Abstract
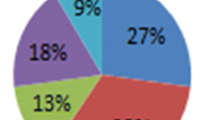
We report in this study the effect of Kankara zeolite-Y-based catalyst on the chemical properties of liquid fuel from mixed waste plastics pyrolysis using a batch reactor. Fourteen set of 200 g of waste plastics comprising 27 wt%HDPE, 33 wt%LDPE, 13 wt%PP, 18 wt%PS, 9 wt%PET were de-polymerized with catalyst and a set without catalyst. The catalyst used comprises of a mixture of zeolite-Y, metakaolin, aluminum hydroxide and sodium silicate all synthesized from Kankara kaolin from Kankara in Katsina state, Nigeria. Fourteen different catalyst combinations derived from design of experiment using Design expert 11.0 were used to produce fourteen different liquid fuel samples, and the liquid sample with the highest yield was determined. Fourier transform infrared spectroscopy was used to detect various characteristic functional groups present in the samples. Furthermore, the various compounds in the uncatalyzed (thermal pyrolysis) and catalyzed samples with highest yield were determined using GC/MS. The results show that the highest yield of liquid fuel from the fourteen catalyst combination was 46.7 wt% while the thermal pyrolysis gave a yield of 66.9 wt%. The GC/MS result shows carbon in the range of C6–C13 and absence of long straight chain paraffins in the catalyzed fuel samples while the uncatalyzed sample has carbon in the range of C7–C20. Consequently, the thermal pyrolysis sample consists of 59%, 36% and 5% of gasoline, diesel and fuel oil, respectively, while the catalyzed sample consists of 93% gasoline and 7% diesel fraction.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The use of plastics is becoming more prevailing and indispensable in all facets of human endeavors, as a result of their inherent properties such as lightweight, durability and energy efficiency, accompanying with a high rate of production and ease of design. These materials are increasingly been used in most industrial and domestic applications; hence, plastics have become crucial materials for advancement of technology in all sectors. Consequently, huge volume of waste from plastics has created a very serious environmental challenge because of their enormous quantities and non-degradability over a reasonable period after disposal [1, 2]. These plastic wastes pose serious danger on soil availability for cultivation and living organisms throughout the ecosystem, with an increasingly high impact on aquatic animals. Evidently, conventional plastics waste management practices (such as landfill, incineration, primary and secondary recycling) have been seen to be inadequate in managing these wastes [3]. The landfill and incineration approach of managing waste plastics are undesirable due to non-biodegradability and harmful gas emissions, respectively [4, 5]. However, mechanical recycling of plastic wastes seems to be a popular alternative process presently, but the recycling operation is quite expensive as it requires high cost for cleaning, sorting and transportation besides the additives used to provide a useable product [6].
Most authorities around the world therefore, have resorted to partial or total ban on single-use plastics in their localities as a way to reduce this disposal problem and to have a greener environment. To this end, many researchers and scientist are working on the exploitation of more sustainable approach to managing this global problem [3]. Again, the high heating values associated with consumable plastics also suggest their suitability as a source of energy or fuel recovery [7, 8]. For this reason, pyrolysis, a thermal decomposition reaction of plastics at temperatures in the range of 350 and 600 °C in the absence of oxygen [9,10,11], could be considered a suitable technique for both the treatment of plastics wastes and fuel production or chemical feedstock from plastics wastes.
Currently, pyrolysis is receiving attention for its flexibility to produce a combination of solid, liquid and gaseous materials in different proportions by varying the operating parameters such as temperature or heating rate [12]. Pyrolysis could be achieved through hydrocracking, thermal cracking and catalytic cracking processes. Nevertheless, the thermal degradation of polymers to low molecular weight materials requires high temperatures. Moreover, the output has been a major limitation as its product has limited use due to broad carbon range products obtained [10]. But catalytic pyrolysis process attempts to address these problems. The presence of catalyst lowers the reaction temperature and time [13], inhibits the creation of unwanted products, increases product yield and production of liquid products with a lower boiling point [14, 15]. Furthermore, catalytic degradation produces a much narrower product distribution of carbon atom number having peaks at lower amount of hydrocarbons and at significantly lower temperatures [10, 15]. This process can be optimized by reuse of catalysts and the use of effective catalysts in lesser quantities. Many studies on the catalytic pyrolysis of plastic wastes have been reported.
