Abstract
Polylactic acid (PLA), a highly promising biodegradable biopolymer, has been extensively studied over the last two decades. The thermoplastic polyester PLA is bio-based, compostable, and eco-friendly. Its monomer, lactic acid, is fermented from renewable plant sources like starch and sugar. Therefore, PLA is regarded as a desirable substitute for conventional petroleum-based polymers. Because of its biocompatible and biodegradable properties, U.S. FDA (Food and Drug Administration) has approved PLA as a biomedical material. PLA has excellent mechanical, physical, structural, and thermal properties, making it suitable for various applications. However, PLA has limitations such as low impact toughness, hydrophobicity, and slow degradation rate at ambient temperatures. This can be improved by different modification methods, such as copolymerization, making composites or blends of PLA with other biodegradable polymers, or incorporating additives. This review presents information about the various methods of synthesis of PLA, its properties, and the environmental degradation of PLA. In addition, the different processing techniques of PLA are also reviewed. Lastly, the applications of PLA in sectors such as packaging, biomedical, agricultural, automotive, and textile are also discussed.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The worldwide annual production of plastic is more than 390 million tons, in which the share of bioplastics is still less than 1%. According to projections, the worldwide production capacity of bioplastics is expected to increase significantly, reaching around 6.3 million tons by 2027, up from approximately 2.2 million tons in 2022 [1]. Bioplastics are plastics which are either bio-based or biodegradable or both that have comparable properties to traditional plastics but they offer especial advantages like lower carbon emissions, improved functionalities, and extra waste management possibilities over conventional plastics to decrease the reliance on finite fossil resources and to minimize greenhouse gas emissions. Due to rising demand and introduction of advanced materials, applications, and products, the bioplastics market is experiencing robust growth [2].
Conventional petroleum-based plastics cause environmental pollution and contribute to global warming. Bio-based and biodegradable plastics are environment-friendly since they reduce CO2 emissions by absorbing CO2 from the atmosphere and degrade at a faster rate than conventional plastics and hence reduce plastic pollution. Polylactic acid (PLA) is a bio-based, biodegradable, biocompatible (compatible with living tissues) and eco-friendly thermoplastic polymer which is widely available and can be decomposed easily after its use without causing harm to the environment. In comparison to other biodegradable polymers, PLA has very good processability, mechanical properties and transparency, making it more suitable and useful biodegradable polymer. PLA is widely used in food packaging, agriculture and biomedical applications [3–4].
Polylactic acid or Polylactide (PLA) is derived from the monomer lactic acid, i.e., 2-hydroxy propionic acid, which is produced from renewable materials like starch or sugar, through fermentation. PLA can be broken down into water and carbon dioxide by microbes and is fully biodegradable. Due to its good biocompatibility and biodegradability, U.S. FDA (Food and Drug Administration) has approved PLA as a biomedical material. PLA is widely regarded as the most promising biodegradable polymer in the market [5].
Biopolymers are obtained from natural resources like starch, cellulose, proteins and chitosan sourced from plants and animals. These natural polymers can become the promising substitutes for synthetic plastic materials, which are non-biodegradable and petroleum-based, due to their renewable, biodegradable and eco-friendly features. Biodegradable polymers can be categorized into various types based on their manufacturing processes as well as resources, as illustrated in Fig. 1. They are obtained from following: (1) directly from biomass (e.g., proteins, and polysaccharides) (2) by microbial fermentation (like polyhydroxyalkanoate (PHA), and polyhydroxybutyrate (PHB)) (3) synthetic biopolymers derived from biomass (e.g., PLA) (4) synthetic biopolymers derived from petrochemicals (like polyglycolic acid (PGA), polycaprolactone (PCL), and polybutylene succinate-co-adipate (PBSA)) [6].
Classification of biodegradable polymers based on two resources [6]
Environmental pollution and the depletion of fossil fuels are the two serious problems associated with the polymers industries. To overcome these problems, one solution is to use renewable resources for production of polymers (i.e. use of bio-based polymers) [7]. Since the PLA monomer (lactic acid) is produced from renewable resources (carbohydrates) by microbial fermentation and PLA biodegradation does not cause any eco-toxicological effect, PLA is considered as an environment friendly sustainable polymeric material [8]. The life cycle of PLA is illustrated in Fig. 2 [9].
Life cycle of PLA [9]
This review article summarizes different synthesis methods of Polylactic acid (PLA), advantages and disadvantages of PLA, PLA properties, degradation, and processing characteristics of PLA. In addition, applications of PLA in various fields are also highlighted.
Synthesis of PLA
Lactic acid
Lactic acid (LA), i.e., 2-hydroxy propionic acid is monomer or starting material for the production of Polylactic acid. LA is the simplest hydroxy acid which contains an optically active asymmetric carbon atom within its molecular structure and, therefore, two optical isomers exist: S or L (+) lactic acid, known as levorotatory form, and R or D (-) lactic acid, referred to as dextrorotatory form, as shown in Fig. 3. The symbols (+) and (-) indicate the direction in which a chemical causes rotation of plane-polarized light [10].
Two optical isomers of lactic acid [10]
Both biological and chemical processes can be used to create LA through synthesis. The chemical synthesis method for industrial production of lactic acid involves the reaction of acetic aldehyde with hydrogen cyanide in presence of catalyst to get cyanohydrin and then it is hydrolyzed to obtain LA, as shown in Fig. 4 [11].
Production of LA from acetic aldehyde and hydrogen cyanide [11]
The biological method for the production of LA is usually preferred, which is based on the bacterial fermentation of carbohydrates, which can be readily obtained from sources such as corn, sugar beet, potatoes, sugar cane, and other biomasses. The bacterial fermentation process, starting with hydrolysis of lactose, is shown in Fig. 5 [11].
Production of LA from bacterial fermentation of carbohydrates [11]
Nowadays, the majority of the world’s commercial production of LA is done by the bacterial fermentation of carbohydrates route. Throughout the fermentation process, various conditions such as pH, temperature and atmosphere are very important to obtain maximum yield with high purity LA. Both D- and L-enantiomers of LA can be produced by selecting suitable microorganism in fermentation process. Lactobacilli amylophilus, L. maltaromicus, L. casei, L. bavaricus, and L. salivarius are the predominant organisms that predominantly produce the L (+)-isomer of lactic acid. On the other hand, L. delbrueckii, L. acidophilus, or L. jensenii yield the D (-)-isomer or a combination of both isomers. Depending on the specific strain of Lactobacillus, different types of carbohydrates may be used in fermentation [12]. The chemical synthesis method results in the production of a racemic mixture containing both D- and L-isomers of the compound, whereas the fermentation method offers an advantage of producing either optically pure L-LA or D-LA and the optical purity significantly impacts the physical properties of PLA. In addition, the production of LA by microbial fermentation process facilitates a decrease in production costs [13]. The equal mixture (50:50) of D-LA and L-LA, known as racemic lactic acid, is not advisable for use in the food, pharmaceutical and beverage industries because of potential metabolic issues associated with D-LA. Also, PLA industry does not recommend the use of racemic lactic acid as it generally needs lactic acid having high degree of optical purity, such as, more than 98 to 99% L-LA as well as less than 1 to 2% D-LA [10].
PLA synthesis methods
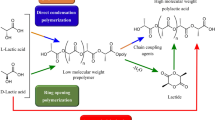
PLA is a linear aliphatic polyester composed of building blocks of lactic acid, which are organic acids found naturally in renewable resources, like sugarcane and corn starch [14]. Carothers was the first to produce low molecular weight PLA in 1932. Later on, DuPont synthesized higher molecular weight PLA and patented in 1954. The first commercial production of LA-based polymers (copolymers of lactic and glycolic acids) was done as fiber materials for resorbable sutures by Ethicon in 1972. Further, they were used for other medical applications, such as implants and controlled drug release. There are mainly three methods of synthesis of high molecular weight PLA: (1) direct condensation polymerization; (2) azeotropic dehydrative condensation polymerization; and (3) ring-opening polymerization, as illustrated in Fig. 6 [15].
Different routes of manufacturing Polylactic acid [15]
In direct condensation polymerization process, polymerization of LA is done in presence of catalyst under reduced pressure conditions. Low molecular weight polymer (Mw less than 10,000 Da) is obtained because it is very tough to eliminate water completely from reaction mixture having high viscosity. Although direct condensation polymerization is simple and least expensive method but the main disadvantage of this process is production of low molecular weight polymer. Also, it is difficult to control the stereoregularity during the course of polymerization, resulting in inferior mechanical properties of the polymer. So, this method is used only when low molecular weight polymer is needed. However, high molecular weight polymer can be produced using chain coupling agents or chain extenders. The function of chain coupling agent is to join low molecular weight polymer chain to chain of high molecular weight by preferentially reacting with hydroxyl or carboxyl end groups of polymer and found to be effective in achieving high molecular weight PLA [16]. The coupling agents like isocyanates, peroxides or epoxides can be utilized to synthesize a range of molecular weights. But, it is found that the chain extenders and polymer impurities can be toxic and non-biodegradable.
PLA of high molecular weight can be produced through azeotropic dehydrative condensation polymerization of lactic acid, eliminating the need for adjuvants or chain extenders. During the process, lactic acid is subjected to reduced pressure distillation at 130 °C for approximately 2–3 h to eliminate a significant portion of the condensed water. Subsequently, the catalyst along with diphenyl ether is introduced, and a reaction vessel is equipped with a tube containing 3A0 molecular sieve. The solvent undergoing reflux is recycled back into the vessel through the molecular sieve over a period of 30–40 h at a temperature of 130 °C. Then, the polymer is segregated by either separation or dissolution and subsequently precipitated for additional purification. By employing this polymerization method, high molecular weight polymers are obtained; however, they may contain notable levels of catalyst impurities [17]. Mitsui Toatsu Chemical Company synthesized Poly-D, L-lactic acid (PDLLA) by direct solution polycondensation method, where lactic acid along with catalysts and high boiling point organic solvent were mixed in a reactor to obtain high molecular weight polymer of about 300,000 Da [18].
Ring-opening polymerization (ROP) is the widely employed method to produce high molecular weight (Mw greater than 100,000 Da) PLA. This method involves ring-opening of the lactide (cyclic dimer of lactic acid) in presence of catalyst. This process includes three steps: (a) polycondensation of lactic acid into low molecular weight PLA; (b) depolymerization of PLA into the lactide; and (c) ring-opening polymerization of lactide intermediate in presence of catalyst to obtain PLA with a controlled molecular weight. The additional purification steps required by this process are relatively complicated and increases the cost of the polymer as compared to the polycondensation method. By ROP method, the molecular weight of the PLA polymer as well as the final polymer’s composition and arrangement of D- and L- LA units can be managed by regulating the residence time, temperature, catalyst type, and concentration. The catalysts used are usually transition metals, such as, tin, zinc, aluminum, iron, lead, bismuth, and yttrium [19]. Tin (II) bis-2-ethylhexanoate, termed as stannous octoate (Sn(Oct)2) also, is the preferred catalyst in PLA synthesis due to its excellent catalytic efficiency, low toxicity, and ability to generate high molecular weight polymers having minimal racemization [20]. The conventional ROP process employed by NatureWorks is shown in Fig. 7. In this process, a continuous conversion of lactic acid takes place, resulting in the formation of low molecular weight pre-polymers of PLA. Then the oligomers undergo a catalytic cyclization depolymerization process in a separate reactor, leading to the formation of lactide. The molten lactide undergoes vacuum distillation in a column to eliminate any remaining lactic acid residues. The purified lactide within the polymer reactor undergoes ring-opening polymerization facilitated by an organic tin catalyst, eliminating the need for costly solvents. The unreacted lactide is extracted through a vacuum process for the purpose of recycling. After purifying the PLA polymer, it is blended with additives and then subjected to extrusion to create resin pellets. These pellets are subsequently utilized for crystallization as well as packaging purposes [10, 21].
