Abstract
This study aimed to assess the relative efficacy and safety of tofacitinib 5 and 10 mg twice daily, or in combination with methotrexate (MTX), in patients with active RA. Randomized controlled trials (RCTs) examining the efficacy and safety of tofacitinib in patients with active RA were included in this network meta-analysis. We performed a Bayesian network meta-analysis to combine the direct and indirect evidence from the RCTs. Ten RCTs including 4867 patients met the inclusion criteria. There were 21 pairwise comparisons including 11 direct comparisons of seven interventions. The ACR20 response rate was significantly higher in the tofacitinib 10 mg + MTX group than in the placebo and MTX groups (OR 7.56, 95 % credible interval (CrI) 3.07–21.16; OR 3.67, 95 % CrI 2.60–5.71, respectively). Ranking probabilities based on the surface under the cumulative ranking curve (SUCRA) indicated that tofacitinib 10 mg + MTX had the highest probability of being the best treatment for achieving the ACR20 response rate (SUCRA = 0.9254), followed by tofacitinib 5 mg + MTX (SUCRA = 0.7156), adalimumab 40 mg + MTX (SUCRA = 0.6097), tofacitinib 10 mg (SUCRA = 0.5984), tofacitinib 5 mg (SUCRA = 0.4749), MTX (SUCRA = 0.1674), and placebo (SUCRA = 0.0086). In contrast, the safety based on the number of withdrawals due to adverse events did not differ significantly among the seven interventions. Tofacitinib, at dosages 5 and 10 mg twice daily, in combination with MTX, was the most efficacious intervention for active RA and was not associated with a significant risk for withdrawals due to adverse events.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rheumatoid arthritis (RA) is a systemic autoimmune disease characterized by chronic synovial joint inflammation, which leads to disability and loss of quality of life [1, 2]. Intracellular pathways that include Janus kinases (JAKs) are critical to immune cell activation, proinflammatory cytokine production, and cytokine signaling [3]. Tofacitinib (CP-690,550) is a novel orally administered JAK inhibitor [4]. It selectively inhibits JAK-1, JAK-2, and JAK-3, with functional cellular specificity for JAK-1 and JAK-3 over JAK-2 [5, 6]. Tofacitinib subsequently modulates adaptive and innate immunity [6].
Disease-modifying antirheumatic drugs (DMARDs) have been used to decrease inflammation, delay bone erosion, and improve functional ability in patients with RA. Several clinical trials have attempted to evaluate the efficacy and safety of tofacitinib in patients with active RA who had an incomplete response to DMARD or methotrexate (MTX) [7–16]. A previous meta-analysis has shown that tofacitinib is effective in active patients with RA who had an inadequate response to DMARD or MTX and has a manageable safety profile [17]. However, the comparative efficacy and safety of tofacitinib in various treatment regimens with different dosages or in combination with MTX remains unclear due to the lack of multiple comparisons.
Standard meta-analysis compares only two treatments at a time [18, 19]. On the other hand, network meta-analysis, also called multiple-treatments meta-analysis, simultaneously combines direct and indirect evidence of the relative treatment effects [20]. Network meta-analysis can assess the comparative effectiveness of multiple interventions and combines evidence across a network of randomized controlled trials (RCTs), even if there are no head-to-head comparisons [21]. The present study aimed to compare the efficacy and safety of tofacitinib 5 and 10 mg twice daily, or in combination with methotrexate (MTX), in patients with active RA, by using a network meta-analysis.