Seo, Lee and Shin [16] reported the effect of a catalyst (ZSM-5) on the degradation of HDPE at 450 °C in a batch reactor. Li et al. [17] also reported the catalytic activity of different kinds of catalysts in the pyrolysis of waste PE and PP mixture using a batch reactor at 500 °C. Three microporous catalysts (HUN-ZSM-5, C-ZSM-5 and β-zeolite) and three mesoporous catalysts (Al-MCM-41, KFS-16B and Al-SBA-15(wo)) were used. They concluded that the acidity and textural properties of the catalysts determined the yield and the catalytic pyrolysis products could be a potential alternative to fossil fuel. Uemichi et al. [18] investigated the catalytic pyrolysis of PE using HZSM-5 catalyst. Aguado et al. [19] employed a two-stage, pyrolysis–catalysis reactor for processing PE using zeolite HZSM-5 and MCM-41 catalysts at temperatures range of 425 and 475 °C.
Presently, the use of kaolin clay or its derived zeolite as catalyst in plastic pyrolysis is gaining attention largely as a result of its high silica-to-alumina ratio which makes it suitable for use as catalyst and has been reported as used on single plastic type [20, 21]. Currently, the use of these catalysts on mixed plastics pyrolysis has not received adequate attention by researchers; using mixed plastics as feed will potentially lower the cost associated with pre-recycling operation (sorting). Nigeria has an abundance of kaolin deposit in deferent geographical locations; it is important to state that the properties and elemental composition of these raw kaolin vary with their geographical source or mining location within the same country; Hakeem et al. [21] reported silica-to-alumina ratio (SiO2/Al2O3) of 3.8 for Ahoko kaolin from Kogi state of Nigeria; Ajibola et al. [20] reported SiO2/Al2O3 of 1.45 for Arobieye kaolin from Sango Ota, Ogun state of Nigeria; Babalola et al. [22] reported silica/alumina molar ratio of 3.90, 2.287, 3.03, 2.55 and 2.76 for raw kaolinite clay from Eket, Etinan, IbionoIbom, Ikot Abasi and UdungUko clay deposits, respectively, in Akwa Ibom state, Nigeria.
But in the present study, the proposed use of combination of local zeolite-Y, metakaolin, aluminum hydroxide and sodium silicate from Kankara kaolin from Katsina state of Nigeria as catalyst for the pyrolysis of mixed plastic waste plastics has the potential of producing liquid fuel with narrow carbon range. Furthermore, the success of this study will reduce significantly the cost associated with the use of analytical grade catalyst for plastics pyrolysis and also open a new market for abundant kaolin deposit in Nigeria and Africa at large [23].
Materials and methods
Mixed waste plastics
Commingled waste plastics were collected from the municipal waste dump site located in Sabon Gari Market, Zaria, Kaduna state, Nigeria.
The plastics type selected are polypropylene, polystyrene, polyethyleneterephtalate, low- and high-density polyethylene waste. Samples were washed, sun dried for 2–4 days and then chopped into 2–4 mm so they can mix properly in the reactor. The composition of MWPs used in this study for each batch is shown in Fig. 1.
Preparation of the catalyst
The zeolite-Y used for this study was received from the petroleum trust fund chair laboratory, Ahmadu Bello University, Zaria, Nigeria. The other catalyst components metakaolin, aluminum hydroxide and sodium silicate including the said zeolite-Y were synthesized from Kankara kaolin a local source in Kankara Local Government Area in Katsina, Nigeria.
The process employed for the preparation of the metakaolin, aluminum hydroxide and sodium silicate from raw Kankara kaolin was adopted from literature;
Beneficiation of Kankara kaolinite clay
Raw kaolin clay from Kankara community in Katsina state, Nigeria, was beneficiated according to the method reported by Ajayi et al. [24]. Raw kaolin was soaked in water for 3 days in a plastic bucket; with periodic stirring, the suspended impurities were decanted each day. The mixture left after 3 days was sieved using 75-micron mesh and allowed to settle for 24 h. After settling, it was decanted and dried atmospherically for 2 days. The beneficiated clay was ground and re-sieved.