Advantages and disadvantages of PLA
There are many benefits of using PLA since it is an eco-friendly polymer. PLA exhibits biodegradability, recyclability, and compostability. PLA can be obtained from renewable agricultural resources, like sugarcane, corn, sugar beets, cassava, potatoes, rice, and wheat. The next advantage of PLA is that it is biocompatible, which is safe to use in food packaging and biomedical applications. Neither toxic nor carcinogenic effects are produced by PLA on the human body. After degradation, PLA decomposes into water and carbon dioxide and does not hinder the tissue healing process [22,23,24,25, 33].
Apart from this, PLA has better thermal processability as compared to other biopolymers like PHAs (polyhydroxyalkanoates), PEG (polyethylene glycol), and PCL (polycaprolactone). Processing of PLA can be done by various processing techniques, such as injection molding, film extrusion, thermoforming, blow molding, and fiber spinning. In addition, the manufacturing of bioplastics based on PLA needs 25 to 55% less energy compared to traditional plastics based on petroleum and this saving of energy will further be reduced by additional 10% in near future. Therefore, the production of PLA offers notable advantages in terms of both energy and cost savings [26].
However, PLA has some disadvantages, including poor toughness, which limits its applications. Due to its brittleness, PLA exhibits less than 10% elongation at break, making it unfavorable for applications that require plastic deformation under high stress levels. Secondly, degradation rate of PLA is significantly slow at normal or room temperatures. The degradation rate of PLA is influenced by several factors, including molecular weight, crystallinity, morphology, stereoisomeric content, molecular weight distribution, and the rate of water diffusion into the polymer. Also, the hydrophobic nature of PLA leads to low cell affinity. When PLA comes into direct contact with biological fluids, it can induce an inflammatory response in the living host organism. Furthermore, PLA demonstrates chemically inertness and lacks reactive side-chain groups, which makes surface as well as bulk modifications of PLA a difficult task [25–26, 33].
Properties of PLA
Physical properties of PLA
PLA, along with majority of its products, is soluble in various solvents, including dioxane, chloroform, acetonitrile, methylene chloride, dichloroacetic acid, and 1, 1, 2-trichloroethane. At low temperatures, PLA products have a partial solubility in ethyl benzene, acetone, toluene, and tetrahydrofuran (THF). Nevertheless, when subjected to their boiling temperatures, PLA and its products can be completely dissolved in the solvents mentioned above. PLA products are incompatible with aliphatic hydrocarbons (like hexane and heptane), water, and alcohol; which can be utilized as antisolvents. PLA having high crystallinity along with high molecular weight cannot be dissolved in acetone, THF or ethyl acetate and only PLA having lower molecular weights can be dissolved in these solvents [27, 30].
The various types of PLA polymer are: pure PLLA (poly-L-lactic acid), pure PDLA (poly-D- lactic acid), and PDLLA (poly-D, L-lactic acid) [28]. PLLA as well as PDLA are semi-crystalline polymers but PDLLA is an amorphous polymer. The presence of crystalline regions in PLLA significantly slows down its degradation rate in comparison to PDLLA. The melting enthalpy of PLA having 100% crystallinity has been found to be 93–148 J/g according to studies conducted on PLA [29,30,31]. The physical properties of PLA are illustrated in Table 1 [32].
Mechanical properties of PLA
The mechanical characteristics of PLA can exhibit a wide range, spanning from soft, elastic plastic to stiff, high strength plastic. Semi-crystalline PLA is favored over amorphous PLA, if higher mechanical properties are needed. Farah et al. [33] found that PLA typically possesses a tensile modulus of approximately 3 GPa, a tensile strength ranging from 50 to 70 MPa, a flexural strength of around 100 MPa, as well as an elongation at break of approximately 4%. The stereo chemical structure of the backbone and molecular weight (Mw) play a significant role in determining the mechanical properties and crystallization behavior of PLA. For example, it was found that by increasing the molecular weight (Mw) of PLLA from 50 to 100 kDa, the tensile modulus doubled [34], and when the Mw was varied from 50 to 150 to 200 kDa, corresponding tensile strengths of 15.5 MPa, 80 MPa, and 150 MPa were obtained [35].
The comparison of mechanical properties of PLA with some other polymers is illustrated in Table 2 [36].
The comparison of mechanical properties of PLA with some typical polymers showed that PLA exhibits a higher tensile modulus than PVC, PP, and Nylon as well as it demonstrates a higher flexural strength than PP, which indicates that PLA has a very good potential to be used in various engineering applications. Moreover, there is significant potential for enhancing the physical and mechanical properties of PLA [37]. Also, PLA can undergo processing through a simple conventional method with lesser energy or time, which makes it an inexpensive and easily available polymer [38,39,40].
Thermal properties of PLA
The amorphous or semi-crystalline nature of PLA is determined by its stereochemistry as well as thermal history. In case of amorphous PLA, the Tg (glass transition temperature) plays a vital role as it indicates the temperature at and above which polymer chain mobility occurs. However, for semi-crystalline PLA, the Tg and the Tm (melting temperature) both are significant factors to consider [41,42,43]. The thermal properties of PLA can be influenced by various parameters, such as molecular weights and composition (stereoisomers content). The Tg is associated with the molecular weight of the polymer by Eq. (1) (Flory-Fox equation):
Where T∞ represents the glass transition temperature when molecular weight of the polymer approaches infinity, Mn refers to the number-average molecular weight, and K denotes constant associated with the free volume of end groups in the polymer chains. This relationship has been investigated by Dorgan et al. [44] on PLA polymers, in which the effect of molecular weights and composition (L/D ratio) on the thermal properties of PLA has been studied. Tg values of various PLA polymers having different molecular weights and compositions are shown in Fig. 8. In this figure, the Tg increases rapidly with increase in molecular weight and after that it reaches a constant value. The effect of L-stereoisomer content on the Tg of the polymer shows that, by increasing the amount of L-stereoisomer, the Tg at the infinite molecular weight increases, as shown in Fig. 8 [44, 53].
The thermal properties of PLA are shown in Table 3 [45].
The modification in morphologies of PLA can be done by different mixtures of L- and D- content. Increasing D-content in PLA, leads to a greater degree of amorphousness, while PLA with L-content exceeding 90% tends to become semi-crystalline, as shown in Table 4 [46].
The determination of PLA crystallinity is generally carried out using DSC (differential scanning calorimetry) by measurement of the heat of fusion, ΔHm, and the heat of crystallization, ΔHc. The crystallinity (C [%]) can be calculated using Eq. (2):
Where constant, 93.1 J/g refers to the ΔHm of PLLA or PDLA homo-polymers having 100% crystallinity [47]. The effect of stereochemistry on the crystallization behavior of PLA has been investigated by Pyda et al. [48].
Figure 9 shows the results from DSC, in which PLA polymers containing ∼8 and ∼ 16% D-stereoisomer is amorphous even after 15 h of isothermally treatment at 1450C, whereas the heat treatment at 1450C of PLA polymers containing 1.5% D-stereoisomer results in a large endothermic melting peak at around 1770C, which suggests that PLA containing 1.5% D-stereoisomer is semi-crystalline [48, 53].
Rheological properties of PLA
Like other thermoplastic polymers, PLA exhibits Newtonian behavior when subjected to low shear rates (< 10 s− 1), whereas at high shear rates (> 10 s− 1), it exhibits non-Newtonian behavior characterized by shear thinning, which is shown in Fig. 10 [49]. Many investigations have been done on the rheological behavior of PLA, which shows that PLA obeys the power law (Eq. (3)) over a definite range of shear rates and temperatures similar to the other polymers [50]:
Where Ʈ represents shear stress, γ refers to shear rate, K denotes consistency index, and n represents non-Newtonian index. The value of n explains the deviation from Newtonian fluid flow behavior, therefore, it is also known as the flow index. A larger value of n shows that the shear rate has less effect on the flow behavior. Also, it has been found that a PLA melt obeys Arrhenius equation (Eq. (4)) at different shear rates and shear stresses [50]:
Where η is shear viscosity, E is flow activation energy at a constant shear rate, A represents equation constant, R denotes gas constant, and T refers to absolute temperature. Flow activation energy characterizes the temperature dependence of the viscosity. Here, a larger E indicates a higher temperature sensitivity of the materials behavior.
The viscosity curve of PLA at 180 °C illustrating shear viscosity versus shear rate [49]
Two types of PLA have been investigated, such as amorphous PLA consisting of 82% L-lactide and 18% D-lactide, and semi-crystalline PLA consisting of 95% L-lactide and 5% D-lactide. The result shows that the shear viscosity of PLA increases with the increase in L-isomer content in L/D-isomer mixture due to increase in crystallinity of PLA [51]. In addition, Dorgan et al. [52] investigated the effect of linear and branched structure of PLA on the rheological properties. The findings show that in the Newtonian range, branched PLA exhibits a higher viscosity compared to linear PLA. However, in the non-Newtonian range, branched PLA exhibits a lesser viscosity compared to linear PLA. This behavior can be attributed to the shear thinning characteristic of the polymer, leading to reduced viscosity at higher shear rates.
Barrier properties of PLA
The barrier properties of PLA, as shown in Fig. 11, reveals that PLA possessed barrier properties higher than that of PE (polyethylene), PP (polypropylene) and similar to PS (polystyrene) but lower than that of PET (polyethylene terephthalate) [53].
Barrier properties of PLA at 30 °C as compared to other common polymers (a) N2, (b) O2, (c) CO2, and (d) CH4 [53]
Auras and coworkers [54, 55] investigated the PLA’s barrier properties and compared them with those of PET and PS. They found that the permeability coefficients of N2, O2, CO2, and H2O (g) for PLA were lesser than that of PS but greater than that of PET. Generally, the crystallinity of PLA strongly influences the barrier properties of the polymer, where the decrease in crystallinity gives relatively lower mechanical and barrier properties.
Many studies have been done for the improvement of barrier properties of PLA. Thellen et al. [56] examined montmorillonite-layered silicate/PLA composites in terms of the barrier properties. The enhancement of 50% in the oxygen barrier properties was found. Bao et al. [57] studied how the process of annealing treatment affects the barrier properties of films made from PLA. It was found that the barrier properties of the PLA films increased after annealing due to the increase in crystallinity induced by annealing.
Degradation of PLA
In nature, both biotic (living) and abiotic (non-living) factors coexist, and hence, the complete degradation process of a material is referred to as environmental degradation. Various material properties of PLA, including molecular weight, optical purity, crystallinity, Tg (glass transition temperature), and Tm (melting temperature) influence the environmental degradation of PLA. Additionally, environmental factors such as humidity, temperature, pH, and the presence of enzymes or microorganisms also play a vital role in the environmental degradation process of PLA. When the molecular weight (Mw) of PLA is low (Mw < 100,000 Da), the material tends to be brittle, cloudy, and opaque. However, as the molecular weight increases, PLA becomes stronger, more transparent, and less prone to degradation. The hydrolysis of PLA occurs at a slower rate in its crystalline regions compared to the amorphous regions. This disparity arises from the fact that water can more readily penetrate the less structured amorphous regions than the well-organized crystalline regions. As a result, the amorphous regions exhibit higher rates of hydrolysis and are more susceptible to biodegradation. Within semi-crystalline PLA, the degradation process initiates in the amorphous regions and progresses at a slower pace in the crystalline areas. Consequently, over time, the proportion of crystalline regions in PLA increases, leading to a decrease in the degradation rate. Higher concentrations of D-isomer content in PLA result in reduced optical purity as well as regularity, causing increased water diffusion via PLA’s amorphous regions. This enhanced water diffusion accelerates the process of hydrolysis. The degradation rate of PLA is significantly higher when the temperature surpasses its glass transition temperature (Tg) range of 55–62 °C. At temperatures above Tg, the polymer chains become more flexible, leading to increased water absorption. This elevated water absorption accelerates both hydrolysis and microbial attachment, causing faster degradation of PLA. Additionally, when PLA is exposed to high relative humidity levels (> 60%), hydrolysis occurs rapidly.