Methods
Identification of eligible studies and data extraction
We performed an exhaustive search for studies that examined the efficacy and safety of tofacitinib in patients with active RA who showed inadequate response to DMARD or MTX. A literature search was performed using MEDLINE, EMBASE, and the Cochrane Controlled Trials Register to identify available articles (up to January 2015). The following key words and subject terms were used in the search: “tofacitinib,” “rheumatoid arthritis,” and “RA.” All references in the studies were reviewed to identify additional works not included in the electronic databases. RCTs were included if they met the following criteria: (1) the study compared tofacitinib with placebo or MTX in the treatment for RA, (2) the study provided end points for the clinical efficacy and safety of tofacitinib, and (3) the study included patients diagnosed with RA based on the American College of Rheumatology (ACR) criteria for RA. The exclusion criteria were as follows: (1) the study included duplicate data, and (2) the study did not contain adequate data for inclusion. Efficacy outcome was the number of patients who achieved an ACR20 response, and the safety outcome was the number of patients withdrawn due to adverse events (AEs). Data were extracted from original studies by two independent reviewers. Any discrepancy between the reviewers was resolved by consensus or a third reviewer. The following information was extracted from each study: first author, year of publication, country in which the study was conducted, tofacitinib dose, length of follow-up, time when outcomes were evaluated, and outcomes for efficacy and safety. We assessed the methodological qualities using the Cochrane Collaboration’s tool for assessing risk of bias in randomized trials [22]. The following parameters were considered: random sequence generation, blinding, concealed allocation, selective reporting, incomplete outcome data, and other biases. We conducted a network meta-analysis in accordance with the guidelines provided by the PRISMA statement [23].
Evaluations of statistical associations for network meta-analysis
For RCTs that compared multiple doses of tofacitinib in different arms, the results from different arms were analyzed simultaneously. The efficacy and safety of tofacitinib in different arms were ordered according to the probability of being ranked as the best performing regimen. We used a Bayesian random-effects model for network meta-analysis using NetMetaXL [24] and WinBUGS statistical analysis program version 1.4.3 (MRC Biostatistics Unit, Institute of Public Health, Cambridge, UK). We used the Markov Chain Monte Carlo method to obtain the pooled effect sizes [21]. All chains were run with 10,000 burn-in iterations followed by 10,000 monitoring iterations. Information of relative effects was converted to a probability that a treatment is best, second best, and so on, or the ranking of each treatment, called the surface under the cumulative ranking curve (SUCRA) [25], which is expressed as a percentage—the SUCRA would be 100 % when a treatment is certain to be the best and 0 % when a treatment is certain to be the worst. The league table arranges the presentation of summary estimates by ranking the treatments in order of the most pronounced impact on the outcome under consideration based on SUCRA [25]. We reported the pairwise OR and 95 % credible interval (CrI) (or Bayesian CI) and adjusted for multiple-arm trials. Pooled results were considered statistically significant if the 95 % CrI did not contain the value 1.
Test for inconsistency
Inconsistency refers to the extent of disagreement between direct and indirect evidence [26]. Assessment of inconsistency is important for conducting a network meta-analysis [27]. We plotted the posterior mean deviance of the individual data points in the inconsistency model against their posterior mean deviance in the consistency model to assess the network inconsistency between direct and indirect estimates in each loop [28]. We assessed the robustness of the results by performing network meta-analysis after eliminating outlier studies.
Results
Studies included in the meta-analysis
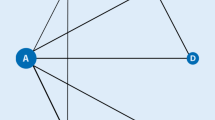
A total of 76 studies were identified by electronic or manual search, 17 were excluded for repeated publications, and 48 were excluded for irrelevance. Eleven studies were selected for a full-text review based on the title and abstract details. However, one of the eleven was excluded because it contained no outcome data (Fig. 1). Thus, 10 RCTs including 4867 patients (2470 events for efficacy and 301 events for safety) met the inclusion criteria [7–16]. There were 21 pairwise comparisons including 11 direct comparisons and seven interventions, such as MTX, tofacitinib 5 mg + MTX, tofacitinib 10 mg + MTX, tofacitinib 5 mg, tofacitinib 10 mg, adalimumab 40 mg once a week + MTX, MTX, and placebo for the network meta-analysis (Fig. 2). The recommended dosage of tofacitinib is 5 mg twice daily [29], but patients may benefit from an increase to 10 mg twice a day. Thus, we chose the dosages of 5 and 10 mg of tofacitinib twice daily. Relevant features of the studies included in the meta-analysis are provided in Table 1. Although sequence generation, concealed allocation, and selective reporting were not mentioned in all the studies, blinding and incomplete outcome data were mentioned in all (Table 1, Supplementary data).
