Abstract
In this study, thermo-catalytic co-pyrolysis of different high-molecular weight hydrocarbon mixtures (Fischer–Tropsch paraffin mixture, heavy residue of waste polyethylene thermal cracking and waste polyethylene from agricultural sector) was studied in a two-zone semi-batch reactor system at 450 °C, using Beta zeolite catalyst. The yields and compositions of co-pyrolysis products were studied depending on feedstock, catalyst and its placement (one or two-step pyrolysis). Some results were compared to our previous work, when catalyst free or thermal pyrolysis was compared with ZSM-5 promoted thermo-catalytic pyrolysis with the same feedstocks and layout as in the present case. It was found that the two-step pyrolysis (placing the Beta-zeolite in the 2nd reactor) resulted more gaseous product with higher hydrogen and methane content. In terms of liquid products, the composition shifted towards the heavy ends, which means that the C21+ and diesel fuel boiling ranged hydrocarbon content became higher, comparing to the one-step pyrolysis. In terms of feedstock composition, it was concluded that the higher Fischer–Tropsch wax and waste polyethylene contents enhance the gas and gasoline formation, while heavy residue obtained from waste polyethylene pyrolysis contributed to the formation of JET fuel-like hydrocarbons in higher amount if the catalyst were placed in the 1st reactor.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The demand for energy is increasing as population increases and living standard improves. Based on projections, crude oil and natural gas will be a significant share in the energy mix even in 2040 (~ 56%), and crude oil will remain the predominant feedstock for transportation fuels (ExxonMobil 2019). Plastic production is also increasing year by year and it can reach 1.124 billion tonnes annually by 2050, which is more than three times higher than as it is nowadays. This capacity will require approximately 20% share of global oil consumption (World Economic Forum 2016). With this conditions, usage of fossil resources is inevitable and fighting for mitigation of the climate change and plastic waste reduction is more important than ever. Although intensive electrification can be observed in transportation, especially in the case of light-duty vehicles (International Energy Agency 2021), there are sectors (e.g. marine, aviation) that cannot be electrified. Marine and aviation sectors can be made more environmentally friendly by using less carbon-intensive alternative fuels (U.S. Department of Energy 2020).
The well-known Fischer–Tropsch synthesis can be a promising way to produce less carbon-intensive alternative fuels (Noureldin et al. 2014). The process was developed by the German scientists Franz Fischer and Hans Tropsch (Fischer and Tropsch 1923) and can use a variety of raw materials, such as coal (Mantripraganda and Rubin 2011), natural gas (Panahi et al. 2018) or biomass (Isaksson et al. 2014). Based on the operational parameters, two types of processes—High Temperature and Low Temperature Fischer–Tropsch Synthesis—can be distinguished. In the case of High Temperature Fischer–Tropsch synthesis the temperature is usually above 320 °C, while the most commonly used reaction temperature is between 170 and 270 °C in the Low Temperature Fischer–Tropsch (LTFT) technology (de Klerk 2016).
In LTFT synthesis, significant amount (40–50%) of C21+ hydrocarbons, so-called Fischer–Tropsch wax is formed (de Klerk 2016). This low-value heavy fraction requires further upgrading, mainly hydrocracking and/or hydroisomerisation to produce engine fuels and base oils (Neuner et al. 2021). Although hydrocracking and/or hydroisomerisation of Fischer–Tropsch (FT) wax results excellent quality middle distillates, many refineries are facing hydrogen shortfall (Ratan et al. 2014); therefore, it is worth investigating other ways for FT wax upgrading. For instance, valuable light α-olefins and high-octane number gasoline can be obtained by thermo-, and thermo-catalytic pyrolysis of FT wax (de Klerk 2007) and waste plastics (Yang et al. 2021).
Thermo- and thermo-catalytic pyrolysis of waste plastics is a widely researched topic these days (Huang et al. 2022), and the number of articles pyrolyzing FT waxes is also increasing (Liang et al. 2022). It is a generally accepted fact that the yield structure of pyrolysis depends on the feedstock composition and the process conditions (e.g. temperature, heating rate, residence time and presence of catalyst) (Maqsood et al. 2021). Among the mentioned parameters, temperature and heating rates are the most vital, which affect the whole pyrolysis process (Yansaneh and Zein 2022). Residence time is another pivotal factor, because short residence times result the formation of primary decomposition products, such as monomers, while the thermodynamically more stable products (e.g. hydrogen, methane and aromatics) are formed by longer residence times (Buekens 2006).