In general, PLA degradation follows a two-step mechanism that involves abiotic factors initially, followed by biotic factors. Abiotic degradation refers to the chemical hydrolysis of PLA at higher temperatures in presence of water. Subsequently, biotic degradation occurs as microorganisms break down the PLA polymer. During biotic degradation, aerobic conditions (in the presence of oxygen) result in the generation of carbon dioxide, water, and biomass, while anaerobic conditions (in the absence of oxygen) lead to the production of methane, hydrocarbons, and biomass through the breakdown of PLA. Microorganisms are capable of degrading PLA only when high molecular weight PLA undergoes hydrolysis, resulting in a reduction of the polymer’s molecular weight (Mw) to 10,000 Da or less. At this lower molecular weight range, microorganisms can effectively break down and degrade the PLA polymer [58,59,60,61,62]. PLA degradation is mainly as a consequence of hydrolysis of ester linkages, which happens randomly along the backbone of PLA polymer. The reaction of PLA with water is shown in Fig. 12. The hydrolytic degradation mechanism is subsequently followed by the bacterial degradation of the fragmented residues [63].
Hydrolysis mechanism of PLA degradation [63]
Under composting conditions, microorganisms present in the compost can degrade PLA within 45–60 days at temperatures ranging from 50 to 60 °C, in which it is first hydrolyzed to smaller molecules (oligomers, dimers, and monomers), and then these molecules are decomposed into carbon dioxide and water by microorganisms in the compost [64]. Studies have indicated that PLA degradation in soil occurs at a slower rate compared to a compost medium. This is attributed to the fact that compost provides more favorable conditions for PLA degradation due to its higher moisture content and a wider range of temperatures [65]. Few studies have been done for PLA biodegradation in aquatic environments also. In case of both static and dynamic sea water, no signs of microbial degradation were observed after 10 weeks, which leads the researchers to propose that marine microorganisms may have limited capability to disintegrate PLA [66, 67]. In a separate study, PLA rods were immersed in seawater for a period of 3 months at 20 °C. It was observed that there was minimal alteration in the molecular weight of the PLA rods under these conditions. However, when the PLA rods were immersed in seawater at a higher temperature of 40 °C for the same duration, a significant 48% reduction in molecular weight was observed. This reduction was primarily attributed to hydrolysis rather than microbial degradation [68].
The process of enzymatic degradation of PLA depends on enzymes secreted by microorganisms. First, microorganisms secrete extracellular PLA depolymerase, then the depolymerase affects the intracellular ester bonds of PLA, producing oligomers, dimers, and monomers. Eventually, these low molecular weight compounds are taken up by microorganisms and further broken down by intracellular enzymes into carbon dioxide, water, and methane. Some of the common enzymes used to degrade PLA are proteinase K, protease, esterase, and lipase. However, it was found that PLA degraded most efficiently by enzymes proteinase K or protease as compared to esterase or lipase [69].
Processing of PLA
Extrusion
The extrusion process involves the uninterrupted melting, conveying, and expulsion of plastic materials via a die. In an extruder, a typical single screw is divided into distinct zones including the feed zone, transition zone, and metering zone, as illustrated in Fig. 13. The L/D ratio and compression ratio are important screw parameters that are relevant to the extrusion process. L/D ratio denotes the ratio between the flight length and the screw outer diameter. Compression ratio refers to the ratio between the depth of flight in the feed section and the depth of flight in the metering section. Typically, for PLA, it is recommended to use L/D ratios ranging from 24 to 30 and compression ratios between 2 and 3 [70].
Extrusion single screw plasticizing unit scheme [70]
A typical single screw extrusion process is illustrated in Fig. 14 [71].
Single Screw Extrusion Process [71]
The plasticization of PLA commences in the conveying zone as the polymer powders or pellets are transported from the hopper to the screw channel. Within the channel, the rotating screw moves the compacted material and the material is sheared and pushed against the wall of the barrel. The material melts due to the friction experienced during the material’s transport via the transition zone. Outside of the barrel, the heat bands are wrapped. The thermal energy from the heater as well as the heat generated by friction during material’s transport increases the temperatures beyond the melting point of PLA (Tm = 170–180 0C). The heater’s temperature is generally set to 200–210 0C to make certain that whole crystalline regions are melted as well as to get an optimum melt viscosity necessary for processing. Following the transition zone, polymer melt passes through the metering zone, generating adequate pressure to propel the material via the die [70, 71].
Extrusion plays a vital role in the shaping processes of blow molding as well as film blowing, where the molten material is expelled through a specially designed die to achieve the desired form. In these processes, high melt stiffness is needed to ensure the stability of the film. Due to its low melt strength, PLA requires improvement to expand the processing range and broaden its application possibilities. Considering this, several investigations have been done to improve the PLA’s melt properties, practicing mainly with chain modification of PLA [72,73,74,75,76,77,78]. In addition, extrusion plays a significant role as a plasticizing unit in both melt spinning [79,80,81,82,83,84] and injection molding [85,86,87,88,89,90,91].
Injection molding
Injection molding stands as a highly significant polymer processing technique, widely employed for various polymers, including PLA [92, 93]. This process is widely used in making complex parts as well as mass production of articles. The major benefits of injection molding process are: (a) direct production from raw material to finished product, (b) without or small post processing operations, (c) fully automated processing can be done, and (d) high production and dimensional precision [94]. In a typical injection molding process, the polymer granules are heated until they melt. The melted material is further injected into a closed mold. Typically, the mold comprises two halves that are compressed together under pressure, surpassing the resistance exerted by the molten material. Subsequently, the injected material is permitted to undergo the cooling and solidification process within the mold. Finally, the two halves of the mold are opened and the molded part (or article) comes out, as shown in Fig. 15. Many design possibilities exist due to very complex geometries of the mold shapes. For rigid packaging container applications, such as food storage containers, generally thin-wall injection molding is used. Injection molding is an excellent choice for producing items with varieties of shapes, such as items for domestic household applications as well as medical applications [95].
Injection molding process [95]
Stretch blow molding
The food industry is increasingly interested in replacing non-biodegradable thermoplastics with PLA for specific beverage products, driven by environmental concerns. To date, PLA bottles have primarily been utilized for beverages that are not oxygen-sensitive, such as still water beverages and pasteurized milk. While there are several technologies available to enhance the barrier properties of PLA bottles (such as multilayer structures, plasma deposition, external coatings, and oxygen scavengers), their adoption is presently restricted because of the comparatively higher manufacturing costs involved. The manufacturing of PLA bottles is done by injection stretch blow molding (ISBM) method, as shown in Fig. 16. First, the process involves the making of preform (called parison also) through an injection molding machine. Then preform is shifted to a blow molding machine, in which it undergoes stretching in axial direction and blowing in hoop direction, resulting in the biaxial orientation of polymer. Within blow molding machine, preform is subjected to infrared heaters that raise its temperature to the appropriate range (85–110 °C) required for the blow molding process (Fig. 16a). Typically, various power settings are employed for the infrared heaters to achieve an optimal temperature profile, ensuring the uniform stretching of the preform and achieving a bottle with a consistent distribution of wall thickness. PLA preforms exhibit a tendency to shrink, particularly in the neck and end cap regions, after reheating, which is attributed to the presence of residual injection molding stresses in those areas. This issue can be mitigated by employing appropriate preform design techniques, incorporating gradual transition regions to alleviate the impact of shrinkage during reheating. Once preform reaches the desired temperature, then it is shifted to blow mold for further processing (Fig. 16b). The blow nozzle is brought down to close off the preform finish, whereas the stretch rod moves in the direction of the preform at a speed of 1 to 1.5 m/s, stretching preform all the way to the base cup (Fig. 16c-e). In pre-blow phase (Fig. 16d & e), compressed air ranging from 0.5 to 2.0 MPa pressure is introduced to the preform via the blow nozzle. This partial inflation of the preform helps prevent contact with stretch rod at the time of axial stretching. Once stretch rod reaches the base cup, it securely holds the preform against the mold base; further air pressure is raised to approximately 3.8 to 4.0 MPa to achieve complete inflation of the preform. As a result, the inflated preform is compelled to conform to the shape of blow mold, thereby imprinting surface details of bottles (Fig. 16f & g). Sustaining high blow pressure for a few seconds is essential to ensure that the bottle adequately cools down before it is discharged, allowing for sufficient cooling time. This manufacturing process is commonly referred to as 2-stage process. But in case of 1-stage process, injection and blow molding of preform are carried out within a single machine that is fitted with both injection and blow molding units. During this method, injection-molded preform is cooled partially to temperatures ranging from 100 to 120 °C and subsequently stretch blown within blow molding station [96].
Injection stretch blow molding (ISBM) of PLA bottle [96]
Thermoforming
Thermoforming process is used to produce trays, plastic cups, blisters, and jars of PLA. First, polymer film is made using flat die extrusion in the thickness range of 50 to 300 μm, and then it is utilized for thermoforming. The polymer is generally heated using an infrared heater at a temperature above its Tg and below its Tm, resulting in a softened sheet. Subsequently, the softened sheet is molded into the desired shape by inserting a mold or applying vacuum pressure, as shown in Fig. 17 [97].
Thermoforming process [97]
Thermoforming of PLA sheets can be accomplished using vacuum forming, compressed air/vacuum forming, or solely with the aid of compressed air. The radiant heater in a PLA thermoforming line needs to be carefully adjusted to operate at very low temperatures. PLA sheets exhibit brittleness at room temperature, necessitating special considerations for handling and storage. There exists a chance of cracking and breaking while in transit in comparison to oriented polystyrene (OPS) or polyethylene terephthalate (PET). It is advisable not to store PLA sheets or finished products at temperatures exceeding 40 °C or in environments with relative humidity higher than 50%. Exposure to elevated temperatures or high humidity can lead to deformation and potential degradation of the PLA material. PLA’s toughness improves with orientation, consequently making thermoformed articles relatively less brittle compared to PLA sheets. Thermoforming of PLA is commonly carried out utilizing forming ovens, molds, and trim tools specifically engineered for PET or polystyrene (PS) materials. Due to higher shrinkage of polypropylene (PP) compared to PLA, molds and trim tools tailored for PP are generally less utilized for thermoforming PLA. Thermoforming PLA often calls for the use of aluminum molds, which are commonly recommended for this purpose. The cooling time required in the mold for PLA is longer compared to PET or PS, as PLA has a lower softening temperature than both PET and PS materials [98].