Evidence network of comparisons for network meta-analysis. The width of each edge in the evidence network is proportional to the number of randomized controlled trials comparing each pair of treatments, and the size of each treatment node is proportional to the number of randomized participants (sample size). A MTX, B tofacitinib 5 mg + MTX, C tofacitinib 10 mg + MTX, D tofacitinib 5 mg, E tofacitinib 10 mg, F adalimumab + MTX, G Placebo
Network meta-analysis of the efficacy of tofacitinib in RCTs
Tofacitinib 10 mg + MTX is listed in the top left of the diagonal of the league table (Table 2) because it was associated with the most favorable SUCRA for the ACR20 response rate, while placebo is listed in the bottom right of the diagonal of the league table because it was associated with the least favorable results. For interpretation purposes, the results are read from top to bottom and left to right. The ACR20 response rate was significantly higher in the tofacitinib 10 mg + MTX group than in the placebo or MTX groups (OR 7.56, 95 % CrI 3.07–21.16; OR 3.67, 95 % CrI 2.60–5.71, respectively) (Table 2; Fig. 3). Similarly, the ACR20 response rate was significantly higher in the tofacitinib 5 mg + MTX group than in the placebo or MTX groups (OR 6.11, 95 % CrI 2.58–18.02; OR 2.97, 95 % CrI 2.17–4.89, respectively) (Table 2; Fig. 3). Compared with the placebo or MTX groups, adalimumab 40 mg + MTX, tofacitinib 10 mg, and tofacitinib 5 mg groups showed a significantly higher ACR20 response rate (Table 2; Fig. 3). A trend of tofacitinib 10 mg + MTX being more efficacious than tofacitinib 5 mg + MTX, adalimumab + MTX, tofacitinib 10 mg, and tofacitinib 5 mg was noted (Table 2; Fig. 3). Ranking probability based on SUCRA indicated that tofacitinib 10 mg + MTX had the highest probability of being the best treatment for achieving the ACR20 response rate (SUCRA = 0.9254), followed by tofacitinib 5 mg + MTX (SUCRA = 0.7156), adalimumab 40 mg + MTX (SUCRA = 0.097), tofacitinib 10 mg (SUCRA = 0.5984), tofacitinib 5 mg (SUCRA = 0.4749), MTX (SUCRA = 0.1674), and placebo (SUCRA = 0.0086) (Table 3).
Network meta-analysis of the safety of tofacitinib in RCTs
We considered the number of patient withdrawals due to AE as the safety outcome. The number of patients withdrawn due to AEs was lower in the placebo group than in the tofacitinib 10 mg + MTX and tofacitinib 5 mg + MTX groups, but the difference did not reach statistical significance (OR 0.95, 95 % CrI 0.24–3.85; OR 0.88, 95 % CrI 0.23–3.68, respectively) (Table 2; Fig. 4). However, the number of patients withdrawn due to AEs did not differ significantly among the seven interventions (Tables 2, 3; Fig. 4).
Inconsistency and sensitivity analysis
An inconsistency plot provides information that can help identify the loops in which inconsistency is present [27]. Although the contributions to the deviance were likely to be similar and close to 1 for both models, two points in both plots of the efficacy (tofacitinib 5 mg of Fleischmann et al. study, tofacitinib 10 mg + MTX of van der Heijde et al. study) and safety (tofacitinib 5 mg of Fleischmann et al. study, MTX of Kremer et al. study) appeared to have a higher than expected posterior mean deviance (Fig. 5). However, a sensitivity analysis removing outlier studies did not meaningfully change the network meta-analysis results. Three studies were considered as low (or unclear) quality [8, 13, 16]. Excluding these studies did not significantly affect the results of the network meta-analysis, indicating statistically robust results from this network meta-analysis.
Discussion
Network meta-analysis, an extension of traditional meta-analysis, synthesizes all available evidence to allow for simultaneous comparisons of different treatment options that lack direct head-to-head comparisons [20, 21]. We conducted a network meta-analysis to compare the efficacy and safety of different tofacitinib interventions in patients with active RA, because this analysis enables an indirect comparison of multiple treatments, which are either lacking in or have insufficient direct head-to-head comparisons.
