+ stress responses and Al3+ tolerance can be a solution and informative work of this major pulse crop.
Materials and method
Root growth, fresh weight and dry weight measurement
The black gram seeds were procured from the Indian Institute of Pulse Research (IIPR), Kanpur, India. Black gram seeds were surface sterilised with 1 % NaClO and kept for germination; the germinated seedlings were transferred to Hoagland nutrient solution (pH 6.2). After 3 days of growth, seedlings were treated with 100 μM of CaCl2 (pH 4.7) for 24 h. Subsequently, seedlings were treated with different concentrations of Al3+ (10, 50 and 100 μM) in 100 μM of CaCl2 (pH 5) and root growth and biochemical parameters were tested after 24, 48 and 72 h of treatment. Fresh weight and dry weight were measured to analyse the effect of Al3+ stress in regulating the biomass of black gram seedlings.
Histochemical assay
Black gram plants were grown in Hoagland nutrient solution and treated with different concentrations of Al3+ as mentioned earlier. After 0, 24, 48 and 72 h of treatment, plant tissues were used for the following histochemical assays. Evan’s blue test, to analyse plasma membrane integrity, was done as per Ikegawa et al. (1998). Haematoxylin assay for aluminium uptake was done as per Ownby (1993). Schiff’s reagent test for lipid peroxidation test was followed from Pompella et al. (1987). After staining, samples were observed under a compound light microscope. Photographs were taken with a Fujifilm camera under ×40 microscopic resolution.
Superoxide, H2O2 and protein carbonylation analysis under Al3+ stress
Superoxide production was measured following Elstner and Heupel (1976). Plant tissue (200 mg) was homogenised with 3 ml of 65 mM (pH 7.8) and centrifuged at 5000 rpm for 10 min. The supernatant was added with 0.9 ml of 65 mM phosphate buffer and 0.1 ml of 10 mM hydroxylamine hydrochloride and incubated at room temperature for 20 min. Then, 1 ml of sulphonylamide and 1 ml of 7 mM ∞-napthyl ethylene dihydrochloride were added. After incubation at room temperature, 1 ml of diethyl ether was added. Centrifugation was at 1500 rpm for 5 min. Then, absorbency was observed at 530 nm. H2O2 was determined by the method of Sagisaka (1976).
Plant tissue (200 mg) was homogenised in 5 % trichloroacetic acid (TCA) followed by centrifugation at 15,000 rpm for 20 min at 4 °C. Thereafter, the reaction mixture was prepared by adding 50 % TCA, 2.5 M potassium thiocyanate (KSCN) and 10 mM ferrous ammonium sulphate ((NH4)2Fe(SO4)2·6H2O). The absorbency was observed at 480 nm by using a spectrophotometer.
Protein carbonylation was done with spectrophotometric DNPH assay of Levine et al. (1994) with some modifications. Total protein was extracted by homogenising 500 mg of root in phosphate buffer (25 mM, pH 7.0) followed by DNPH reaction. The carbonyl content was calculated by measuring absorbency at 374 nm. The extinction coefficient of hydrazones, i.e. 221 m/mol/cm, was used for the calculation of carbonylated proteins.
Metabolic profile analysis under Al3+ stress by an 1H-NMR approach
Black gram plants were grown under the above-mentioned hydroponic culture condition for further 1H-NMR analysis following Kruger et al. (2008) with some modifications. NMR signals were analysed after Kim et al. (2010) to detect the primary metabolites under normal and stress conditions. Two hundred milligrams of fresh treated and untreated root and shoot tissues was homogenised with liquid nitrogen (LN2). The ground sample was added with 1 ml of 3 M HClO4 and vortexed to mix well. Samples were incubated on ice for 15 min. Again, 1 ml of 1 M HClO4 was added and the solution was shaken vigorously to get a homogenised suspension. Thereafter, samples were incubated on ice for 30 min and then centrifuged at 15,000 rpm for 20 min at 4 °C. The supernatant was collected in a new tube. The pellet was added with 2 ml of 1 M HClO4 to re-suspend the pellet and centrifuged again. Both supernatants were combined together and pH was adjusted to 6.0 to neutralise the supernatant with 2 M KOH. Then, samples were kept on ice for 30 min to precipitate the KClO4. After incubation, the pH was again adjusted to 6.0. The upper solution part was taken in a centrifuge tube and centrifuged at 15,000 rpm for 15 min at 4 °C. Again, the supernatant was collected and lyophilised. The freeze-dried samples were dissolved in 1 ml of 25 mM Na-phosphate buffer containing EDTA. After that, samples were centrifuged at 10,000 rpm for 15 min at 4 °C to remove KClO4 precipitation. The supernatant was again lyophilised and dissolved in 0.3 ml of 10 % D2O in Na-phosphate buffer (pH 6.8) containing trimethyl silyl propionic acid sodium salt 2,2,3,3-D4 (TMSP), as a concentration standard and chemical shift reference (δ = 0 ppm). Thereafter, the samples were centrifuged at 10,000 rpm for 30 min at 4 °C to get a final supernatant and to remove probable remaining KClO4. Required amounts of samples were transferred into a NMR tube (Shigemi), and spectra were measured and recorded in 1H-NMR (Bruker Biospin AVANCE III 600). Measurement parameters were as follows: probe temperature—299 K, scan number—256, receiver gain—203.