Applications of PLA
Packaging/food packaging applications
Food packaging plays a crucial role in the food supply chain, ensuring the protection, preservation, and safe delivery of food products. Apart from safeguard food from potential deterioration or spoilage, the packaging improves cleanliness and reduces food wastes and, therefore, it is very important to public health. Over 30% of food production is wasted and disposed of in landfills due to spoilage and rotting. Food packaging also prevents unwanted alterations in food quality (such as color, taste, and other sensory attributes), impede the rapid growth of microbes and the deterioration of nutritional content in the food [99,100,101]. Traditional packaging is usually discarded after the packaged material is received or consumed by the user. Food packaging materials include paper, plastic, glass, aluminum, steel etc. In spite of very good recycling rates (more than 20% of certain types of paper and paperboard), traditional packaging causes a major environmental burden. The recycling rate of plastics is comparatively low, at less than 20% [102]. Petroleum-based polymers, comprising polyethylene (PE), polypropylene (PP), and polyethylene terephthalate (PET), cause significant environmental as well as health issues. But, these materials are more often utilized because of their convenience, lower weights, and relatively simple shape-forming properties [103, 104]. Most of these materials used in food packaging are both non-renewable and non-biodegradable, often ending up in landfills or polluting the oceans. Utilizing such materials for food packaging can pose risks to human health [99,100,101,102]. PLA is renewable, biodegradable and its manufacturing entails 25 to 55% less fossil energy in comparison to polymers based on petroleum [105]. The manufacturing of PLA results in negligible greenhouse gas emissions since the carbon dioxide released during its biodegradation is offset by the carbon dioxide absorbed from the environment during the growth of the agricultural feedstock used as a renewable resource for PLA production [106]. PLA derived from either biomass or agricultural waste may serve as a carbon dioxide sink, contributing to the long-term reduction of greenhouse gas emissions [107]. PLA is used in food packaging films, food-contact articles, food containers, bottle labels, tea bags, grocery bags, etc. applications, as mentioned in Table 5. Under composting conditions characterized by elevated temperatures and humidity, PLA undergoes breakdown within a matter of weeks. The breakdown of PLA consists of two steps: hydrolysis and bacterial attack on fragmented residues. First, high molecular weights PLA polymers (polyester chains) are degraded into low molecular weight LA (lactic acid) oligomers. After the molecular weight of oligomers reduces to 10,000 Da or less, then the soil microbes’ breakdown the oligomers to produce carbon dioxide and water [108]. PLA is widely used among bio-based plastics. Some packaging applications of PLA are shown in Table 6 [109].
Medical/biomedical applications
The bioresorbability and biocompatible properties of PLA make it a widely used material in various medical applications within the human body. The most commonly used biodegradable polymers in healthcare industry are: PLA, PGA (polyglycolic acid), and PDS (polydioxanone). PLA finds application in various biomedical fields, including tissue engineering, sutures, drug delivery systems, implants, wound management, as well as the development of biomaterials for possible biomedical uses. The advantage of using biodegradable biomaterials over non-biodegradable biomaterials is that they do not need implant removal and give long-term biocompatibity. Some of the biomedical applications of PLA are shown in Fig. 18 [19, 108].
Tissue engineering
Tissue engineering involves solving medical problems, like tissue injury and organ damage. Since tissues cannot regenerate themselves in case of extreme bone defects attributed to injury or inborn diseases, therefore, additional intervention are needed to facilitate the healing process. Many metals and ceramics are inappropriate for tissue engineering applications (such as scaffolds) due to lack of biodegradability and limited processability, whereas polymers possess very good flexibility in design since their composition as well as structure can be changed according to the particular needs. Linear aliphatic polyesters like PLA, PGA, and their copolymer PLGA are extensively utilized polymers in bone tissue engineering applications. These polymers are biocompatible and have been approved by FDA for certain human use. The degradation products of PLA, PGA, and PLGA are non-toxic, harmless, natural metabolites, and finally excreted from the body in the form of CO2 and H2O. By changing the chemical composition (for example, LA/GA ratio in copolymers of PLGA), molecular weight, crystallinity, and molecular weight distribution, the degradation rates of these polymers can be modified from several weeks to several years to satisfy the needs for specific applications [110].
The primary role of a scaffold is to serve as an extracellular matrix (ECM) that facilitates cell adhesion and growth. Its purpose is to guide the formation of new, entirely working tissues. The preliminary function of scaffold is to maintain the structural integrity of the cells and tissues even during partial degradation. Biodegradable polymeric scaffolds bring together the benefits of both synthetic and natural materials. PLA, whether used alone or in conjunction with some other biodegradable polymers, offers excellent support for growth of cells. A fibrous scaffold contains substantial level of porosity, which is required to fit massive number of cells. In this context, the pore diameter assumes significance for facilitating cell growth and vascularization as well as the diffusion of nutrients. PLA can be fabricated to different shapes (for example, filament, knitted, braided, non-woven or film) according to the requirement of the organ construction [111].
Due to its remarkable biocompatible properties, PLA has been extensively studied for tissue engineering purposes, particularly in the development of bone scaffolds. The mechanical properties of PLA for tissue engineering applications can be improved by various methods such as, blending, composites formation, and co-polymerization. 3D (Three-dimensional) porous scaffolds made of PLA have been successfully made for culturing various types of cell, with applications in cell-based gene therapy used for cardiovascular diseases, regeneration of muscle tissues, cartilage and bone, as well as treatments for cardiovascular, orthopedic, and neurological conditions. The other two investigations have reported that they placed osteogenic stem cells onto scaffolds composed of this material. These scaffolds were then inserted either into bone defects or just under the skin, aiming to replicate the processes of bone formation known as endochondral and intramembranous ossification. The superior strength of PLLA mesh permits the creation of three-dimensional structures like trays and cages. The degradation process of PLA can range from 10 months to 4 years, and depends on various microstructural features like chemical composition, crystallinity, and porosity. These factors can have an impact on the tensile strength of PLA for specific applications. In addition, PLA has the capability to stimulate isolated cells for tissue regeneration and facilitate the controlled release of drugs like painkillers, anti-inflammatories, and antibiotics. This characteristic has motivated recent research in PLA as scaffolds for cell transplantation [112].
Drug delivery
PLA in the form of microspheres and microcapsules is suitable for sustained drug release of diverse medicinal agents, comprising contraceptives, narcotic antagonists, vaccines, local anesthetics, proteins, and peptides for various durations. Polymeric drugs can be released through any of three mechanisms: erosion, diffusion, or swelling. In case of PLA, the hydrolytic cleavage of ester bonds causes random breakage, leading to the erosion of the device. After degradation, the hydrolytic byproducts of PLA are transformed into non-toxic substances that can be eliminated from the body by means of normal cellular processes and urine. PLA as well as its copolymers can be utilized in the shape of micro- or nano-particles to encapsulate a wide range of drugs, including psychotic, restenosis, hormones, oridonin, dermatotherapy, and protein (BSA). PLA nano-particles can be made by solvent evaporation technique and are found suitable for the development of drug delivery systems. The challenge of achieving controlled drug release may be addressed by modifying the mechanical stability as well as crystallinity level of PLA within the formulation [32, 33, 53, 113,114,115,116,117,118].
During the 1970s, researchers developed a complex that combined naltrexone within a PLA structure, which aimed to investigate its potential for drug delivery purposes. The results indicated that, after a period of 35 days, around 67% of the drug was released in vitro (in laboratory conditions). Subsequent in vivo tests (tests conducted on living organisms) confirmed the complex’s ability to inhibit the effects of morphine. PLA copolymers were used to create microspheres consisting of PLA-PEG-PLA, and these microspheres encapsulated paclitaxel [119, 120]. The sizes of the microsphere were uniform and inside it, there was a porous structure which assisted the drug release. After duration of 1 month, the in vitro release rate was measured at 49%, which extended the duration of drug activity within the body and enhanced its effectiveness. The inconsistent local concentration by drug release was avoided by this sustained-release drug delivery system and therefore, improved the therapeutic effect. The slow release smart drug delivery system offers several advantages over traditional dosage system like; it reduces the negative consequences of medications on gastrointestinal tract and prevents excessive local drug concentrations inside a specific timeframe, which can cause allergies or even toxicity. In addition, the degradable materials can undergo degradation in certain environment, which means that the external polymeric materials can be modified to obtain controlled drug release [121,122,123].
In case of few specific diseases like tuberculosis and tumors, therapeutic drugs may exhibit elevated toxicity and large side effects. Besides their intended effect of eliminating the targeted lesions, these drugs can also inflict considerable harm on normal tissues, leading to significant damage. Also, the treatment procedure needs repeated and frequent administrations of the drugs [124]. Therefore, the investigation of targeted drug delivery system is very important [125]. By utilizing a specific type of coating material and employing target selection based on the affinity of functional groups, it becomes possible to effectively direct the drug to the site of the lesion. This targeted approach enhances the bioavailability of the drug, reduces its toxic side effects, and minimizes toxicity to other tissues [126, 127]. But, the polymer needs to undergo chemical modifications to become more effective for targeting or inducing a stimulatory response. A type of pH-sensitive linkage has been innovated to modify PLA nano-particles loaded with doxorubicin, permitting them to easily disintegrate within the tumor microenvironment, as illustrated in Fig. 19. Hence, modified PLAs such as PLGA (poly lactic-co-glycolic acid) are generally utilized in drug-delivery systems. For magnetic nano-particles, ferric oxide is very crucial in drug delivery systems. PLGA was first embedded with ferric oxide and afterwards doxorubicin (DOX) was encapsulated inside to obtain a nanocomposite carrier. Due to high sensitivity of the carrier to the external magnetic field, magnetic targeting was fulfilled by the carrier. Also, this drug possesses very good ability to inhibit or suppress the growth and development of tumors [128].
High drug loading and pH-responsive PLA-g-doxorubicin (DOX) nanoparticles [128]
Implants
In recent years, there has been a shift towards employing biodegradable materials instead of metallic materials for fractured bones fixation, which can take the form of plates, screws, pins, and wires. High strength is needed for bone fixation materials, and therefore, PLA finds broad application in this area [19]. Haers et al. (1998) [129] investigated an enhancement in mechanical characteristics of PLA by controlling the ratio of L-LA / D-LA in polymer. By taking L-LA/D-LA (85/15) ratio, production of PLA was done and successfully utilized in making screws and plates for fixation of fractures. The results also showed the possibility of using the plates independently without requiring extra support for fracture fixation. Van Sliedregt et al. (1992) [130] studied the biocompatibility of 4 PLA films for orthopedic applications by taking 5 types of cell: ear canal, middle ear, and nasal septum of epithelial cells along with fibroblasts and osteosarcoma cells. The study yielded favorable results, indicating satisfactory biocompatibility of PLA.
The biodegradable polymer implants readily undergo degradation within the human body, eliminating the need for additional surgical procedures to remove them once the defect site is repaired. This characteristic holds significant importance in the medical field. Applications that demand prolonged strength retention like reconstruction of ligament and tendon along with stents used in vascular and urological surgery, often favor the use of PLLA fibers (Durselen et al. 2001) [131]. Bioresorbable plates and screws are extensively utilized in various maxillofacial osteosynthetic surgeries as implants (Kanno et al. 2018) [132]. Injectable microspheres made of PLLA have been employed as temporary fillers in facial reconstructive surgery. The microspheres made of PLLA have also been reported to be utilized as embolic material in trans catheter procedures. This application plays a significant role in the successful treatment of fistulas, arteriovenous anomalies, severe bleeding, and tumors [133, 134].
Sutures and stents
Medical sutures synthesized from PLA are non-toxic, biocompatible, biodegradable, and have high strength. PLA breaks down into carbon dioxide and water gradually during wound healing process, which avoids the pain related to wound removal [135, 136]. PLA sutures are used in different kinds of surgery, such as facial, neck, colostomy, circumcision, etc [137]. . . Various types of wounds need various healing times and degradation rate of sutures of PLA is fixed, therefore, the degradation rate is altered by manipulating the diameter of the suture, copolymerizing PLA with other polymers like PEG (polyethylene glycol), and PGA (polyglycolic acid), or blending with nano-materials like nano-silica and nano-montmorillonite, but these processes only shorten the degradation time. Taking this into consideration, Liu et al. (2019) [138] compounded carbon nanotube (CNT) by adding PLA sutures and prolonged the degradation time of resulting PLA composite. The total degradation period of CNT/PLA sutures was increased from 49 weeks to a range of 63 to 73 weeks as compared to pure PLA sutures, with effectively extending the strength also.