Calcination of beneficiated of Kankara kaolin clay
The dried kaolinite powder was calcined in an electric furnace at 750 °C for 5 h to obtain the more reactive phase of kaolin known as metakaolin. The resulting metakaolin were characterized, for elemental composition as shown in Table 1.
Part of the metakaolin was used for synthesis of sodium silicate and aluminum hydroxide, and the procedure was adopted from the literature [25,26,26].
Dealumination of metakaolin using sulfuric acid
One hundred fifty grams of the metakaolin was mixed with 3000 ml of distilled water and stirred to achieve homogenous mixture. Calculated quantity of 98% sulfuric acid that will give acid concentration of 60 wt% was measured and then added to the metakaolin suspension in the conical flask and the exothermic reaction was left for 30 min, after which additional distilled water was then added to quench the reaction.
The resulting mixture consists of silica component which was inert to the sulfuric acid used during the reaction and was obtained as a solid product. The alumina which reacted with the sulfuric acid was obtained in liquid form as aluminum sulfate, known as alum. The two components were separated and the solid (silica) washed with distilled water several times filtered and dried for further processing into sodium silicate. The aluminum sulfate (alum) was collected for further processing into aluminum hydroxide.
Processing aluminum hydroxide and sodium silicate
Aluminum hydroxide was precipitated from aqueous solutions of aluminum sulfate by alkaline solution of sodium hydroxide; the precipitation was carried out at 25 °C and pH below 9. The precipitate was characterized as shown in Table 1.
Hundred grams of silica were dissolved in 2.5 M of sodium hydroxide in distilled water and placed in a plastic bottle and heated at 90 °C with constant stirring on a magnetic stirrer for 1–3 h. The resulting mixture was allowed to cool to room temperature and then filtered. Hot deionized water at 100 °C was used to wash the residue. The resulting product was characterized (Table 1).
To examine the composition and the textural properties of the zeolite-Y, metakaolin, aluminum hydroxide and sodium silicate, the X-ray fluorescence (XRF) and (Brunauer–Emmett–Teller) BET analyses were conducted. The composition of the zeolite-Y and other catalyst components is shown in Table 1. The high amount of SiO2 and Al2O3 in the zeolite-Y and metakaolin showed that they are proper to be used as a catalyst. These compounds could increase the cracking efficiency and improve the properties of liquid pyrolytic products.
The textural properties of the catalyst components were determined from BET technique by N2 adsorption at − 196 °C using Tristar 3000 Micrometrics equipment, Table 2.
The various catalyst components and the design of experiment (DOE) for the fourteen runs are shown in Table 2.
Pyrolysis experiment
A diagram of the experimental setup is as shown in Fig. 2. The setup consists of a stainless steel (reactor) heated with gas source, a galvanized steel container, two condensers, oil collectors. The maximum loading capacity of the reactor is 1 kg. The reactor consists of inner stainless steel container and an outer galvanize steel container and in between is clay as lagging material. A cast iron pipe of 17 cm diameter and 60 cm length is connected to the top cover of the reactor. A double pipe counterflow heat exchanger of length 90 cm connected in series functions as the condenser. Water at 28 °C (room temperature) was used as the coolant in the first condenser, and the temperature of water being supplied to the second heat exchanger was not more than 10 °C. The waste plastic was placed inside the stainless steel container of 15 cm diameter and 20 cm height.

Source: Eze et al. [23]
Setup for the pyrolysis.
An amount of 200 g of MWPs and 20 g of the compounded catalyst was put into reactor locked to airtight. The reaction time was 120 min for thermal pyrolysis (uncatalyzed) and 90 min for catalyzed pyrolysis experiments. Heat was supplied from external gas source; the temperature was monitored using a digital thermocouple and regulated from the gas cylinder. The temperature range for the uncatalyzed pyrolysis was between 450 and 490 °C, while for the catalyzed pyrolysis the maximum temperature was 350 °C. The gas produced was flowed into the first condenser to the shell and tube condensers at 28 °C while the second condenser was maintained at 10 °C maximum. The experiments were conducted for fourteen different catalyst combination as indicated in Table 3 to determine the catalyst composition that gives optimum yield. The char remained in the reactor was collected after each batch of pyrolysis reaction was finished. The various liquid fuels are shown in Fig. 3.