Statistical analysis
All experiments were performed thrice in replicate to get a significant variation between the control and stress samples. All statistical analyses of the data were performed by using Microsoft Excel and data were expressed as means ± SE. Significant differences between the stress and control samples were determined by Student’s t test at p ≤ 0.05.
Results
Root growth, fresh weight and dry weight measurement
The root growth inhibition test was performed in hydroponic culture with different concentrations of Al3+ (10, 50 and 100 μM, at pH 5). In this study, 10 μM of Al3+ also induces root growth inhibition (Fig. 1a). The rate of root growth inhibition increases as the time interval of stress and concentration of Al3+ increased. The low pH root growth inhibition analysis (Fig. 1b) showed that only low pH can reduce the root elongation rate, but with aluminium, it was more toxic to black gram. Similarly, under aluminium stress, decreased fresh (FW) and dry weight (DW) of root was observed in black gram. As the concentration of aluminium increased, the reduction of mass also increased, whereas significant increased DW was observed after 72 h of treatment among all aluminium concentrations in this study.
a Root growth analysis of black gram plant in response to Al3+ (10, 50 and 100 μM with pH 4.7) after 24, 48 and 72 h. b Comparative root growth inhibition analysis between different pH values (pH 6, 5.5, 5, 4.5) and different Al3+ concentrations (10, 50 and 100 μM with low pH 4.7) for 24 h only. c Fresh weight and d dry weight of root were measured in response to Al3+ (10, 50 and 100 μM) after 24, 48 and 72 h. Data presented are means ± SE. Asterisks indicate significant difference from control at p < 0.05 by Student’s t test (*p < 0.05, **p < 0.01 and ***p < 0.001)
Histochemical assay
Histochemical assay can visualise the plasma membrane integrity lost. Evan’s blue test is well known to check the integrity of the plasma membrane. It can only enter through a disintegrated plasma membrane caused by different stress media. Control roots did not show Evan’s blue uptake whereas the Al3+-treated roots showed gradual Evan’s blue uptake, and in response to an increase in Al3+ concentration, an increased intensity of the blue colour was also observed (Fig. 2a). Haematoxylin test is specific to aluminium ion uptake by plant root systems, which is being represented by haematoxylin brown-coloured regions in the root tip zone. In black gram, the haematoxylin assay (Fig. 2b) reveals Al3+ ion uptake through the root system. Higher concentrations of Al3+ (50 μM)-treated plants showed more haematoxylin (∼Al3+) uptake in the root tip region. Schiff’s reagent test was done specially to detect lipid peroxidation in the cell membrane. Due to Al3+ toxicity, the lipid molecules were peroxidised and some free aldehydes were released. Schiff’s reagent binds with those free aldehydes and forms a magenta-coloured product, i.e. fuchsin. In the black gram plant, under Al3+ stress, in the root tip zone, a distinct magenta colour was spotted, while in the normal root tip, no such colour was observed. The production of magenta colour was greater in the 48-h-treated root (Fig. 2c).
Superoxide, H2O2 and protein carbonylation analysis under Al3+ stress
Superoxide (O2 ·−) content was measured in response to different Al3+ concentrations as well as with different time intervals in both root (Fig. 3a) and shoot (Fig. 3b). Al3+ stress-induced ROS activity was observed as the concentration and duration of stress gradually increased. But a decreased superoxide content was observed after 48 h of treatment as compared to control in shoot only. It was also observed that at 10 and 50 μM, the superoxide content was greater as compared to that at 100 μM of Al3+ in both root and shoot. Most interestingly, in both root and shoot, a highly increasing order of superoxide content was observed after 24 h of treatment, but it was more significant in root rather than shoot.