Catalysts have a beneficial effect on pyrolysis, due to the increased yield of light products, lower temperatures required for decomposition, and higher reaction rates. The most commonly used catalysts are aluminosilicates, such as ZSM-5 and Beta zeolites (Almeida et al. 2016). These catalysts are suitable to convert polyolefins (Santos et al. 2019) waste plastics (Ates et al. 2013) and FT waxes (Komvokis et al. 2012) into lighter hydrocarbon fractions in a temperature range of 300–500 °C and to catalyse the co-pyrolysis, which is also another popular research topic.
To determine the fuel potential—yield and usability of the products as fuels—and the optimal process parameters, Ansari et al. (2021) reviewed various co-pyrolysis experiments with biomass and plastic waste. In the co-pyrolysis experiments high-quality liquid fuels were produced and between the two reactants synergistic effects were prevailed. The studied catalyst facilitated the multiple parallel reactions such as depolymerization, dehydration, deoxygenation, hydrogenation hydrodeoxygenation, aromatization, and condensation; therefore, the produced oil was suitable for direct use or blend in the existing fuel. Wu et al. (2020) conducted experiments for co-pyrolysis of corn stover and polypropylene, while investigating the effect of the feedstock composition, and catalyst (ZSM-5) on the yields and properties of pyrolysis products. It was found that co-pyrolysis of corn stover and polypropylene can increase the yield of pyrolysis oil and addition of catalyst reduces the proportion of oxygenates and promotes the generation of aromatic hydrocarbons. Burra et al. (2018) also reported the increased carbon conversion efficiency and volatile yield of co-pyrolysis, as also confirmed by Ryu et al. (2020) and Paradela et al. (2009) in the case of biomass and plastic waste feedstock.
About co-pyrolysis of by-product waxes and waste plastics limited information is available. Motawie et al. (2015) co-pyrolyzed high-density polyethylene and petroleum wax in a thermogravimetric equipment and a stirred batch auto-clave. The results showed that the mixed plastic-wax samples can be converted into gases, gasoline and middle distillates, but the feedstock composition has a significant effect on the product yields. Based on this fact investigation of possible raw materials would be particularly important. Nevertheless, to the best of the authors’ knowledge, co-pyrolysis of FT wax, heavy residue of waste polyethylene cracking (PEWAX) and waste polyethylene (WPE) has not been performed before.
Some research groups carried out two-step pyrolysis experiments for conversion of polyethylene and polypropylene, too (Sakata et al. 1999). Although in these experiments the thermal volatiles were brought into direct contact with the zeolite placed in the second stage, the main aim was the “in-situ” quality improvement and not the assessment of the catalyst placement. Hence catalyst placement needs further investigation especially in the case of feedstocks listed above. Since the pyrolysis reactors can have multiple configurations Sharuddin et al. (2016), it seems to be important how the feedstock material comes into contact with the applied catalyst in the point of view of technology implementation. Layout of the system and placement of the catalysts have grate influence on the products yields and composition and the catalysts durability. This is also important for technology development and determination of optimums.
Materials and methods
Based on the aforementioned the aim of the experimental work was to study the thermo-catalytic co-pyrolysis of high molecular weight hydrocarbon mixtures (FTWAX, PEWAX, WPE) as the function of the raw material composition and catalyst placement.
Feedstocks
The commercial Fischer–Tropsch paraffin mixture (FTWAX, Sasolwax C80, Sasol, South Africa) contained mainly C21+ n-paraffins (99.2%) and was characterised by a C13-C69 carbon number range based on GC results (see in ‘Analysis chapter’). The PEWAX was obtained from pyrolysis of waste polyethylene and also comprised aliphatic hydrocarbons (n-paraffins (19.0%) and n-olefins (63.8%) from the carbon number range of C9-C48. Details about the production process can be found elsewhere (Tomasek et al. 2020). Shredded and crashed (particle size < 5 mm) WPE mixture was originated from Hungarian agricultural sector: bags, packaging material etc. Based on IR spectroscopy analysis (see in ‘Analysis chapter’), it was found that the mixture was contained almost entirely (99.5%) high-density polyethylene (HDPE).
To increase the pyrolysis efficiency, commercial Beta-zeolite was used. This zeolite (Si/Al molar ratio: 25, SSA: 680 m2/g) was purchased from Alfa Aesar in ammonium form, thus a thermal pretreatment was also required prior to the experiments. During the thermal pretreatment the catalyst sample was heated in a furnace to 500 °C in a stream of oxygen at a heating rate of 10 °C/min and maintained at this temperature for at least 1 h. As a result of the heat, ammonia was released from the NH4+ zeolite and equivalent amount of proton (H+) remained.