Surgical sutures are wound closure filaments manufactured in different shapes. These sutures secure tissues in position until the wound naturally heals and gives enough tissue strength. The U.S. Federal Drug Administration (FDA) has approved PLA for utilizing as a suture material due to its advantageous features such as biocompatibility, bioresorbability, and biodegradability with non-toxic degradation products. But, the inherent properties of PLA like high crystallinity (around 40%), and slow rate of degradation, impose restrictions on its application as material for suture. Because of this, copolymerization of lactic acid (LA) with biodegradable monomers, like glycolic acid (GA), is done to obtain the PGA/PLA copolymer, which has better properties than PLA for suture applications. PGA/PLA copolymer with 90:10 mol ratio of GA: LA has been commercialized as Vicryl by Ethicon. This amorphous copolymer has lower glass transition temperature (Tg) and higher degradation rate than that of PLA and has been found very much useful as a suture material [111, 139].
A stent is a small mesh tube used to hold open passages in the human body, such as weak or narrowed arteries. There exist three major kinds of stents: (a) bare-metal stents, (b) drug-eluting stents, and (c) biodegradable / bioresorbable stents. Bare-metal stents and drug-eluting stents may leave residues in the body that can affect further treatment and recovery process, whereas bioresorbable stents, providing both mechanical support and drug delivery to the vessel wall, can be fully absorbed by the body, and therefore, permits better vascular remodeling and decreases the triggering of persistent inflammation. Among different biodegradable polymeric stents, PLA (especially PLLA-based stents) has been proven to be a reliable and effective biomaterial. In order to improve the mechanical properties and degradation characteristics of PLA-based stents, different PLA-matrix composites have been studied over the past many years. In the field of bone tissue engineering, research has focused on combining PLLA with other biomaterials like bio-ceramics, metals, or polymers. This approach aims to enhance the mechanical properties, promote improved tissue grafting with implants, and achieve a controlled degradation rate. The rate of polymer degradation is governed by several factors, including the degree of crystallinity, the distribution of crystallites, and the polymer’s water uptake. By controlling these factors, the development of PLA-based biodegradable stents can be achievable [140].
Agricultural applications
PLA is used in agricultural applications, such as mulch films, delivery system for fertilizers and pesticides, temporary replanting pots, compost bags, agricultural nets etc [16]. . . The use of plastic in agriculture is known as plasticulture, which began in 1950s to increase the production. The main reasons for using plastic in agriculture are: (a) Protection of soil from erosion and to protect plants from weeds, insects, and birds with the use of mulch films, (b) Use of drip irrigation tubing, and (c) To shield the tunnels of greenhouses. At present, the non-degradable traditional plastics are being replaced by the environment-friendly biodegradable plastics such as PLA, PHAs (polyhydroxyalkanoates), and PBAT (polybutylene adipate terephthalate) in many agricultural applications.
Due to inferior mechanical and thermal properties of pure / homopolymer PLA, its use as homopolymer is limited in agricultural applications. Therefore, blending of PLA is done with biodegradable polyesters, such as PBAT, to make commercial PLA/PBAT mulch films. In addition, plasticizers are also used to make biodegradable PLA-based mulch films because the addition of plasticizer promotes the hydrolysis and accelerate the biodegradation of PLA-based mulch films. The use of bioplastics in agriculture is currently new and still under development and therefore, needs improvement in many areas, including the high cost of the polymers [141].
Automotive applications
PLA is used in automotive industry in many engineering parts, especially in interior components and under-the-hood gears. Since PLA is biodegradable and compostable, it reduces the carbon footprint and gives several advantages, such as high tensile strength and stiffness, improved UV resistance, high gloss, and dimensional stability. Due to these features, PLA can replace many conventional thermoplastics, such as PET (polyethylene terephthalate), ABS (acrylonitrile butadiene styrene), PBT (polybutylene terephthalate), and polyamides, which are generally utilized in automobile interiors and engine compartments [142].
PLA composites incorporating biofibers are used globally in various applications, including automotive interiors. Biofibers or fillers are eco-friendly materials, which can be used to improve the mechanical and thermal characteristics of PLA. The plant fiber is extensively used in E-class models of Mercedes-Benz. These biofiber composites are used in different components of an automobile like dashboards, package trays, door panels, headliners, and various interior components [143]. The performance of PLA composites can be improved by using nanofillers and suitable processing methods. PLA nanocomposites reinforced with graphene can improve thermal stability and high moisture absorption characteristics, which can be useful in automotive applications [144]. PLLA biopolymers can be used in many automotive components, especially automotive interior parts. Today, bio-based PLLA blends are utilized by various automobile companies, such as Toyota, Ford, Mazda, and Hyundai Motors [145].
Textile applications
PLA fibers manufactured by melt spinning process have physical properties comparable to PET and Nylon. Due to its aliphatic polyester nature and the absence of aromatic rings, PLA exhibits superior moisture regain and wicking properties as compared to PET. Clothing items manufactured from PLA or blended with wool or cotton offer a high level of comfort and provide a luxurious, silky touch sensation. PLA fibers possess improved self-extinguishing characteristics because PLA is a non-flammable polymer [146,147,148].Fibers can be synthesized either by solvent or by melt spinning techniques. The fibers made by solvent spinning have better mechanical properties than the fibers made by melt spinning due to the occurrence of thermal degradation while undergoing the melt spinning process [149].
The use of biodegradable polymer PLA in the textile industries is increasing. Garment industries (e.g., apparel, homeware) are currently using PLA textiles. Because of its breathability and wicking properties, PLA is considered as a comfortable biomaterial for making apparel. The Hohenstein Research Institute examined the application of PLA as well as PLA-cotton blends in clothing by conducting various tests, and it was found that the thermal insulation and sweat absorption abilities of PLA makes it highly appropriate material for sports apparel in addition to its other features. PLA is used for making jackets due to its good resilience property. PLA finds application in knitted and embroidered textiles because of its good retention and crimp properties. PLA textiles are used in homeware, such as curtains, pillowcases, and rugs. However, PLA textiles present certain challenges, including lower pressing and ironing temperatures in comparison to cotton and PET fabrics. Dyeing and finishing operations of textiles go through the conditions, such as temperature, time, and pH, which impose challenges for PLA because PLA is prone to degradation under the above mentioned conditions [150, 151]. In brief, PLA has huge potential to be used in fiber and textile industries, but it requires more development and modifications to compete with conventional petroleum-based polymers due to its limitations.
Conclusions and future perspectives
Over the last few years, bio-based and biodegradable polymers have been emerged as a promising and environment friendly alternative to conventional petroleum-based polymers. PLA is one of the most favorable biodegradable biopolymers because of its excellent physical, mechanical, and thermal properties as well as very good processability. Due to bioresorbability and biocompatibility properties of PLA, it is extensively used in biomedical applications. But, in comparison to certain traditional thermoplastics, PLA demonstrates inferior toughness, heat stability, and water barrier characteristics. Therefore, great efforts have been undertaken for improving the performance characteristics of PLA by applying various modification methods, such as copolymerization, making composites or blends of PLA, incorporation of different additives etc.
PLA has great potential to be utilized in number of applications due to its very good processing characteristics and therefore, it can play an important role in the form of copolymers, composites and blends by exhibiting different properties for various applications. In this way, the renewable, biodegradable and compostable polymer PLA can replace conventional petroleum-based polymers in many applications. Indeed, the cost of PLA is still higher than many conventional thermoplastics and therefore, further research is required in this area to manufacture PLA at lower cost. By using the appropriate method of synthesis, optimizing the synthesis parameters (like temperature, time, and catalyst type), and using cheaper substrates as well as high-performance microorganisms to increase the production efficiency of lactic acid, are some of the methods by which cost of producing PLA can be reduced in the near future, but still further research and development is needed in this field for the accomplishment of this challenging task.
Abbreviations
- PLA:
-
Polylactic acid
- FDA:
-
Food and Drug Administration
- PHA:
-
Polyhydroxyalkanoate
- PHB:
-
Polyhydroxybutyrate
- PGA:
-
Polyglycolic acid
- PCL:
-
Polycaprolactone
- PBSA:
-
Polybutylene succinate-co-adipate
- LA:
-
Lactic acid
- PDLLA:
-
Poly-D, L-lactic acid
- ROP:
-
Ring-opening polymerization
- Sn(Oct)2 :
-
Stannous octoate
- PHAs:
-
Polyhydroxyalkanoates
- PEG:
-
Polyethylene glycol
- THF:
-
Tetrahydrofuran
- PLLA:
-
Poly-L-lactic acid
- PDLA:
-
Poly-D- lactic acid
- Tg :
-
Glass transition temperature
- Tm :
-
Melting temperature
- Mw :
-
Molecular Weight
- PVC:
-
Polyvinyl chloride
- PP:
-
Polypropylene
- PS:
-
Polystyrene
- DSC:
-
Differential Scanning Calorimetry
- PET:
-
Polyethylene terephthalate
- ISBM:
-
Injection stretch blow molding
- OPS:
-
Oriented polystyrene
- PDS:
-
Polydioxanone
- PLGA:
-
Poly lactic-co-glycolic acid
- DOX:
-
Doxorubicin
- CNT:
-
Carbon nanotube
- PBAT:
-
Polybutylene adipate terephthalate
- ABS:
-
Acrylonitrile butadiene styrene
- PBT:
-
Polybutylene terephthalate
References
European-Bioplastics.Org/Market (2022)
European, Bioplastics (2022) Bioplastics facts and figures
Jem KJ, Tan B (2020) The development and challenges of poly (lactic acid) and poly (glycolic acid). Adv Ind Eng Polym Res 3(2):60–70. https://doi.org/10.1016/j.aiepr.2020.01.002
Ramezani Dana H, Ebrahimi F (2023) Synthesis, properties, and applications of polylactic acid-based polymers. Polym Eng Sci 63(1):22–43. https://doi.org/10.1002/pen.26193
Li G, Zhao M, Xu F, Yang B, Li X, Meng X, Li Y (2020) Synthesis and biological application of polylactic acid. Molecules 25(21):5023. https://doi.org/10.3390/molecules25215023
Zhong Y, Godwin P, Jin Y, Xiao H (2020) Biodegradable polymers and green-based antimicrobial packaging materials: a mini-review. Adv Ind Eng Polym Res 3(1):27–35. https://doi.org/10.1016/j.aiepr.2019.11.002
Babu RP, O’connor K, Seeram R (2013) Current progress on bio-based polymers and their future trends. Prog Biomater 2:1–16. https://doi.org/10.1186/2194-0517-2-8
Averous L (2004) Biodegradable multiphase systems based on plasticized starch: a review. J Macromol Sci C Polym Rev 44(3):231–274. https://doi.org/10.1081/MC-200029326
Arjmandi R, Hassan A, Zakaria Z (2017) Polylactic acid green nanocomposites for automotive applications. Green biocomposites: design and applications. Springer, Cham, pp 193–208. https://doi.org/10.1007/978-3-319-49382-4_9.
Jem KJ, van der Pol JF, de Vos S (2010) Microbial lactic acid, its polymer poly (lactic acid), and their industrial applications. Plastics from Bacteria: natural functions and applications. Springer, Berlin, Heidelberg, pp 323–346. https://doi.org/10.1007/978-3-642-03287-5_13.