Changes in the activity of superoxide content in root (a) and shoot (b), H2O2 content in root (c) shoot (d) and protein carbonyl content in root (e) shoot (f) of black gram seedlings after 24, 48 and 72 h of aluminium treatment [control (0), 10, 50 and 100 μM]. Data presented are means ± SE. Asterisks indicate significant difference from control at p < 0.05 by Student’s t test (*p < 0.05, **p < 0.01 and ***p < 0.001). The graphical representation of these data was created by using ggplot2 package of R programme (Wickham 2009; R Core Team 2013)
Hydrogen peroxide (H2O2) content was also measured in both root (Fig. 3c) and shoot (Fig. 3d) under different Al3+ concentrations. The H2O2 content was increased gradually in all treated samples with the increasing duration of stress and concentration of Al3+. But under 100 μM of Al3+ stress, less H2O2 content was observed than the other two concentrations (10 and 50 μM) in both root and shoot. Under 50 μM of Al3+ stress, a high level of H2O2 content was quantified in both root and shoot. Time-dependent analysis showed an increasing order of H2O2 level even in the control, but the amount was lesser than that in the stressed one in both root and shoot. A significant Al3+ stress-induced H2O2 production was observed in this study. In root, H2O2 production was greater under 10 and 50 μM Al3+, whereas in shoot, a high level of H2O2 production was observed under 50 μM Al3+ only.
Carbonylated protein (PC) content was also measured in both root (Fig. 3e) and shoot (Fig. 3f) under different Al3+ stress conditions. The stressed plants showed a greater content of carbonyl proteins. The level of carbonyl-bound proteins increased with increasing Al3+ concentration, after 24 h of treatment. After 48 and 72 h of treatment, the carbonylated protein content was greater as the concentration increased. But as compared to control plants at 48 and 72 h, the carbonylated protein content was less in the 24 h control plant. Under 100 μM Al3+ concentration, the plant showed more carbonyl-bound proteins in root. In shoot, after 48 h of treatment at higher concentration, carbonyl-bound protein content was decreased, whereas in root, after 48 h of treatment, the carbonyl-bound protein content was greater and gradually increased as the concentration increased.
Primary metabolite content analysis under Al3+ stress by the 1H-NMR approach
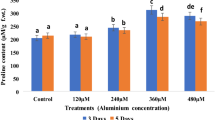
1H-NMR spectra of the stressed and control (root (Fig. 4) and shoot (Fig. 4)) samples were obtained based on their X: ppm value with standard spectra as per Kim et al. 2010. The spectra obtained from control samples showed a characteristic difference with the stress samples. Time-dependent analysis of metabolite change was also done and a significant difference was observed. Under stress condition, very low metabolite synthesis was observed after 24 h as compared to that in control samples. Based on the peak intensity of different samples, the level of expressed metabolites can be measured. Characteristic NMR signals (δ) are the determinants of a particular metabolite in NMR spectra. In this NMR analysis, we tried to detect certain amino acids, organic acids, sucrose and non-phenolic compounds. In this study, we were able to measure the characteristic 1H-NMR signals for tryptophan (δ = 7.20), proline (δ = 4.08), tyrosine (δ = 6.86), alanine (δ = 1.48), valine (δ = 1.00), threonine (δ = 1.32), formic acid (δ = 8.46), fumaric acid (δ = 6.56), malonate (δ = 3.36), citric acid (δ = 2.74), succinic acid (δ = 2.56), glutamine (δ = 2.15), malic acid (δ = 2.68), γ-amino butyric acid (δ = 1.90), choline (δ = 3.24), methanol (δ = 3.36), inositol (δ = 4.0) and sucrose (δ = 5.4). Most of them are interrelated and connected to the citric acid cycle which is a major concern for aluminium stress. Most of the metabolites get lowered in both root (Fig. 4) and shoot (Fig. 5) after 24 h of aluminium treatment as compared to control samples. But after 72 h of treatment, all peaks were detected to be increased in the stressed samples as compared to the control samples.