This network meta-analysis assessed seven kinds of interventions on the number of patients who achieved an ACR20 response and the number of patients withdrawn due to AEs in patients with active RA. In regard to efficacy, our network meta-analysis suggests that tofacitinib 10 mg + MTX is most effective in the treatment for active RA, followed by tofacitinib 5 mg + MTX, adalimumab 40 mg + MTX, tofacitinib 10 mg, tofacitinib 5 mg, MTX, and placebo. Tofacitinib 10 mg + MTX and tofacitinib 5 mg + MTX are more efficacious than tofacitinib monotherapy. Tofacitinib with MTX had a higher probability of being the best treatment for achieving an ACR20 response than tofacitinib monotherapy. This may be explained by the inhibitory action of MTX on the activation and proliferation of lymphocytes; thus, the combination of tofacitinib with MTX fills the gap associated with each drug with respect to the mode of action [30].
With respect to safety based on the number of withdrawals due to AEs, tofacitinib 10 mg + MTX and tofacitinib 5 mg + MTX showed more withdrawals due to AEs and had a lower probability of being the best in terms of the number of withdrawals due to AEs than placebo. However, no significant difference was observed in withdrawals due to AEs among seven interventions, suggesting comparable safety among the different tofacitinib dosages, with or without MTX, and placebo.
Although this network meta-analysis showed that the number of tofacitinib-treated patients who discontinued medication due to AEs was not different from placebo groups, tofacitinib has been reported to have the risk of infections, cancer, and cytopenias [31, 32]. The common serious infections reported with tofacitinib included pneumonia, cellulitis, herpes zoster, and urinary tract infection [31]. Tuberculosis (TB) infection has been reported in the trials of tofacitinib [33, 34]. A study using a mouse model indicated a reactivation of LTBI in the presence of tofacitinib and suggested that tofacitinib should be prescribed with caution in patients with chronic inflammation, and screening for LTBI is necessary prior to use [35]. With respect to malignancy, lung cancer and renal cell carcinoma have been reported in the tofacitinib group [36, 37]. Tofacitinib reported other side effects, such as hypercholesterolemia, and rise in liver enzymes and serum creatinine [38]. Thus, monitoring should be conducted during the use of tofacitinib, and larger trials with longer duration of study with pharmacovigilance are needed to confirm the long-term safety.
Network meta-analysis synthesizes all available evidence to allow for simultaneous comparisons of different treatment options that lack direct head-to-head comparisons, optimizing the use of all available data [21]. In comparison with the individual studies, network meta-analysis provides more accurate data by increasing the statistical power and resolution through pooling the results of independent analyses. Use of network meta-analysis has increased, but this is the first network meta-analysis that evaluated comprehensive and simultaneous assessment of tofacitinib for RA.
This network meta-analysis results, which combined evidence from both direct and indirect comparisons for evaluating the relative efficacy and safety of tofacitinib, were in agreement with a meta-analysis of direct comparisons showing that tofacitinib provided a statistically significant improvement according to the response criteria (ACR20) compared to placebo, and there were no statistically significant differences between tofacitinib and placebo in terms of treatment discontinuation due to adverse reactions [39]. However, our network meta-analysis differs from the previous meta-analysis, because we could generate a rank order for the efficacy and safety of tofacitinib or in combination with MTX in patients with active RA.
Our results should be interpreted with caution because of the several shortcomings of our study. First, the follow-up time points ranged widely from 3 to 24 months, with most being of a short duration (<6 months). The follow-up duration was therefore too short for an evaluation of the long-term effects. Longer comparative studies in the future are warranted. Second, there was heterogeneity in the design and patient characteristics of the included trials; thus, there is the possibility that these differences across studies affected the results of this network meta-analysis. Third, this study did not comprehensively address the efficacy and safety outcomes of tofacitinib in RA. This study only focused on the effectiveness based on the number of patients who achieved an ACR20 response and on the safety according to the number of patients withdrawn due to AEs, without assessing various outcomes. Specifically, the number of withdrawals due to AEs may not be sufficient for the safety outcome measure because of its frequency.
In conclusion, by using a Bayesian network meta-analysis involving 10 RCTs comparing seven different interventions, we found that tofacitinib 5 and 10 mg twice daily, in combination with MTX, was most efficacious for active RA and was not associated with a significant risk for withdrawals due to AEs. Long-term studies are needed to determine the relative efficacy and safety of tofacitinib in a large number of patients with active RA.