Pyrolysis experiments
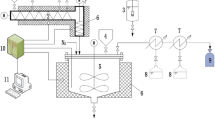
The pyrolysis experiments were carried out in an electrically heated semi-batch reactor system (Figs. 1 and 2) at a maximum reactor temperature of 450 °C in case of both vessel. The reactor system had an attachable second reaction vessel with individual heating jacket and a catalyst holder, which was also used in our present and previous work as well (Horváth et al. 2022). The height/diameter ratio of the reactor system is 0.95 with 240 ml feedstock holder. During the experiments 75 ml/min nitrogen flow was set to maintain the inert atmosphere and to avoid the oxidation and unfavoured secondary reactions. Before the experiments, 50.0 g of feedstock was placed in the 1st reactor. Also 1.0 g of Beta-zeolite catalyst was added to the feedstock or was arranged in the 2nd reaction vessel. One-step thermo-catalytic experiments were carried out when the catalyst was mixed with the feedstock in the 1st reactor. In the case of two-step pyrolysis, the catalyst was placed in the 2nd reactor, therefore only vapours from 1st reactor (thermal step) could contact with the catalyst (thermo-catalytic step). The temperature of the reactor and the heating rate (5 °C/min) was determined by preliminary experiments, where the focus was on reducing the amount of unconverted components and maximizing the yield of the liquid products. To control the temperature of the reactors PID controllers were used. The obtained pyrolysis vapours were condensed in a heat exchanger at 90 °C (the condenser temperature was set 10 °C above the drop melting point of FTWAX for safety consideration). The non-condensable pyrolysis products were collected in a gas bag. The amount of the liquid products and the residue was determined by weight measurement and the amount of the gas product was calculated by the difference.
Analysis
In order to get information on pyrolysis behaviour of the individual feedstocks, thermogravimetric analysis (TGA) was carried out. During the analysis TG 209 F1 Libra equipment was used, with 30–900 °C temperature range (heating rate: 25 °C/min). The TGA was conducted in constant nitrogen flow (20 ml/min), in order to maintain inert atmosphere.
To characterize the hydrocarbon feedstocks and the liquid state pyrolysis products gas chromatography (GC) was used. The DANI type GC was fitted with an RTx-1 column (30 m × 0.53 mm × 0.25 µm) and a flame ionization detector (FID). During the analysis the temperature of both the injector and the detector was 340 °C, and the following heating program was set: holding at 40 °C for 5 min, heating to 340 °C with a heating rate of 8 °C/min and holding at 340 °C for 28 min.
In case of waste polyethylene feedstock, the plastic composition were determined via Fourier transform infrared spectroscopy based on spectra comparison. Measurements were carried out from multiple representative sample taken from different batch using Bruker Tensor 27 FTIR-ATR equipment. The weight of each particle was measured with laboratory scale.
The composition of the gas products was determined with also a DANI type GC comprising a FID and a thermal conductivity detector (TCD). To analyse the gas components Rtx-1 PONA (100 m × 0.25 mm × 0.5 μm) and Carboxen TM 1006 PLOT (30 m × 0.53 mm) columns were used. For the Rtx-1 PONA column isothermal condition (T = 35 °C) and an injector and detector temperature of 230 °C was used. In the case of the Carboxen TM 1006 PLOT column the following heating program was applied: 35 °C for 18 min, heating to 120 °C with a heating rate of 15 °C/min and held at 120 °C for 2 min. The retention times of the components were determined using gas mixtures and individual analytical standards.
The aromatic contents were measured on a Shimadzu LC-20AD type HPLC instrument equipped with a Shimadzu RID-10A type refractory index detector, using n-heptane diluents.
Results and discussion
Thermal decomposition behaviour
During the thermogravimetric analysis 20–40 mg of the individual feedstocks was pyrolyzed in the TG equipment (crucible: Al2O3). Figure 3 depicts the weight change as function of temperature (TG) and the derivative weight changes (DTG). As data shows, the samples contained negligible amount of moisture evidenced by the absence of a peak around 100 °C. However, significant differences were observed in their thermal decomposition, which were attributed to the different compositions. Despite the different tendencies, the decomposition step ended up to 510 °C. It is well shown that the decomposition intensity of FTWAX had a peak maximum at 405 °C while the peak maximum of PEWAX and WPE decomposition was around 480 °C. In the case of thermogravimetric analysis of polyethylene Wong et al. (2023) reported similar peak temperature (~ 470 °C), while Liang et al. (2022) found that peak maximum of Fischer–Tropsch wax decomposition is around 420 °C.