Discussion
Aluminium responses in pulse crop are not yet well understood by researchers. Little work has been done by some researchers in pulse crops in response to proton and aluminium toxicity. Because of aluminium contamination in agricultural fields, the productivity of pulse crops is decreasing. Our soil analysis data showed (data not shown) that in the north-east region (Assam), aluminium content in soil increased to 10–11 %, whereas in normal soil, it should be 7–8 %. This elevated percentage of aluminium added more acidity to this region. Field observation of this area proves that the productivity of crops is decreasing in order every year. In this study, we tried to understand the aluminium responses in black gram. We tried to understand the primary responses of the black gram plant under aluminium stress conditions. More than 50 % root growth inhibition was observed under the 100 μM Al3+ stress condition in black gram (Fig. 1a). Aluminium-induced root growth inhibition was increased by a low pH condition. Our proton toxicity analysis showed that root growth inhibition under low pH (5–4.5) is less toxic to black gram than aluminium with low pH (50 μM Al3+ + pH 4.5) (Fig. 1b). Similar results on root growth inhibition induced by Al3+ were also reported in previous studies in other plant species such as sorghum (Ohki 1987), soybean (Hai et al. 1989), wheat (Ryan et al. 1992), squash (Van et al. 1994), green gram (Panda et al. 2003), maize (Boscolo et al. 2003) and pea (Panda and Matsumoto 2010). Besides root growth inhibition analysis, we also observed a decrease in fresh and dry weight of root samples under aluminium stress. Under 50 μM Al3+ concentration, plants were found with decreased fresh and dry weight (Fig. 1c, d). Time interval analysis showed that after 24 h of treatment, the fresh and dry weight of plants decreased more than that of plants after 48 and 72 h of treatment. Meriga et al. (2003) suggested that the root is the major site of cytokinin-like hormones, and under aluminium stress, the production of such hormone can be reduced, which results in reduced root growth and dry mass production.
Histochemical analysis of aluminium-treated roots revealed the primary target of aluminium toxicity. From this study, it was observed that the root tip and elongation zone are the major sites of aluminium interaction (Taylor 1995). Foy (1988) found that aluminium-like toxic species can cause reduced root cell division by increasing the rigidity of the double helix of DNA. Aluminium causes plasma membrane integrity loss which was confirmed by Evan’s blue test. In this assay, a gradual uptake of the blue colour indicates that long treatment with aluminium causes plasma membrane disintegration (Fig. 2a). This result was also supported by the haematoxylin assay (Cançado et al. 1999), where it was observed that with the increased duration of aluminium treatment, the uptake of aluminium was also increased in terms of haematoxylin colour (Fig. 2b). Haematoxylin can bind to aluminium to form a complex which gives a reddish brown colour. The brown colour that appeared in the root tips of aluminium-treated plants after 24, 48 and 72 h indicates the uptake of aluminium in the root tip region at the preliminary stage of stress. But more interestingly, it was observed that after 48 h of stress condition, the root tips showed a more intense colour which was less after 72 h of stress. Wu et al. (2012) found that ROS production and lipid peroxidation were higher during the early stage of aluminium treatment and they gradually declined as the duration of stress increased. After entering into the root tip of the plant, aluminium starts modifying the cellular content and integration, which was characterised by Schiff’s reagent test. This test showed that under aluminium stress, roots undergo lipid peroxidation. After 24 h of treatment, root tips showed pink-coloured lesions. These lesions are the complexes of Schiff’s reagent, and free aldehydes are released from the lipid peroxidation process, which was caused by aluminium. In the 0-h-treated root, no such lesions were observed. Moreover, the pink-coloured lesions were more prominent in the 48-h-treated root samples (Fig. 2c). From this point, we can suggest that more aluminium uptake can cause more lipid peroxidation in cells, due to oxidative damage of polyunsaturated fatty acids by excessive ROS production induced by aluminium in cells promoting lipid peroxidation (Andrews et al. 1965).