Source: Eze et al. [23]
Mixed waste plastic pyrolysis liquid samples.
The solid (S), gaseous (G) and liquid product (L) yields were calculated using the formula given below as shown in Eqs. 1, 2, 3 (Source: Anene et al. [27]):
Results and discussion
Characterization of the catalyst
The chemical composition of the zeolite-Y, metakaolin, aluminum hydroxide and sodium silicate were analyzed using X-ray fluorescence (XRF) analysis (Table 1). Table 2 shows their textural properties. The zeolite is described as a near micrporous material because it has a pore size of 3.4 nm which is slightly greater than 2 nm for microporous materials. However, its large surface area (321 m2 g) and silica-to-alumina ratio of 1.981 makes it adequate for use as catalyst for cracking hydrocarbon [7]. The mesoporous metakaolin has pore size, surface area and silica-to-alumina ratio of 25.96991 nm, 9.1217 m2 g and 1.331, respectively. These results show that the Si/Al ratio is within the acceptable ranges of 1–2 for the metakaolin and 1.5–3.8 for the zeolite-Y [13].
Yield of MPWs liquid fuel
Table 4 shows the percentage yield of uncatalyzed and catalyzed pyrolysis; it is evident that the yield of liquid fuel decreased significantly with the use of the catalyst at all combination under investigation. The uncatalyzed pyrolysis (thermal pyrolysis) has a percentage yield of 66.9% while the maximum yield for the catalyzed pyrolysis is 46.7% (Table 4 and Fig. 4). This decrease in yield for catalyzed reaction is in agreement with findings of Rehan et al. [28] who studied the effect of zeolite catalysts on pyrolysis liquid oil and reported that thermal pyrolysis produced maximum liquid oil (80.8%) with gases (13%) and char (6.2%), while catalytic pyrolysis using synthetic and natural zeolite decreased the liquid oil yield (52%) with an increase in gases (17.7%) and char (30.1%) production.
In this study, the optimum yield (sample 2) was obtained with 10.4902%:32.4225%:27.0872%:30% of zeolite-Y, metakoalin, aluminum hydroxide and sodium silicate catalyst composition, respectively.
Similar observation as above was also reported by Seo et al. [12] who studied the catalytic degradation of HDPE using a batch reactor at a temperature of 450 °C and noted that the pyrolysis performed with the zeolite ZSM-5 had higher yield of the gaseous fraction and smaller liquid fraction when compared with thermal cracking.
Figure 4 shows the combine effects of catalyst component on the yield of waste plastic fuel; it is clear from the chart that the variation of the catalyst components has significant effect on the yield. The yield increased significantly with the decrease in fraction of zeolite-Y and increased with higher fraction of metakaolin and aluminum hydroxide for all the experiment under investigation. This can be attributed to pore size of the catalyst components; metakaolin has a larger pore size while the zeolite has smaller pore size and has higher selectivity.
The lower yield but improved quality of liquid oil through catalytic pyrolysis is due to catalytic features of zeolites such as very small pore size and high BET surface area. However, the larger pore size of aluminum hydroxide lowers molecular sieving activity and tends to favor more liquid yield.
FTIR analysis of MPWs liquid fuel
FTIR analysis of the liquid sample obtained by thermal pyrolysis and the liquid fuel at optimum catalytic yield condition was carried out to reveal the presence of different functional groups within the waveband of 400–4000 cm−1 with the highest signal occurring at 98%T showing different types of vibrations (Figs. 5, 6). A close study on the spectra was done to reveal the difference is the functional groups appearing in the thermal pyrolysis sample and the catalyzed sample. A broadband of 2922.2–3026.6 cm−1 indicates the presence of aromatic alkyl groups (C–H stretch) in both samples. The sample from thermal pyrolysis (control sample) shows higher peak intensity in this range, which is an indication of higher concentration and relative abundance of aromatic compounds due to lack of cracking. On the contrary, the catalyzed sample with ultimate yield shows weak peaks in the same wavelength range of 2922.2–3026.6 cm−1.