Wider decomposition range was observed in case of PEWAX (120–500 °C) while narrower peaks were typically found for FTWAX and WPE feedstocks. This behaviour was also be attributed to the different chemical composition. Based on the results, it was found that FTWAX had the highest volatile matter content (98.4%) and the minimum pyrolysis temperature should be between 400 and 500 °C to achieve sufficient conversion of the feedstocks. The volatile matter contents of PEWAX and WPE were similar (89.6 and 87.5%) as there were no significant differences in the amount of residues (PEWAX: 10.4%, WPE: 12.5%).
Product yields
As it was published in our earlier article, in case of thermal pyrolysis, 19.2–35.8% gas and 57.6–74.4% liquid product was obtained (Horváth et al. 2022), meanwhile the gas and liquid product yields of Beta zeolite catalysed pyrolysis were in the range of 19.2–40.0% and 57.6–79.4%, respectively (Fig. 4).
As Fig. 4 shows, more significant gas formation was occurred when the Beta-zeolite was placed in the 2nd reaction zone. The reason for this was that pores of the Beta zeolite were fully accessible to the smaller molecule fragments formed in the first step, and the small crystal size of the catalyst provided a short diffusion path for reactants and products, as also reported by (Vlasenko et al. 2019). In contrast, when the catalyst was mixed with the raw material the high molecular weight hydrocarbons were degraded on the outer surface of the catalyst (Sotoudehnia et al. 2021) and further degradation of primary crack products could only begin later in the internal pores (Miandad et al. 2016) when the molecular size became smaller and the molten feedstock was able to enter the internal pores to undergo secondary reactions (Daligaux et al. 2021).
It is also clear that FTWAX- and WPE-rich feedstocks resulted in higher gas formation, while in case of PEWAX, the product formation shifted towards the liquid products, mainly due to the fact that lighter components are more stable in terms of cracking reactions. According to β-scission mechanism, the reactivity of hydrocarbons in cracking reactions related to stability of the forming carbenium ion. The stability of secondary linear carbenium ions (from linear alkanes) increases with the chain length, due to that fact that the positive charge can be delocalized among more carbon atoms. These long chained highly stable carbenium ions participate more likely to the cracking and isomerization reactions, than those with less delocalized positive charge—therefore less stable—shorter chained carbenium ions (Cnudde et al. 2018).
Comparing the two different zeolites, the narrow zigzag channels of the ZSM-5 zeolite produced gases in significantly higher proportion than Beta-zeolite in both system layout (Horváth et al. 2022), which can be attributed to the zeolite structure and also to the longer diffusion path length.
Composition of gaseous products
Gaseous products contained hydrogen and C1–C5+ hydrocarbons (Fig. 5). Glancing the results, it is clear that Beta zeolite can significantly increase the proportion of C2+ hydrocarbons in those cases when the catalyst and feedstock are placed in the same reactor. In contrast, when the catalyst was present in the upper reactor (2nd reactor) and was only in contact with product vapours of thermal pyrolysis, proportion of hydrogen and methane becomes more significant. These tendencies were caused by the fully accessible zeolite pores and could be attributed to dehydrogenation-aromatization reactions. Propane-butane produced in the 1st reactor (direct contact) could be participated in dehydrogenation and aromatization reactions in the 2nd reactor (two-step pyrolysis).
The largest share of methane and hydrogen was observed in product obtained from the pyrolysis of 0.25 FTWAX/0.25 PEWAX/0.50 WPE; and 0.25 FTWAX/0.50 PEWAX/0.25 WPE feedstocks (catalyst in the 2nd reactor). This leads to the conclusion that both PEWAX and WPE feedstock increase the hydrogen and methane content.
Among the two zeolites—not surprisingly—the use of 10-membered ring ZSM-5 resulted the formation of more hydrogen and methane. The higher hydrogen and methane contents are in well agreement with our previous results and the longer residence time. Longer residence times lead to the formation of thermodynamically more stable products (e.g. hydrogen, methane and aromatics) (Buekens 2006).
Composition of liquid products
Figure 6 depicts the hydrocarbon composition of liquid products. The result of the GC analysis was categorized by four different carbon-number ranges: C6–C9 gasoline, C10–C14 JET fuel, C15–C21 gasoil range and C21+ heavy hydrocarbons. It is well shown that thermo-catalytic pyrolysis resulted a more intense C–C bond scission and thus was able to significantly reduce the concentration of the C21+ hydrocarbons, in contrast to catalyst free or thermal pyrolysis where the share of C21+ varied between 19.0 and 46.8%, respectively (Horváth et al. 2022).