Stefaniak K, Masek A (2021) Green copolymers based on poly (lactic Acid)—Short review. Materials 14(18):5254. https://doi.org/10.3390/ma14185254
Hartmann MH (1998) High molecular weight polylactic acid polymers. Biopolymers from renewable resources. Springer, Berlin, Heidelberg, pp 367–411. https://doi.org/10.1007/978-3-662-03680-8_15
Li X, Lin Y, Liu M, Meng L, Li C (2023) A review of research and application of polylactic acid composites. J Appl Polym Sci 140(7):e53477. https://doi.org/10.1002/app.53477
Peres C, Matos AI, Conniot J, Sainz V, Zupančič E, Silva JM, Graça L, Gaspar RS, Préat V, Florindo HF (2017) Poly (lactic acid)-based particulate systems are promising tools for immune modulation. Acta Biomater 48:41–57. https://doi.org/10.1016/j.actbio.2016.11.012
Lunt J (1998) Large-scale production, properties and commercial applications of polylactic acid polymers. Polym Degrad Stab 59(1–3):145–152. https://doi.org/10.1016/S0141-3910(97)00148-1
Gupta AP, Kumar V (2007) New emerging trends in synthetic biodegradable polymers–Polylactide: a critique. Eur Polym J 43(10):4053–4074. https://doi.org/10.1016/j.eurpolymj.2007.06.045
Garlotta D (2001) A literature review of poly (lactic acid). J Polym Environ 9:63–84. https://doi.org/10.1023/A:1020200822435
Cheng Y, Deng S, Chen P, Ruan R (2009) Polylactic acid (PLA) synthesis and modifications: a review. Front Chem China 4:259–264. https://doi.org/10.1007/s11458-009-0092-x
Singhvi MS, Zinjarde SS, Gokhale DV (2019) Polylactic acid: synthesis and biomedical applications. J Appl Microbiol 127(6):1612–1626. https://doi.org/10.1111/jam.14290
Kricheldorf HR, Sumbel M (1989) Polylactones—18. Polymerization of l, l-lactide with sn (II) and sn (IV) halogenides. Eur Polym J 25(6):585–591. https://doi.org/10.1016/0014-3057(89)90010-4
Gruber P (2004) Chemicals from renewable resources
Vink ET, Rabago KR, Glassner DA, Gruber PR (2003) Applications of life cycle assessment to Nature Works™ polylactide (PLA) production. Polym Degrad Stab 80(3):403–419. https://doi.org/10.1016/S0141-3910(02)00372-5
Shih YF, Huang CC, Chen PW (2010) Biodegradable green composites reinforced by the fiber recycling from disposable chopsticks. Mater Sci Eng A 527(6):1516–1521. https://doi.org/10.1016/j.msea.2009.10.024
Athanasiou KA, Niederauer GG, Agrawal CM (1996) Sterilization, toxicity, biocompatibility and clinical applications of polylactic acid/polyglycolic acid copolymers. Biomaterials 17(2):93–102. https://doi.org/10.1016/0142-9612(96)85754-1
Abd Alsaheb RA, Aladdin A, Othman NZ, Abd Malek R, Leng OM, Aziz R, El Enshasy HA (2015) Recent applications of polylactic acid in pharmaceutical and medical industries. J Chem Pharm Res 7(12):51–63
Taib NAAB, Rahman MR, Huda D, Kuok KK, Hamdan S, Bakri MKB, Julaihi MRMB, Khan A (2023) A review on poly lactic acid (PLA) as a biodegradable polymer. Polym Bull 80(2):1179–1213. https://doi.org/10.1007/s00289-022-04160-y
Nampoothiri KM, Nair NR, John RP (2010) An overview of the recent developments in polylactide (PLA) research. Bioresour Technol 101(22):8493–8501. https://doi.org/10.1016/j.biortech.2010.05.092
Griffith LG (2000) Polymeric biomaterials. Acta Mater 48(1):263–277. https://doi.org/10.1016/S1359-6454(99)00299-2
Casalini T, Rossi F, Castrovinci A, Perale G (2019) A perspective on polylactic acid- based polymers use for nanoparticles synthesis and applications. Front Bioeng Biotechnol 7:259. https://doi.org/10.3389/fbioe.2019.00259
Davachi SM, Kaffashi B (2015) Polylactic acid in medicine. Polym Plast Technol Eng 54(9):944–967. https://doi.org/10.1080/03602559.2014.979507
Yamane H, Sasai K (2003) Effect of the addition of poly (D-lactic acid) on the thermal property of poly (L-lactic acid). Polymer 44(8):2569–2575. https://doi.org/10.1016/S0032-3861(03)00092-2
Lasprilla AJ, Martinez GA, Lunelli BH, Jardini AL, Maciel Filho R (2012) Poly-lactic acid synthesis for application in biomedical devices—A review. Biotechnol Adv 30(1):321–328. https://doi.org/10.1016/j.biotechadv.2011.06.019
Farah S, Anderson DG, Langer R (2016) Physical and mechanical properties of PLA, and their functions in widespread applications—A comprehensive review. Adv Drug Deliv Rev 107:367–392. https://doi.org/10.1016/j.addr.2016.06.012
Södergård A, Stolt M (2002) Properties of lactic acid-based polymers and their correlation with composition. Prog Polym Sci 27(6):1123–1163. https://doi.org/10.1016/S0079-6700(02)00012-6
Van de Velde K, Kiekens P (2002) Biopolymers: overview of several properties and consequences on their applications. Polym Test 21(4):433–442. https://doi.org/10.1016/S0142-9418(01)00107-6
Ranakoti L, Gangil B, Mishra SK, Singh T, Sharma S, Ilyas RA, El-Khatib S (2022) Critical review on polylactic acid: Properties, structure, processing, biocomposites, and nanocomposites. Materials 15(12):4312. https://doi.org/10.3390/ma15124312
Bajpai PK, Singh I, Madaan J (2014) Development and characterization of PLA-based green composites: a review. J Thermoplast Compos Mater 27(1):52–81. https://doi.org/10.1177/0892705712439571
Mangaraj S, Yadav A, Bal LM, Dash SK, Mahanti NK (2019) Application of biodegradable polymers in food packaging industry: a comprehensive review. J Package Technol Res 3:77–96. https://doi.org/10.1007/s41783-018-0049-y
Ranakoti L, Gupta MK, Rakesh PK (2019) Analysis of mechanical and tribological behavior of wood flour filled glass fiber reinforced epoxy composite. Mater Res Express 6(8):085327. https://doi.org/10.1088/2053-1591/ab2375
Ranakoti L, Rakesh PK (2020) Physio-mechanical characterization of tasar silk waste/jute fiber hybrid composite. Compos Commun 22:100526. https://doi.org/10.1016/j.coco.2020.100526
Ebrahimi F, Ramezani Dana H (2022) Poly lactic acid (PLA) polymers: from properties to biomedical applications. Int J Polym Mater 71(15):1117–1130. https://doi.org/10.1080/00914037.2021.1944140
Auras R, Harte B, Selke S (2004) An overview of polylactides as packaging materials. Macromol Biosci 4(9):835–864. https://doi.org/10.1002/mabi.200400043
Tsuji H, Ikada Y (2000) Properties and morphology of poly (L-lactide) 4. Effects of structural parameters on long-term hydrolysis of poly (L-lactide) in phosphate- buffered solution. Polym Degrad Stab 67(1):179–189. https://doi.org/10.1016/S0141-3910(99)00111-1
Dorgan JR, Janzen J, Clayton MP, Hait SB, Knauss DM (2005) Melt rheology of variable L-content poly (lactic acid). J Rheol 49(3):607–619. https://doi.org/10.1122/1.1896957
Mehta R, Kumar V, Bhunia H, Upadhyay SN (2005) Synthesis of poly (lactic acid): a review. J Macromol Sci C Polym Rev 45(4):325–349. https://doi.org/10.1080/15321790500304148
Bigg DM (2005) Polylactide copolymers: Effect of copolymer ratio and end capping on their properties. Adv Polym Technol 24(2):69–82. https://doi.org/10.1002/adv.20032
Hughes J, Thomas R, Byun Y, Whiteside S (2012) Improved flexibility of thermally stable poly-lactic acid (PLA). Carbohydr Polym 88(1):165–172. https://doi.org/10.1016/j.carbpol.2011.11.078
Pyda M, Bopp RC, Wunderlich B (2004) Heat capacity of poly (lactic acid). J Chem Thermodyn 36(9):731–742. https://doi.org/10.1016/j.jct.2004.05.003
Lehermeier HJ, Dorgan JR (2001) Melt rheology of poly (lactic acid): consequences of blending chain architectures. Polym Eng Sci 41(12):2172–2184. https://doi.org/10.1002/pen.10912
Hamad K, Ko YG, Kaseem M, Deri F (2014) Effect of acrylonitrile–butadiene–styrene on flow behavior and mechanical properties of polylactic acid/low density polyethylene blend. Asia-Pac. J Chem Eng 9(3):349–353. https://doi.org/10.1002/apj.1802
Fang Q, Hanna MA (1999) Rheological properties of amorphous and semicrystalline polylactic acid polymers. Ind Crops Prod 10(1):47–53. https://doi.org/10.1016/S0926-6690(99)00009-6
Dorgan JR, Lehermeier H, Mang M (2000) Thermal and rheological properties of commercial-grade poly (lactic acid) s. J Polym Environ 8:1–9. https://doi.org/10.1023/A:1010185910301
Hamad K, Kaseem M, Yang HW, Deri F, Ko YG (2015) Properties and medical applications of polylactic acid: a review. Express Polym Lett 9(5):435–455. https://doi.org/10.3144/expresspolymlett.2015.42
Auras R, Harte B, Selke S (2004) Effect of water on the oxygen barrier properties of poly (ethylene terephthalate) and polylactide films. J Appl Polym Sci 92(3):1790–1803. https://doi.org/10.1002/app.20148
Auras RA, Singh SP, Singh JJ (2005) Evaluation of oriented poly (lactide) polymers vs. existing PET and oriented PS for fresh food service containers. Packag Technol Sci 18(4):207–216. https://doi.org/10.1002/pts.692
Thellen C, Orroth C, Froio D, Ziegler D, Lucciarini J, Farrell R, D’Souza NA, Ratto JA (2005) Influence of montmorillonite layered silicate on plasticized poly (l-lactide) blown films. Polymer 46(25):11716–11727. https://doi.org/10.1016/j.polymer.2005.09.057
Bao L, Dorgan JR, Knauss D, Hait S, Oliveira NS, Maruccho IM (2006) Gas permeation properties of poly (lactic acid) revisited. J Membr Sci 285(1–2):166–172. https://doi.org/10.1016/j.memsci.2006.08.021
Karamanlioglu M, Preziosi R, Robson GD (2017) Abiotic and biotic environmental degradation of the bioplastic polymer poly (lactic acid): a review. Polym Degrad Stab 137:122–130. https://doi.org/10.1016/j.polymdegradstab.2017.01.009
Hakkarainen M (2002) Aliphatic polyesters: abiotic and biotic degradation and degradation products. Adv Polym Sci 157:113–138. https://doi.org/10.1007/3-540-45734-8_4
Saha SK, Tsuji H (2006) Effects of molecular weight and small amounts of d-lactide units on hydrolytic degradation of poly (l-lactic acid) s. Polym Degrad Stab 91(8):1665–1673. https://doi.org/10.1016/j.polymdegradstab.2005.12.009
Ho KLG, Pometto AL, Hinz PN (1999) Effects of temperature and relative humidity on polylactic acid plastic degradation. J Environ Polym Degrad 7:83–92. https://doi.org/10.1023/A:1021808317416
Sangwan P, Wu DY (2008) New insights into polylactide biodegradation from molecular ecological techniques. Macromol Biosci 8(4):304–315. https://doi.org/10.1002/mabi.200700317
Henton DE, Gruber P, Lunt J, Randall J (2005) Polylactic acid technology. Natural fibers. Biopolymers Biocomposites 16:527–577
Tokiwa Y, Calabia BP (2006) Biodegradability and biodegradation of poly (lactide). Appl Microbiol Biotechnol 72:244–251. https://doi.org/10.1007/s00253-006-0488-1
Itävaara M, Karjomaa S, Selin JF (2002) Biodegradation of polylactide in aerobic and anaerobic thermophilic conditions. Chemosphere 46(6):879–885. https://doi.org/10.1016/S0045-6535(01)00163-1
Tsuji H, Suzuyoshi K (2002) Environmental degradation of biodegradable polyesters 1. Poly (ε-caprolactone), poly [(R)-3-hydroxybutyrate], and poly (L-lactide) films in controlled static seawater. Polym Degrad Stab 75(2):347–355. https://doi.org/10.1016/S0141-3910(01)00240-3
Tsuji H, Suzuyoshi K (2002) Environmental degradation of biodegradable polyesters 2. Poly (ε-caprolactone), poly [(R)-3-hydroxybutyrate], and poly (L-lactide) films in natural dynamic seawater. Polym Degrad Stab 75(2):357–365. https://doi.org/10.1016/S0141-3910(01)00239-7
Le Duigou A, Davies, P E T E R, Baley C (2009) Seawater ageing of flax/poly (lactic acid) biocomposites. Polym Degrad Stab 94(7):1151–1162. https://doi.org/10.1016/j.polymdegradstab.2009.03.025
Richert A, Dąbrowska GB (2021) Enzymatic degradation and biofilm formation during biodegradation of polylactide and polycaprolactone polymers in various environments. Int J Biol Macromol 176:226–232. https://doi.org/10.1016/j.ijbiomac.2021.01.202
Kühnert I, Spörer Y, Brünig H, Tran NHA, Rudolph N (2018) Processing of poly (lactic acid). Adv Polym Sci 1–33. https://doi.org/10.1007/12_2017_30
Kazmer D (2017) Design of plastic parts. Applied Plastics Engineering Handbook, 2nd edn. William Andrew Publishing, pp 593–615. https://doi.org/10.1016/B978-0-323-39040-8.00028-6.