A peak at 2855.1 cm−1 appeared in both thermal and catalyzed samples; again the catalyzed sample shows weak peak compared to the control sample with high intensity. This peak is the characteristic peak of alkane (paraffin).
A characteristic alkene stretch was noticed in both catalyzed and control sample at 1695.9 cm−1 with both peaks having similarly intensity. Similarly, a weak and sharp peak at 2549.5 cm−1 appeared in both spectra denoting the presence of thiol (S–H) which can be attributed to the sulfur present in the natural gas, the monomer of the polymers, mixed waste plastics.
Short waveband 1285.9 cm−1 denotes the presence of thiocarbonyl groups (C=S stretch); this is expected as the monomers are derived from natural gas.
Spectrum in the waveband of 1818.9 cm−1 confirms the presence of hydroxyl and carbonyl groups (O–H, C=O stretch); the presence of hydroxyl groups can be largely attributed to the additives added during polymer processing. It was reported by Panda et al. [10] that polypropylene has higher affinity to oxidation than other polymeric materials because of the presence of tertiary carbon which is bonded to a methyl group and can easily form peroxide in the chemical form of (–C–O–O–H) in the presence of oxygen even at lower temperature of about 150 °C. Peroxides are further decomposed to a more stable oxygen containing compounds such as hydroxyl (–O–H), carbonyl (–C–O) and nitro (–N–O) groups.
Other functional groups present were amine (N–H, C=O) with a waveband of 1602.8 cm−1, aromatic rings (C–C double bond) of wavelength 1453.7 cm−1 and organohalogens (C–Cl and C–Br) with a wavelength of 697.0.26 cm−1 and 909.5 cm−1, respectively. The presence of traces of impurities in the Kankara kaolin used might be a possible reason for the presence of amine, organohalogens functional groups. Tables 5 and 6 show the summary of the FTIR analysis.
Gas chromatography and mass spectrometer (GC/MS) analysis of MWPs liquid fuel
In GC/MS analysis result, compounds were detected based on retention time and trace mass. The GC-MS analysis of the catalyzed liquid product with optimum yield (Sample 2) and uncatalyzed (thermal pyrolysis) liquid product was carried out and compounds having peaks with quality ranging from 38 to 97% were identified, and their tentative assignments were confirmed using the Chemical Abstract Service (CAS) registry of mass spectra.
Compounds assigned and their peak qualities are as shown in Tables 7 and 8 representing thermal pyrolysis and catalyzed pyrolysis samples with optimum yield, respectively. It can be seen from GC–MS results that the mixed waste plastic pyrolysis gave liquid products that are very complex mixture, containing many aliphatic and cyclic compounds as presented in Figs. 7 and 8. The liquid product from the thermal pyrolysis of mixed waste plastics is made up of numerous hydrocarbons, alkane, alkene, cyclic and halogenated hydrocarbons. Other compounds detected include halogenated, oxygenated alcoholic and nitrogenated compounds.
However, the liquid product from the catalytic pyrolysis of mixed waste plastics shows absence of long-chain alkanes and alkenes (C14–C20) such as eicosane, nonadecane, heptadecane, octadecene, pentadecane, tetradecane and undecene. This could be attributed to the ability of the catalyst to crack large hydrocarbon compounds into lower hydrocarbons. Similar observation has been reported by Kumar et al. [29]; ‘ZY catalyst in powder form is reported to promote the formation of olefins, while the same catalyst in pellet form promotes the formation of paraffins and naphthalenes in HDPE pyrolysis.’
The uncatalyzed pyrolysis liquid is dominated by styrene, also present in high concentration are; single ring toluene, benzene and ethylbenzene which are lesser in the catalyzed sample.
Generally, both catalyzed and uncatalyzed samples contain aromatics, naphthenes, paraffins and other trace compounds. However, the uncatalyzed sample shows higher concentration of aromatics as well as long-chain paraffins. The range of paraffins in the catalyzed sample is between C6 and C13 while that of uncatalyzed samples is between C7 and C20.
Furthermore, Table 9 reveals the percentage composition of the fuel fraction in the thermal pyrolysis sample to be 59%, 36% and 5% of gasoline, diesel and fuel oil, respectively, while the catalyzed sample consist of 93% gasoline and 7% diesel fraction. This suggests that the catalyst combination is effective in cracking large hydrocarbon molecules in the liquid fuel to lighter molecules with lower carbon chain length.