ROS can be generated by cellular metabolism in plants (Kim et al. 2008; Song et al. 2010; Elstner 1982). But excess ROS production can be induced by certain stresses, which is toxic to plants. Most plant defence mechanisms can scavenge the excess ROS activity via various metabolic processes. In this study, it was observed that the ROS level was induced by aluminium. Under 10 μM Al3+ concentration, plants showed a high level of superoxide content, which indicates that a lower concentration of aluminium can also enhance the production of ROS and can cause oxidative damages. As compared to shoot (Fig. 3b), the superoxide level was higher in root (Fig. 3a), because the root is the primary site of aluminium toxicity. In this study, we observed a significant increment of H2O2 after 24 h of aluminium treatment as compared to control (−Al) in both root and shoot. For 24 h of treatment, H2O2 content was less increased than that for 48 and 72 h of treatment in both root (Fig. 3c) and shoot (Fig. 3d). A gradual increased level of H2O2 was observed with the duration of stress increased. From this data, we assumed that aluminium can induce more ROS activity. Wu et al. (2012) suggested that H2O2 might work as a signalling molecule to activate the tolerance mechanism. The high amount of H2O2 can cause stomata closure and decrease the photosynthetic rate; as a result, crop productivity becomes less (Aftab et al. 2010). Another effect of excessive ROS production was observed by means of carbonyl content in both root and shoot. Interestingly, after aluminium stress, an increased level of carbonyl protein content was observed. When excessive ROS production is not scavenged by antioxidant regulatory mechanisms, protein carbonyl content is suggested to be an indicator for oxidative damage (Juszczuk et al. 2008 and Achary et al. 2008). Accordingly, in this study, we observed that after 24 h of treatment, the protein carbonyl content was greater in the control than that in both root (Fig. 3e) and shoot (Fig. 3f), but it was found to be less increased after 48 and 72 h of treatment. This might be because of the induction of antioxidative defence mechanism that may give resistance to aluminium stress in black gram. This was also supported by our 1H-NMR analysis of the metabolic profile of black gram. We observed that after 24 h of aluminium treatment, black gram plants showed a decreased level of certain metabolic products, but after 72 h of treatment, it was increased as compared to untreated samples. In our 1H-NMR data, we have observed certain metabolites which play a critical role in metabolic processes in plants, such as tryptophan which acts as a precursor in auxin, protein biosynthesis. Fumarate is an intermediate in the citric acid cycle. Fumarate can be converted into malate with the help of the enzyme fumarase and gives malate as a product, which is a major detoxifying organic acid involved in aluminium stress in plants. Changes in sucrose content were also observed in our data, which is a major source for energy production. On the other hand, proline (a major non-enzymatic antioxidant in plants) content was also increased with the time of stress increased. But, as compared to control, the content was less/decreased at the initial stage of stress; it indicates that aluminium stress induces proline production after long exposure. It can act as an osmo-protectant and keeps proteins safe against a stressed environment (Pollard and Wyn Jones 1979). Proline also helps to scavenge hydroxyl radicals produced from stressful conditions (Smirnoff and Cumbes; 1989). Proline formation is implicated as a mechanism of alleviating cytoplasmic acidosis and may maintain NADPH/NADP+ ratios at values compatible with metabolism (Hare and Cress 1997). Another important metabolite that has been detected by our study is GABA, which can be converted into succinic acid semialdehyde and take part in the citric acid cycle by oxidising itself into succinic acid. This condition was the same in both root and shoot. Metabolic processes get interrupted after treating with aluminium stress which caused damage to black gram, but as long as we kept the stress on the plants, defence mechanisms also activate possibly to revive its metabolic homeostasis.
Conclusion
From this investigation, we conclude that aluminium-induced ROS production caused major oxidative damage like lipid peroxidation and protein carbonylation and ROS activity and also altered the metabolic status at the early stage of stress. From the ROS and NMR quantification data, it was observed that prolonged aluminium treatment may be managed by the activation of metabolic processes that may have a chance of either tolerating or falling prey to Al stress. Our investigation also supports the previous findings in terms of root growth inhibition and primary root cell injury. Histochemical assay indicates the post-detoxification mechanism involved in black gram, which means long treatment with aluminium in black gram may be managed by several detoxification mechanisms that again will depend on the genotype and severity of Al stress.