References
Harris ED Jr (1990) Rheumatoid arthritis. Pathophysiology and implications for therapy. N Engl J Med 322:1277–1289
Choi SJ, Rho YH, Ji JD, Song GG, Lee YH (2006) Genome scan meta-analysis of rheumatoid arthritis. Rheumatology (Oxford) 45:166–170
Ghoreschi K, Laurence A, O’Shea JJ (2009) Janus kinases in immune cell signaling. Immunol Rev 228:273–287
Changelian PS, Flanagan ME, Ball DJ, Kent CR, Magnuson KS, Martin WH, Rizzuti BJ, Sawyer PS, Perry BD, Brissette WH, McCurdy SP, Kudlacz EM, Conklyn MJ, Elliott EA, Koslov ER, Fisher MB, Strelevitz TJ, Yoon K, Whipple DA, Sun J, Munchhof MJ, Doty JL, Casavant JM, Blumenkopf TA, Hines M, Brown MF, Lillie BM, Subramanyam C, Shang-Poa C, Milici AJ, Beckius GE, Moyer JD, Su C, Woodworth TG, Gaweco AS, Beals CR, Littman BH, Fisher DA, Smith JF, Zagouras P, Magna HA, Saltarelli MJ, Johnson KS, Nelms LF, Des Etages SG, Hayes LS, Kawabata TT, Finco-Kent D, Baker DL, Larson M, Si MS, Paniagua R, Higgins J, Holm B, Reitz B, Zhou YJ, Morris RE, O’Shea JJ, Borie DC (2003) Prevention of organ allograft rejection by a specific Janus kinase 3 inhibitor. Science 302:875–878
Chrencik JE, Patny A, Leung IK, Korniski B, Emmons TL, Hall T, Weinberg RA, Gormley JA, Williams JM, Day JE, Hirsch JL, Kiefer JR, Leone JW, Fischer HD, Sommers CD, Huang HC, Jacobsen EJ, Tenbrink RE, Tomasselli AG, Benson TE (2010) Structural and thermodynamic characterization of the TYK2 and JAK3 kinase domains in complex with CP-690550 and CMP-6. J Mol Biol 400:413–433
Meyer DM, Jesson MI, Li X, Elrick MM, Funckes-Shippy CL, Warner JD, Gross CJ, Dowty ME, Ramaiah SK, Hirsch JL, Saabye MJ, Barks JL, Kishore N, Morris DL (2010) Anti-inflammatory activity and neutrophil reductions mediated by the JAK1/JAK3 inhibitor, CP-690,550, in rat adjuvant-induced arthritis. J Inflamm 7:41
Lee EB, Fleischmann R, Hall S, Wilkinson B, Bradley JD, Gruben D, Koncz T, Krishnaswami S, Wallenstein GV, Zang C, Zwillich SH, van Vollenhoven RF, Investigators OS (2014) Tofacitinib versus methotrexate in rheumatoid arthritis. N Engl J Med 370:2377–2386
Kremer J, Li ZG, Hall S, Fleischmann R, Genovese M, Martin-Mola E, Isaacs JD, Gruben D, Wallenstein G, Krishnaswami S, Zwillich SH, Koncz T, Riese R, Bradley J (2013) Tofacitinib in combination with nonbiologic disease-modifying antirheumatic drugs in patients with active rheumatoid arthritis: a randomized trial. Ann Intern Med 159:253–261
van der Heijde D, Tanaka Y, Fleischmann R, Keystone E, Kremer J, Zerbini C, Cardiel MH, Cohen S, Nash P, Song YW, Tegzova D, Wyman BT, Gruben D, Benda B, Wallenstein G, Krishnaswami S, Zwillich SH, Bradley JD, Connell CA, Investigators OS (2013) Tofacitinib (CP-690,550) in patients with rheumatoid arthritis receiving methotrexate: twelve-month data from a twenty-four-month phase III randomized radiographic study. Arthritis Rheum 65:559–570
Burmester GR, Benda B, Gruben D, Bradley J, Mebus C (2013) Tofacitinib for rheumatoid arthritis—Authors’ reply. Lancet 381:1812–1813
van Vollenhoven RF, Fleischmann R, Cohen S, Lee EB, Garcia Meijide JA, Wagner S, Forejtova S, Zwillich SH, Gruben D, Koncz T, Wallenstein GV, Krishnaswami S, Bradley JD, Wilkinson B, Investigators OS (2012) Tofacitinib or adalimumab versus placebo in rheumatoid arthritis. N Engl J Med 367:508–519