Corre YM, Duchet J, Reignier J, Maazouz A (2011) Melt strengthening of poly (lactic acid) through reactive extrusion with epoxy-functionalized chains. Rheol Acta 50:613–629. https://doi.org/10.1007/s00397-011-0538-1
Ljungberg N, Andersson T, Wesslén B (2003) Film extrusion and film weldability of poly (lactic acid) plasticized with triacetine and tributyl citrate. J Appl Polym Sci 88(14):3239–3247. https://doi.org/10.1002/app.12106
Zhou ZF, Huang GQ, Xu WB, Ren FM (2007) Chain extension and branching of poly (L-lactic acid) produced by reaction with a DGEBA-based epoxy resin. Express Polym Lett 1(11):734. https://doi.org/10.3144/expresspolymlett.2007.101
Anderson KS, Hillmyer MA (2004) The influence of block copolymer microstructure on the toughness of compatibilized polylactide/polyethylene blends. Polymer 45(26):8809–8823. https://doi.org/10.1016/j.polymer.2004.10.047
Ljungberg N, Wesslen B (2002) The effects of plasticizers on the dynamic mechanical and thermal properties of poly (lactic acid). J Appl Polym Sci 86(5):1227–1234. https://doi.org/10.1002/app.11077
Kulinski B, Piorkowska E (2005) Crystallization, structure and properties of plasticized poly (L-lactide). Polymer 46(23):10290–10300. https://doi.org/10.1016/j.polymer.2005.07.101
Martin O, Avérous L (2001) Poly (lactic acid): plasticization and properties of biodegradable multiphase systems. Polymer 42(14):6209–6219. https://doi.org/10.1016/S0032-3861(01)00086-6
Hagen R (2013) The potential of PLA for the fiber market. Bioplastic Magazine 8:12–15
Schmack G, Tändler B, Optiz G, Vogel R, Komber H, Häußler L, Voigt D, Weinmann S, Heinemann M, Fritz HG (2004) High-speed melt spinning of various grades of polylactides. J Appl Polym Sci 91(2):800–806. https://doi.org/10.1002/app.13170
Perepelkin KE (2002) Polylactide fibres: fabrication, properties, use, prospects. A review. Fibre Chem 34(2):85–100. https://doi.org/10.1023/A:1016359925976
Beyreuther R, Brünig H (2006) Dynamics of fibre formation and processing: modeling and application in fibre and textile industry. Springer Science and Business Media
Hahn J, Breier A, Brünig H, Heinrich G (2016) Mechanical adapted embroidered scaffolds based on polylactic acid melt spun multifilaments for ligament tissue engineering. Annual report of Leibniz-Institut für Polymerforschung Dresden e. V., Dresden, chapter: Biologyinspired interface and material design, pp 42–44
An Tran NH, Brünig H, Hinüber C, Heinrich G (2014) Melt spinning of biodegradable nanofibrillary structures from poly (lactic acid) and poly (vinyl alcohol) blends. Macromol Mater Eng 299(2):219–227. https://doi.org/10.1002/mame.201300125
Endres HJ, Siebert-Raths A (2011) Manufacture and chemical structure of biopolymers. Engineering Biopolymers: Markets, Manufacturing, Properties ans Applications, pp 71–148
Ghosh S, Viana JC, Reis RL, Mano JF (2007) Effect of processing conditions on morphology and mechanical properties of injection-molded poly (l-lactic acid). Polym Eng Sci 47(7):1141–1147. https://doi.org/10.1002/pen.20799
Ghosh S, Viana JC, Reis RL, Mano JF (2008) Oriented morphology and enhanced mechanical properties of poly (l-lactic acid) from shear controlled orientation in injection molding. Mater Sci Eng A 490(1–2):81–89. https://doi.org/10.1016/j.msea.2008.01.003
Kuehnert I (2009) Cold and hot interfaces during injection molding. In: PPS-25 Polymer Processing Society, Goa, Indien
Kuehnert I, Pompsch I (2011) Morphology and strength of injection molded parts with interfaces. In: Proceedings of SPE Antec
Kuehnert I, Schoenfeldt A, Auf der Landwehr M (2013) New insights into interfaces in injection molded parts. In: Proceedings of SPE Antec, Society of Plastics Engineers, Cincinnati
Kuehnert I, Spoerer Y, Zimmermann M (2016) Weld lines in injection molded parts: strength, morphology and improvement. In: Proceedings of SPE Antec
Ren J (ed) (2011) Biodegradable poly (lactic acid): synthesis, modification, processing and applications. Springer Science and Business Media
Fachagentur Nachwachsender Rohstoffe (FNR) (2016) Processing of bioplastics – a guideline. Report
Kamal MR, Isayev AI (eds) (2012) Injection molding: technology and fundamentals. Carl Hanser Verlag GmbH Co KG
Maddah HA (2016) Polypropylene as a promising plastic: a review. Am J Polym Sci 6(1):1–11. https://doi.org/10.5923/j.ajps.20160601.01
Lim LT, Auras R, Rubino M (2008) Processing technologies for poly (lactic acid). Prog Polym Sci 33(8):820–852. https://doi.org/10.1016/j.progpolymsci.2008.05.004
Reichert CL, Bugnicourt E, Coltelli MB, Cinelli P, Lazzeri A, Canesi I, Braca F, Martínez BM, Alonso R, Agostinis L, Verstichel S, Six L, Mets SD, Gómez EC, Ißbrücker C, Geerinck R, Nettleton DF, Campos I, Sauter E, Pieczyk P, Schmid M (2020) Bio-based packaging: materials, modifications, industrial applications and sustainability. Polymers 12(7):1558. https://doi.org/10.3390/polym12071558
Jamshidian M, Tehrany EA, Imran M, Jacquot M, Desobry S (2010) Poly-lactic acid: production, applications, nanocomposites, and release studies. Compr Rev Food Sci Food Saf 9(5):552–571. https://doi.org/10.1111/j.1541-4337.2010.00126.x
Aguirre-Joya JA, De Leon-Zapata MA, Alvarez-Perez OB, Torres-León C, Nieto-Oropeza DE, Ventura-Sobrevilla JM, Aguilar MA, Ruelas-Chacón X, Rojas R, Ramos-Aguiñaga ME, Aguilar CN (2018) Basic and applied concepts of edible packaging for foods. In: Food Packaging and Preservation, Academic Press, pp 1–61. https://doi.org/10.1016/B978-0-12-811516-9.00001-4
Saklani P, Siddhnath DS, Singh SM (2019) A review of edible packaging for foods. Int J Curr Microbiol Appl Sci 8(07):2885–2895
Jeevahan J, Anderson A, Sriram V, Durairaj RB, Britto Joseph G, Mageshwaran G (2021) Waste into energy conversion technologies and conversion of food wastes into the potential products: a review. Int J Ambient Energy 42(9):1083–1101. https://doi.org/10.1080/01430750.2018.1537939
Jeevahan J, Chandrasekaran M (2019) Nanoedible films for food packaging: a review. J Mater Sci 54(19):12290–12318. https://doi.org/10.1007/s10853-019-03742-y
Ahmadi P, Jahanban-Esfahlan A, Ahmadi A, Tabibiazar M, Mohammadifar M (2022) Development of ethyl cellulose-based formulations: a perspective on the novel technical methods. Food Rev Int 38(4):685–732. https://doi.org/10.1080/87559129.2020.1741007
Vasile C (2018) Polymeric nanocomposites and nanocoatings for food packaging: a review. Materials 11(10):1834. https://doi.org/10.3390/ma11101834
Xiao L, Wang B, Yang G, Gauthier M (2012) Poly (lactic acid)-based biomaterials: synthesis, modification and applications. Biomedical Sci Eng Technol 11:247–282
Bátori V, Åkesson D, Zamani A, Taherzadeh MJ, Horváth IS (2018) Anaerobic degradation of bioplastics: a review. Waste Manage 80:406–413. https://doi.org/10.1016/j.wasman.2018.09.040
Mohanty AK, Misra M, Drzal LT (2005) Natural fibers, biopolymers, and biocomposites. CRC
Ahmad A, Banat F, Alsafar H, Hasan SW (2022) An overview of biodegradable poly (lactic acid) production from fermentative lactic acid for biomedical and bioplastic applications. Biomass Convers Biorefin 1–20. https://doi.org/10.1007/s13399-022-02581-3
Jabeen N, Majid I, Nayik GA (2015) Bioplastics and food packaging: a review. Cogent Food Agric 1(1):1117749. https://doi.org/10.1080/23311932.2015.1117749
Liu X, Ma PX (2004) Polymeric scaffolds for bone tissue engineering. Ann Biomed Eng 32:477–486. https://doi.org/10.1023/B:ABME.0000017544.36001.8e
Gupta B, Revagade N, Hilborn J (2007) Poly (lactic acid) fiber: an overview. Prog Polym Sci 32(4):455–482. https://doi.org/10.1016/j.progpolymsci.2007.01.005
Ilyas RA, Zuhri MYM, Aisyah HA, Asyraf MRM, Hassan SA, Zainudin ES, Sapuan SM, Sharma S, Bangar SP, Jumaidin R, Nawab Y, Faudzi AAM, Abral H, Asrofi M, Syafri E, Sari NH (2022) Natural fiber-reinforced polylactic acid, polylactic acid blends and their composites for advanced applications. Polymers 14(1):202. https://doi.org/10.3390/polym14010202
Leroux JC, Allémann E, De Jaeghere F, Doelker E, Gurny R (1996) Biodegradable nanoparticles—from sustained release formulations to improved site specific drug delivery. J Control Release 39(2–3):339–350. https://doi.org/10.1016/0168-3659(95)00164-6
Fishbein I, Chorny M, Rabinovich L, Banai S, Gati I, Golomb G (2000) Nanoparticulate delivery system of a tyrphostin for the treatment of restenosis. J Control Release 65(1–2):221–229. https://doi.org/10.1016/S0168-3659(99)00244-8
Matsumoto J, Nakada Y, Sakurai K, Nakamura T, Takahashi Y (1999) Preparation of nanoparticles consisted of poly (L-lactide)–poly (ethylene glycol)–poly (L-lactide) and their evaluation in vitro. Int J Pharm 185(1):93–101. https://doi.org/10.1016/S0378-5173(99)00153-2