Abbreviations
- ROS:
-
Reactive oxygen species
- Al3+ :
-
Aluminium
- PC:
-
Protein carbonylation
- H2O2 :
-
Hydrogen peroxide
- FW:
-
Fresh weight
- DW:
-
Dry weight
References
Achary VM, Jena S, Panda KK, Panda BB (2008) Aluminium induced oxidative stress and DNA damage in root cells of Allium cepa L. Ecotoxicol Environ Saf 70(2):300–310
Aftab T, Khan MMA, Idress M, Naeem M, Moinuddin (2010) Effects of aluminum exposures on growth, photosynthetic efficiency, lipid peroxidation, antioxidation, antioxidant enzymes and artemisinin content of Artemisia annua L. J Phytol 2:23–37
Andrews F, Johan Bjorkten FB, Trenk AS, Koch RB (1965) The reaction of an auto oxidized lipids with proteins. J Am Oil Chem Soc 42:779–781
Boscolo PRS, Menossi M, Jorge R a (2003) Aluminum-induced oxidative stress in maize. Phytochemistry 62(2):181–189
Cançado GMA, Loguercio LL, Martins PR, Parentoni SN, Paiva E, Borém A, Lopes MA (1999) Hematoxylin staining as a phenotypic index for aluminum tolerance selection in tropical maize (Zea mays L.). TAG Theor Appl Genet 99(5):747–754
Carvalho MCS, van Raij B (1997) Calcium sulphate, phosphogypsum and calcium carbonate in the amelioration of acid subsoils for root growth. Plant Soil 192(1):37–48
Elstner EF (1982) Oxygen activation and oxygen toxicity. Annu Rev Plant Physiol 33(1):73–96
Elstner EF, Heupel A (1976) Formation of hydrogen peroxide by isolated cell walls from horseradish (Armoracia lapathifolia Gilib.). Planta 130(2):175–80
Elstner EF, Wagner GA, Schutz W (1988) Activated oxygen in green plants in relation to stress situations. Curr Top Plant Biochem Physiol 7:159–187
Foy CD (1988) Plant adaptation to acid, aluminium toxic soils. Commun Soil Sci Plant Anal 19:959–987
Hai TV, Nga TT, Laudelout H (1989) Effect of aluminium on the mineral nutrition of rice. Plant Soil 114(2):173–185
Hare PD, Cress WA (1997) Metabolic implications of stress-induced proline accumulation in plants. Plant Growth Regul 21:79–102
Hippeli S, Heiser I, Elstner EF (1999) Activated oxygen and free oxygen radicals in pathology: new insights and analogies between animals and plants. Plant Physiol Biochem 37(3):167–178
Ikegawa H, Yamamoto Y, Matsumoto H (1998) Cell death caused by a combination of aluminum and iron in cultured tobacco cells. Physiol Plant 104(3):474–478
Ikegawa H, Yamamoto Y, Matsumoto H (2000) Responses to aluminum of suspension-cultured tobacco cells in a simple calcium solution. Soil Sci Plant Nutr 46:503–514
Juszczuk IM, Tybura A, Rychter AM (2008) Protein oxidation in the leaves and roots of cucumber plants (Cucumis sativus L.), mutant MSC16 and wild type. J Plant Physiol 165(4):355–365
Kim C, Meskauskiene R, Apel K, Laloi C (2008) No single way to understand singlet oxygen signalling in plants. EMBO Rep 9(5):435–9
Kim HK, Choi YH, Verpoote R (2010) NMR based metabolomic analysis of plants. Nat Protoc 5:536–549
Kruger NJ, Troncoso-Ponce MA, Ratcliffe RG (2008) 1H NMR metabolite fingerprinting and metabolomic analysis of perchloric acid extracts from plant tissues. Nat Protoc 3(6):1001–12
Levine RL, Williams JA, Stadtman ER, Shacter E (1994) Carbonyl assays for determination of oxidatively modified proteins. Methods Enzymol 233:346–357
Meriga B, Krishna Reddy B, Rajender Rao K, Ananda Reddy L, Kavi Kishor PB (2003) Alleviating effect of citrate on aluminium toxicity of rice (Oryza sativa L.) seedling. Curr Sci 85(3):63–68
Meriga B, Krishna Reddy B, Rajender Rao K, Ananda Reddy L, Kavi Kishor PB (2004) Aluminium-induced production of oxygen radicals, lipid peroxidation and DNA damage in seedlings of rice (Oryza sativa). J Plant Physiol 161(1):63–68
Ohki K (1987) Aluminum stress on sorghum growth and nutrient relationships. Plant Soil 98(2):195–202
Ownby JD (1993) Mechanisms of reaction of hematoxylin with aluminium-treated wheat roots. Physiol Plant 87(3):371–380
Panda SK, Matsumoto H (2010) Changes in antioxidant gene expression and induction of oxidative stress in pea (Pisum sativum L.) under Al stress. BioMetals 23(4):753–762