Conclusion
Waste plastics are a great potential raw material to be converted into liquid fuel by pyrolysis. The result of this study shows that the liquid fuel obtained from the uncatalyzed and catalyzed (optimum yield) pyrolysis of MWPs contains alkenes, alkanes, naphthene and aromatics.
The catalyzed liquid fuel contains light liquid product with carbon in the range of C6–C13 and absence of long-chain alkanes and alkenes such as eicosane, nonadecane, heptadecane, octadecene, pentadecane, tetradecane and undecene compared to the uncatalyzed liquid product with carbon in the range of C7–C20. Consequently, the catalyst combination was effective in cracking the hydrocarbon in the MWPs such that the catalyzed liquid sample consists of predominantly gasoline (93%) and 7% diesel range of fuel compared to the thermal pyrolysis liquid sample with 59%, 36% and 5% of gasoline, diesel and fuel oil, respectively.
The study further shows that the optimum yield was obtained with 10.4902%:32.4225%:27.0872%:30% of zeolite-Y, metakoalin, aluminum hydroxide and sodium silicate catalyst composition, respectively.
Combination of zeolite-Y, metakaolin, aluminum hydroxide and sodium silicate from Kankara Kaolin has proven effective in cracking heavy hydrocarbon into lighter liquid product which suggests that the liquid fuel so produced will deposit less sooth upon burning.
References
Behera AK, Avancha S, Basak RK, Sen R, Adhikari B (2012) Fabrication and characterizations of biodegradable jute reinforced soy based green composites. Carbohydr Polym 88(1):329–335
Singh P, Sharma V (2016) Integrated plastic waste management: environmental and improved health approaches. Procedia Environ Sci 35:692–700
Lee H, Park Y-K (2018) Catalytic pyrolysis of polyethylene and polypropylene over desilicated beta and Al-MSU-F. Catalysts 8(11):501
Hafeez S, Manos G, Al-Salem S, Aristodemou E, Constantinou A (2018) Liquid fuel synthesis in microreactors. React Chem Eng 3:414–432
Wang T, Xiao F, Zhu X, Huang B, Wang J, Amirkhanian S (2018) Energy consumption and environmental impact of rubberized asphalt pavement. J Clean Prod 180:139–158
La Mantia F (2002) Handbook of plastics recycling. iSmithers Rapra Publishing, Shrewsbury
Sharuddin SDA, Abnisa F, Daud WMAW, Aroua MK (2016) A review on pyrolysis of plastic wastes. Energy Convers Manag 115:308–326
Sim J-W, Kim S-S (2017) Recovery of polyethylene telephthalate monomer over Cu or Mn/gamma-Al2O3 catalysts. Appl Chem Eng 28(4):485–489
Lee H, Kim Y-M, Lee I-G, Jeon J-K, Jung S-C, Do Chung J, Park Y-K (2016) Recent advances in the catalytic hydrodeoxygenation of bio-oil. Korean J Chem Eng 33(12):3299–3315
Panda AK, Singh RK, Mishra D (2010) Thermolysis of waste plastics to liquid fuel: a suitable method for plastic waste management and manufacture of value added products—a world prospective. Renew Sustain Energy Rev 14(1):233–248
Shafaghat H, Rezaei PS, Ro D, Jae J, Kim B-S, Jung S-C, Park Y-K (2017) In-situ catalytic pyrolysis of lignin in a bench-scale fixed bed pyrolyzer. J Ind Eng Chem 54:447–453
Czajczyńska D, Anguilano L, Ghazal H, Krzyżyńska R, Reynolds A, Spencer N, Jouhara H (2017) Potential of pyrolysis processes in the waste management sector. Therm Sci Eng Progr 3:171–197
Zhang X, Lei H, Yadavalli G, Zhu L, Wei Y, Liu Y (2015) Gasoline-range hydrocarbons produced from microwave-induced pyrolysis of low-density polyethylene over ZSM-5. Fuel 144:33–42
López A, De Marco I, Caballero B, Laresgoiti M, Adrados A, Aranzabal A (2011) Catalytic pyrolysis of plastic wastes with two different types of catalysts: ZSM-5 zeolite and Red Mud. Appl Catal B 104(3–4):211–219