Xing J, Zhang D, Tan T (2007) Studies on the oridonin-loaded poly (D, L-lactic acid) nanoparticles in vitro and in vivo. Int J Biol Macromol 40(2):153–158. https://doi.org/10.1016/j.ijbiomac.2006.07.001
Rancan F, Papakostas D, Hadam S, Hackbarth S, Delair T, Primard C, Verrier B, Sterry W, Blume-Peytavi U, Vogt A (2009) Investigation of polylactic acid (PLA) nanoparticles as drug delivery systems for local dermatotherapy. Pharm Res 26:2027–2036. https://doi.org/10.1007/s11095-009-9919-x
Gao H, Wang YN, Fan YG, Ma JB (2005) Synthesis of a biodegradable tadpole- shaped polymer via the coupling reaction of polylactide onto mono (6-(2- aminoethyl) amino-6-deoxy)-β-cyclodextrin and its properties as the new carrier of protein delivery system. J Control Release 107(1):158–173. https://doi.org/10.1016/j.jconrel.2005.06.010
Dong Y, Feng SS (2006) Nanoparticles of poly (D, L-lactide)/methoxy poly (ethylene glycol)-poly (D, L-lactide) blends for controlled release of paclitaxel. J Biomed Mater Res A 78(1):12–19. https://doi.org/10.1002/jbm.a.30684
Pan J, Feng SS (2008) Targeted delivery of paclitaxel using folate-decorated poly (lactide)–vitamin E TPGS nanoparticles. Biomaterials 29(17):2663–2672. https://doi.org/10.1016/j.biomaterials.2008.02.020
Betancourt T, Byrne JD, Sunaryo N, Crowder SW, Kadapakkam M, Patel S, Casciato S, Brannon-Peppas L (2009) PEGylation strategies for active targeting of PLA/PLGA nanoparticles. J Biomed Mater Res A 91(1):263–276. https://doi.org/10.1002/jbm.a.32247
Gref R, Minamitake Y, Peracchia MT, Trubetskoy V, Torchilin V, Langer R (1994) Biodegradable long-circulating polymeric nanospheres. Science 263(5153):1600–1603. https://doi.org/10.1126/science.8128245
Italia JL, Bhardwaj V, Kumar MR (2006) Disease, destination, dose and delivery aspects of ciclosporin: the state of the art. Drug Discov Today 11(17–18):846–854. https://doi.org/10.1016/j.drudis.2006.07.015
Zhao J, Mi Y, Liu Y, Feng SS (2012) Quantitative control of targeting effect of anticancer drugs formulated by ligand-conjugated nanoparticles of biodegradable copolymer blend. Biomaterials 33(6):1948–1958. https://doi.org/10.1016/j.biomaterials.2011.11.051
Cheng CJ, Tietjen GT, Saucier-Sawyer JK, Saltzman WM (2015) A holistic approach to targeting disease with polymeric nanoparticles. Nat Rev Drug Discov 14(4):239–247. https://doi.org/10.1038/nrd4503
Kamaly N, Xiao Z, Valencia PM, Radovic-Moreno AF, Farokhzad OC (2012) Targeted polymeric therapeutic nanoparticles: design, development and clinical translation. Chem Soc Rev 41(7):2971–3010. https://doi.org/10.1039/C2CS15344K
Mura S, Nicolas J, Couvreur P (2013) Stimuli-responsive nanocarriers for drug delivery. Nat Mater 12(11):991–1003. https://doi.org/10.1038/nmat3776
Yu Y, Chen CK, Law WC, Weinheimer E, Sengupta S, Prasad PN, Cheng C (2014) Polylactide-graft-doxorubicin nanoparticles with precisely controlled drug loading for pH-triggered drug delivery. Biomacromolecules 15(2):524–532. https://doi.org/10.1021/bm401471p
Haers PE, Suuronen R, Lindqvist C, Sailer H (1998) Biodegradable polylactide plates and screws in orthognathic surgery. J Cranio-maxillofacial Surg 26(2):87–91. https://doi.org/10.1016/S1010-5182(98)80045-0
Van Sliedregt A, Radder AM, De Groot K, Van Blitterswijk CA (1992) In vitro biocompatibility testing of polylactides part I proliferation of different cell types. J Mater Sci: Mater Med 3:365–370. https://doi.org/10.1007/BF00705369
Dürselen L, Dauner M, Hierlemann H, Planck H, Claes LE, Ignatius A (2001) Resorbable polymer fibers for ligament augmentation. J Biomed Mater Res 58(6):666–672. https://doi.org/10.1002/jbm.1067
Kanno T, Sukegawa S, Furuki Y, Nariai Y, Sekine J (2018) Overview of innovative advances in bioresorbable plate systems for oral and maxillofacial surgery. Jpn Dent Sci Rev 54(3):127–138. https://doi.org/10.1016/j.jdsr.2018.03.003
Eppley BL, Morales L, Wood R, Pensler J, Goldstein J, Havlik RJ, Habal M, Losken A, Williams JK, Burstein F, Rozzelle AA, Sadove AM (2004) Resorbable PLLA-PGA plate and screw fixation in pediatric craniofacial surgery: clinical experience in 1883 patients. Plast Reconstr Surg 114(4):850–856. https://doi.org/10.1097/01.PRS.0000132856.69391.43
Imola MJ, Schramm VL (2002) Resorbable internal fixation in pediatric cranial base surgery. Laryngoscope 112(10):1897–1901. https://doi.org/10.1097/00005537-200210000-00038
Güleçyüz MF, Mazur A, Schröder C, Braun C, Ficklscherer A, Roßbach BP, Müller PE, Pietschmann MF (2015) Influence of temperature on the biomechanical stability of titanium, PEEK, poly-l-lactic acid, and β–tricalcium phosphate poly-l-lactic acid suture anchors tested on human humeri in vitro in a wet environment. Arthrosc: J Arthrosc Relat Surg 31(6):1134–1141. https://doi.org/10.1016/j.arthro.2014.12.021
Huh BK, Kim BH, Kim SN, Park CG, Lee SH, Kim KR, Heo CY, Choy YB (2017) Surgical suture braided with a diclofenac-loaded strand of poly (lactic-co- glycolic acid) for local, sustained pain mitigation. Mater Sci Eng C 79:209–215. https://doi.org/10.1016/j.msec.2017.05.024
Schrumpf MA, Lee AT, Weiland AJ (2011) Foreign-body reaction and osteolysis induced by an intraosseous poly-l-lactic acid suture anchor in the wrist: case report. J Hand Surg 36(11):1769–1773. https://doi.org/10.1016/j.jhsa.2011.08.012
Liu S, Wu G, Chen X, Zhang X, Yu J, Liu M, Zhang Y, Wang P (2019) Degradation behavior in vitro of carbon nanotubes (CNTs)/poly (lactic acid) (PLA) composite suture. Polymers 11(6):1015. https://doi.org/10.3390/polym11061015
Benicewicz BC, Hopper PK (1990) Polymers for absorbable surgical sutures—part I. J Bioact Compat Polym 5(4):453–472. https://doi.org/10.1177/088391159000500407
Hasanpur E, Ghazavizadeh A, Sadeghi A, Haboussi M (2021) In vitro corrosion study of PLA/Mg composites for cardiovascular stent applications. J Mech Behav Biomed Mater 124:104768. https://doi.org/10.1016/j.jmbbm.2021.104768
Castro-Aguirre E, Iniguez-Franco F, Samsudin H, Fang X, Auras R (2016) Poly (lactic acid)—Mass production, processing, industrial applications, and end of life. Adv Drug Deliv Rev 107:333–366. https://doi.org/10.1016/j.addr.2016.03.010
Balla E, Daniilidis V, Karlioti G, Kalamas T, Stefanidou M, Bikiaris ND, Vlachopoulos A, Koumentakou I, Bikiaris DN (2021) Poly (lactic acid): a versatile biobased polymer for the future with multifunctional properties—from monomer synthesis, polymerization techniques and molecular weight increase to PLA applications. Polymers 13(11):1822. https://doi.org/10.3390/polym13111822
Kabirian F, Ditkowski B, Zamanian A, Heying R, Mozafari M (2018) An innovative approach towards 3D-printed scaffolds for the next generation of tissue-engineered vascular grafts. Mater Today: Proc 5(7):15586–15594. https://doi.org/10.1016/j.matpr.2018.04.167
Adesina OT, Jamiru T, Sadiku ER, Ogunbiyi OF, Adegbola TA (2019) Water absorption and thermal degradation behavior of graphene reinforced poly(lactic) acid nanocomposite. IOP Conf Ser: Mater Sci Eng 627:012015. https://doi.org/10.1088/1757-899X/627/1/012015
Bouzouita A, Notta-Cuvier D, Raquez JM, Lauro F, Dubois P (2018) Poly (lactic acid)-based materials for automotive applications. Adv Polym Sci 177–219. https://doi.org/10.1007/12_2017_10
Kim K, Luu YK, Chang C, Fang D, Hsiao BS, Chu B, Hadjiargyrou M (2004) Incorporation and controlled release of a hydrophilic antibiotic using poly (lactide- co-glycolide)-based electrospun nanofibrous scaffolds. J Control Release 98(1):47–56. https://doi.org/10.1016/j.jconrel.2004.04.009
Cicero JA, Dorgan JR (2001) Physical properties and fiber morphology of poly (lactic acid) obtained from continuous two-step melt spinning. J Polym Environ 9:1–10. https://doi.org/10.1023/A:1016012818800
Agrawal AK, Bhalla R (2003) Advances in the production of poly (lactic acid) fibers. A review. J Macromol Sci C: Polym Rev 43(4):479–503. https://doi.org/10.1081/MC-120025975
Penning JP, Dijkstra H, Pennings AJ (1993) Preparation and properties of absorbable fibres from L-lactide copolymers. Polymer 34(5):942–951. https://doi.org/10.1016/0032-3861(93)90212-S
Farrington DW, Lunt J, Davies S, Blackburn RS (2005) Poly (lactic acid) fibers. Biodegradable Sustainable Fibres 6:191–220
Auras R, Lim LT, Selke S, Tsuji H (2010) Poly (lactic acid): synthesis, structures, properties, Processing, and applications. John Wiley and Sons, Inc., New Jersey, pp 469–476
Acknowledgements
No financial support is taken from any funding agencies for the present work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shekhar, N., Mondal, A. Synthesis, properties, environmental degradation, processing, and applications of Polylactic Acid (PLA): an overview. Polym. Bull. 81, 11421–11457 (2024). https://doi.org/10.1007/s00289-024-05252-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-024-05252-7