Fleischmann R, Kremer J, Cush J, Schulze-Koops H, Connell CA, Bradley JD, Gruben D, Wallenstein GV, Zwillich SH, Kanik KS, Investigators OS (2012) Placebo-controlled trial of tofacitinib monotherapy in rheumatoid arthritis. N Engl J Med 367:495–507
Kremer JM, Cohen S, Wilkinson BE, Connell CA, French JL, Gomez-Reino J, Gruben D, Kanik KS, Krishnaswami S, Pascual-Ramos V, Wallenstein G, Zwillich SH (2012) A phase IIb dose-ranging study of the oral JAK inhibitor tofacitinib (CP-690,550) versus placebo in combination with background methotrexate in patients with active rheumatoid arthritis and an inadequate response to methotrexate alone. Arthritis Rheum 64:970–981
Fleischmann R, Cutolo M, Genovese MC, Lee EB, Kanik KS, Sadis S, Connell CA, Gruben D, Krishnaswami S, Wallenstein G, Wilkinson BE, Zwillich SH (2012) Phase IIb dose-ranging study of the oral JAK inhibitor tofacitinib (CP-690,550) or adalimumab monotherapy versus placebo in patients with active rheumatoid arthritis with an inadequate response to disease-modifying antirheumatic drugs. Arthritis Rheum 64:617–629
Tanaka Y, Suzuki M, Nakamura H, Toyoizumi S, Zwillich SH, Tofacitinib Study I (2011) Phase II study of tofacitinib (CP-690,550) combined with methotrexate in patients with rheumatoid arthritis and an inadequate response to methotrexate. Arthritis Care Res 63:1150–1158
Kremer JM, Bloom BJ, Breedveld FC, Coombs JH, Fletcher MP, Gruben D, Krishnaswami S, Burgos-Vargas R, Wilkinson B, Zerbini CA (2009) The safety and efficacy of a JAK inhibitor in patients with active rheumatoid arthritis: Results of a double-blind, placebo-controlled phase IIa trial of three dosage levels of CP-690,550 versus placebo. Arthritis Rheum 60:1895–1905
Song GG, Bae S-C, Lee YH (2014) Efficacy and safety of tofacitinib for active rheumatoid arthritis with an inadequate response to methotrexate or disease-modifying antirheumatic drugs: a meta-analysis of randomized controlled trials. Korean J Intern Med 29:656–663
Lee YH, Bae SC, Choi SJ, Ji JD, Song GG (2011) Associations between vitamin D receptor polymorphisms and susceptibility to rheumatoid arthritis and systemic lupus erythematosus: a meta-analysis. Mol Biol Rep 38:3643–3651
Lee YH, Rho YH, Choi SJ, Ji JD, Song GG (2007) PADI4 polymorphisms and rheumatoid arthritis susceptibility: a meta-analysis. Rheumatol Int 27:827–833
Catalá-López F, Tobías A, Cameron C, Moher D, Hutton B (2014) Network meta-analysis for comparing treatment effects of multiple interventions: an introduction. Rheumatol Int 34:1489–1496
Caldwell DM, Ades A, Higgins J (2005) Simultaneous comparison of multiple treatments: combining direct and indirect evidence. BMJ 331:897
Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savović J, Schulz KF, Weeks L, Sterne JA (2011) The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343:d5928
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 151:264–269
Brown S, Hutton B, Clifford T, Coyle D, Grima D, Wells G, Cameron C (2014) A Microsoft-Excel-based tool for running and critically appraising network meta-analyses—an overview and application of NetMetaXL. Syst Rev 3:110
Salanti G, Ades A, Ioannidis JP (2011) Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol 64:163–171
Dias S, Welton NJ, Sutton AJ, Caldwell DM, Lu G, Ades A (2013) Evidence synthesis for decision making 4 inconsistency in networks of evidence based on randomized controlled trials. Med Decis Making 33:641–656