Auxilio AR, Choo W-L, Kohli I, Srivatsa SC, Bhattacharya S (2017) An experimental study on thermo-catalytic pyrolysis of plastic waste using a continuous pyrolyser. Waste Manag 67:143–154
Seo Y-H, Lee K-H, Shin D-H (2003) Investigation of catalytic degradation of high-density polyethylene by hydrocarbon group type analysis. J Anal Appl Pyrol 70(2):383–398
Li K, Lee SW, Yuan G, Lei J, Lin S, Weerachanchai P, Wang J-Y (2016) Investigation into the catalytic activity of microporous and mesoporous catalysts in the pyrolysis of waste polyethylene and polypropylene mixture. Energies 9(6):431
Uemichi Y, Hattori M, Itoh T, Nakamura J, Sugioka M (1998) Deactivation behaviors of zeolite and silica–alumina catalysts in the degradation of polyethylene. Ind Eng Chem Res 37(3):867–872
Aguado J, Serrano D, San Miguel G, Castro M, Madrid S (2007) Feedstock recycling of polyethylene in a two-step thermo-catalytic reaction system. J Anal Appl Pyrol 79(1–2):415–423
Ajibola AA, Omoleye AJ, Efeovbokhan VE (2018) Catalytic cracking of polyethylene plastic waste using synthesized zeolite Y from Nigerian kaolin deposit. Appl Petrochem Res 8:211–217
Hakeem IG, Aberuagba F, Musa U (2018) Appl Petrochem Res. https://doi.org/10.1007/s13203-018-0207-8
Babalola R, Bassey EN, Brown IL, Salahudeen N (2017) Identification of kaolinite clay in five local government areas of Akwa Ibom State, Nigeria for zeolite synthesis as a way of import substitution. J Emerg Trends Eng Appl Sci 8(5):215–218
Eze WU, Madufor IC, Onyeagoro GN, Obasi HC (2019) The effect of Kankara zeolite-Y-based catalyst on some physical properties of liquid fuel from mixed waste plastics (MWPs) pyrolysis. Polym Bull. https://doi.org/10.1007/s00289-019-02806-y
Ajayi AO, Atta AY, Aderemi BO, Adefila SS (2010) Novel method of metakaolin dealumination—preliminary investigation. J Appl Sci Res 6(10):1539–1546
Aderemi BO, Edomwonyi-Otu L, Adefila SS (2009) A new approach to metakaolin dealumination. Aust J Basic Appl Sci 3(3):2243–2248
Bawa SG, Ahmed AS, Okonkwo PC, Waziri SM (2017) Developmemt of pilot scale dealumination unit of 2.5 kg metakaolin per batch capacity. Niger J Technol 36(3):829–834
Anene AF, Fredriksen SB, Sætre KA, Tokheim L-A (2018) Experimental study of thermal and catalytic pyrolysis of plastic waste components. Sustainability 10(11):3979
Rehan M, Miandad R, Barakat MA et al (2017) Effect of zeolitecatalysts on pyrolysis liquid oil. Int Biodeterior Biodegrad 119:162–175
Kumar S, Singh RK (2011) Recovery of hydrocarbon liquid from waste high density polyethylene by thermal pyrolysis. Braz J Chem Eng 28:659–667
Acknowledgements
We deeply acknowledge the support received from Dr. Solomon Gajere Bawa of Petroluem Technology Laboratory, Ahmadu Bello University Zaria, Nigeria, for providing us with the zeolite-Y and assistance in the synthesis of the other catalyst components. The authors also appreciate the valuable contributions of Dr. T. K. Bello of the Chemical Engineering Department and Dr. Bashir of the multi-user laboratory both in Ahmadu Bello University, Zaria Nigeria.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Eze, W.U., Madufor, I.C., Onyeagoro, G.N. et al. Study on the effect of Kankara zeolite-Y-based catalyst on the chemical properties of liquid fuel from mixed waste plastics (MWPs) pyrolysis. Polym. Bull. 78, 377–398 (2021). https://doi.org/10.1007/s00289-020-03116-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-020-03116-4