Panda SK, Singha LB, Khan MH (2003) Does aluminium phytotoxicity induce oxidative stress in greengram (Vigna radiata)? Bulg J Plant Physiol 29(1–2):77–86
Panda SK, Baluska F, Matsumoto H (2009) Aluminum stress signaling in plants. Plant Signal Behav 4(7):592–7
Pollard A, Wyn Jones RG (1979) Enzyme activities in concentrated solutions of glycinebetaine and other solutes. Planta 144:291–298
Pompella A, Maellaro E, Casini AF, Comporti M (1987) Histochemical detection of lipid peroxidation in the liver of bromobenzene-poisoned mice. Am J Pathol 129(2):295–301
R Core Team (2013) A language and environment for statstical computing. R foundation for statstical computing, Vienna, Austria. URL http://www.R-project.org/
Ryan PR, Shaff JE, Kochian LV (1992) Aluminum toxicity in roots: correlation among ionic currents, ion fluxes, and root elongation in aluminum-sensitive and aluminum-tolerant wheat cultivars. Plant Physiol 99(3):1193–1200
Sagisaka S (1976) The occurrence of peroxide in a perennial plant Populas gelrica. Plant Physiol 57:308–309
Shao HB, Chu LY, Lu ZH, Kang CM (2008) Primary antioxidant free radical scavenging and redox signaling pathways in higher plant cells. Int J Biol Sci 4(1):8–14
Sharma M, Trofimova M, Sharma V, Tripathi BN (2015) Genotypic variation to aluminium sensitivity in chickpea depends on its ability to efficiently accumulate nitrate. Adv Agron Plant Sci 01(01):1–12
Singh D, Singh NP, Chauhan SK, Singh P (2011) Developing aluminium-tolerant crop plants using biotechnological tools. Curr Sci 100(12):1807–1814
Smirnoff N, Cumbes QJ (1989) Hydroxyl radical scavenging activity of compatible solutes. Phytochemistry 28:1057–1060
Song H, Xu X, Wang H, Wang H, Tao Y (2010) Exogenous gamma-aminobutyric acid alleviates oxidative damage caused by aluminium and proton stresses on barley seedlings. J Sci Food Agric 90(9):1410–6
Taylor GJ (1995) Overcoming barriers to understanding the cellular basis of aluminium resistance. Plant Soil 171(1):89–103
Van HL, Kuraishi S, Sakuraj N (1994) Aluminium-induced rapid root inhibition and changes in cell-wall components of squash seedlings. Plant Physiol 106:971–976
Von Uexküll HR, Mutert E (1995) Global extent, development and economic impact of acid soils. Plant Soil 171(1):1–15
Wang YS, Wang J, Yang ZM, Wang QY, Lu B, Li SQ, Lu YP, Wang SH, Sun X (2004) Salicylic acid modulates aluminum-induced oxidative stress in roots of Cassia tora. Acta Bot Sin-Eng Edn 46:819–828
Wang L, Fan X-W, Pan J-L, Huang Z-B, Li Y-Z (2015) Physiological characterization of maize tolerance to low dose of aluminum, highlighted by promoted leaf growth. Planta. doi:10.1007/s00425-015-2376-3
Wickham H (2009) ggplot2: Elegant graphics for data analysis. Springer-Verlag New York
Wu K, Xiao S, Chen Q, Wang Q, Zhang Y, Li K, Chen L (2012) Changes in the activity and transcription of antioxidant enzymes in response to Al stress in black soybeans. Plant Mol Biol Report 31(1):141–150
Yamamoto Y (2001) Lipid peroxidation is an early symptom triggered by aluminum, but not the primary cause of elongation inhibition in pea roots. Plant Physiol 125(1):199–208
Acknowledgments
We sincerely thank DBT, Govt. of India, for providing the facility for the research work. We thank IIPR, Kanpur, for providing black gram seeds for the present research work. We also sincerely thank DBT-Biotech Hub, Assam University, Silchar, for providing the facility for the research work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Handling Editor: Néstor Carrillo
Rights and permissions
About this article
Cite this article
Chowra, U., Yanase, E., Koyama, H. et al. Aluminium-induced excessive ROS causes cellular damage and metabolic shifts in black gram Vigna mungo (L.) Hepper. Protoplasma 254, 293–302 (2017). https://doi.org/10.1007/s00709-016-0943-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-016-0943-5