Higgins J, Jackson D, Barrett J, Lu G, Ades A, White I (2012) Consistency and inconsistency in network meta-analysis: concepts and models for multi-arm studies. Res Synth Methods 3:98–110
Valkenhoef G, Lu G, Brock B, Hillege H, Ades A, Welton NJ (2012) Automating network meta-analysis. Research synthesis. Methods 3:285–299
Traynor K (2012) FDA approves tofacitinib for rheumatoid arthritis. Am J Health Syst Pharm 69:2120
Herman S, Zurgil N, Deutsch M (2005) Low dose methotrexate induces apoptosis with reactive oxygen species involvement in T lymphocytic cell lines to a greater extent than in monocytic lines. Inflamm Res 54:273–280
Kaur K, Kalra S, Kaushal S (2014) Systematic review of tofacitinib: a new drug for the management of rheumatoid arthritis. Clin Ther 36:1074–1086
Lundquist LM, Cole SW, Sikes ML (2014) Efficacy and safety of tofacitinib for treatment of rheumatoid arthritis. World J Orthop 5:504
Kremer J, Li Z-G, Hall S, Fleischmann R, Genovese M, Martin-Mola E, Isaacs JD, Gruben D, Wallenstein G, Krishnaswami S (2013) Tofacitinib in combination with nonbiologic disease-modifying antirheumatic drugs in patients with active rheumatoid arthritis: a randomized trial. Ann Intern Med 159:253–261
van Vollenhoven RF, Fleischmann R, Cohen S, Lee EB, García Meijide JA, Wagner S, Forejtova S, Zwillich SH, Gruben D, Koncz T (2012) Tofacitinib or adalimumab versus placebo in rheumatoid arthritis. N Engl J Med 367:508–519
Maiga M, Lun S, Guo H, Winglee K, Ammerman NC, Bishai WR (2012) Risk of tuberculosis reactivation with tofacitinib (CP-690550). J Infect Dis 205:1705–1708
Burmester GR, Blanco R, Charles-Schoeman C, Wollenhaupt J, Zerbini C, Benda B, Gruben D, Wallenstein G, Krishnaswami S, Zwillich SH (2013) Tofacitinib (CP-690,550) in combination with methotrexate in patients with active rheumatoid arthritis with an inadequate response to tumour necrosis factor inhibitors: a randomised phase 3 trial. Lancet 381:451–460
Fleischmann R, Cutolo M, Genovese MC, Lee EB, Kanik KS, Sadis S, Connell CA, Gruben D, Krishnaswami S, Wallenstein G (2012) Phase IIb dose-ranging study of the oral JAK inhibitor tofacitinib (CP-690,550) or adalimumab monotherapy versus placebo in patients with active rheumatoid arthritis with an inadequate response to disease-modifying antirheumatic drugs. Arthritis Rheum 64:617–629
He Y, Wong AY, Chan EW, Lau WC, Man KK, Chui CS, Worsley AJ, Wong IC (2013) Efficacy and safety of tofacitinib in the treatment of rheumatoid arthritis: a systematic review and meta-analysis. BMC Musculoskelet Disord 14:298
Kawalec P, Mikrut A, Wiśniewska N, Pilc A (2013) The effectiveness of tofacitinib, a novel Janus kinase inhibitor, in the treatment of rheumatoid arthritis: a systematic review and meta-analysis. Clin Rheumatol 32:1415–1424
Acknowledgments
This study was supported in part by a grant of the Korea Healthcare technology R&D Project, Ministry for Health and Welfare, Republic of Korea (HI13C2124).
Conflict of interest
We have no financial and non-financial conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lee, Y.H., Bae, SC. & Song, G.G. Comparative efficacy and safety of tofacitinib, with or without methotrexate, in patients with active rheumatoid arthritis: a Bayesian network meta-analysis of randomized controlled trials. Rheumatol Int 35, 1965–1974 (2015). https://doi.org/10.1007/s00296-015-3291-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-015-3291-4