During the ZSM-5 catalysed pyrolysis in both reactor layouts, compounds with gasoline carbon number range were present in the largest proportion (Horváth et al. 2022) except in one case (0.75 FTWAX/0.25 PEWAX with catalyst in the 1st reactor). Using Beta zeolite in the 2nd reactor resulted in heavier fractions. Therefore, the proportion of C21+ hydrocarbons and gasoil increased, meanwhile yield of gasoline fraction decreased. This is less advantageous, since as a result of pyrolysis, olefin-rich products are formed, which have negative effect on cetane number of gasoil fractions. The share of hydrocarbons in the JET carbon number range was practically independent from the feedstock composition in that case when the catalyst was placed in the 2nd reactor. In both reactor layouts, liquid product obtained from the 0.75 FTWAX/0.25 WPE feedstock contained the highest amount of C6–C9 hydrocarbons, but the share was 21.8% more when the Beta-zeolite was mixed with the feedstock in the 1st reactor.
From the point of view of feedstock composition, it can be said that FTWAX and WPE containing feedstocks resulted mainly in the formation of C6–C9 hydrocarbons. The highest gasoline content was obtained by pyrolysis of 0.75FTWAX/0.25WPE; meanwhile PEWAX-rich feedstocks have been favourable for the formation of C10–C14 hydrocarbons when the catalysts were placed mixed with the feedstock to the 1st reactor. Based on the results it can be concluded that shorter-chain hydrocarbons of PEWAX had a higher stability in pyrolysis and a weaker adsorption on the catalytically active sites which resulted in a lower extent secondary cracking.
In order to evaluate the efficiency of the pyrolysis, it is also important to determine the yields of valuable products (e.g. gasoline, JET, gasoil and aromatic hydrocarbons). As it was concluded earlier, catalyst free or thermal pyrolysis products contained C21+ hydrocarbons in significantly high amount (Horváth et al. 2022).
As Fig. 7 shows, usage of Beta zeolite was more advantageous for production of aromatics (1.5–2.8%), gasolines (yield: 39.1–55.1%) and C10-C14 hydrocarbons (yield: 13.6–29.6%) when the catalysts were placed to the 1st reaction zone.
When Beta-zeolite was used in the 1st reactor, the yield of valuable components was higher, because the two-step pyrolysis resulted lower liquid product yield and higher proportion of C21+ hydrocarbons, as it was mentioned before. In most cases, the yield of gasoil ranged hydrocarbons is higher when the catalyst is used in the 2nd reactor, in addition, the gasoil/gasoline ratio is lower, which is less beneficial, however, the aromatic yield is higher also. Using beta-zeolite in two-step pyrolysis, the JET yield varied between 15.1 and 19.1%, respectively, so it was almost independent of feedstock composition.
Comparing the two different system layouts, a more significant difference between total valuable product yields can only be observed in the case of PEWAX and WPE mixtures, however, a more significant difference can also be observed in the gas yield of these products. In terms of aromatic hydrocarbon yield, more significant differences can be observed, especially for PEWAX-rich materials.
The highest fuel yield was observed in Beta-zeolite promoted one-step pyrolysis of 0.25FTWAX/0.75PEWAX where 71.1% of the feedstock mixture was converted into fuel range hydrocarbons, mainly into gasoline. From the point of view of gasoline production pyrolysis of 75%FTWAX/25%WPE feedstock seemed to be the most preferred generally if the catalyst was Beta zeolite, although this synergic effect of FTWAX and WPE in terms of gasoline production was reduced during the two-step pyrolysis where no major differences in gasoline yield can be observed in function of feedstock composition comparing to the one-step pyrolysis experiments.
Conclusion
Based on results of the performed one- and two-step pyrolysis experiments, it was found that that if the catalyst was placed in the 2nd reactor, the product yield structure and composition were changed. Due the pre-thermal cracking, shorter chained molecules can contact with the catalyst’s active sites, resulting different product yields and compositions comparing to that layout when the catalyst was mixed with the feedstock.
Application of Beta-zeolite in two-step pyrolysis resulted more gaseous products from the same raw materials, which gas products contained more hydrogen and methane, when the catalysts were placed to the 1st reactor mixed with the feedstocks. On the other hand, liquid product contained more C21+ and gasoil fuel ranged hydrocarbons. Due to these effects, the valuable fuel ranged liquid hydrocarbon yield decreased with this arrangement, therefore mixing the Beta-zeolite into the feedstock was more recommended in the present catalytic system.
Regarding the feedstock composition it can be concluded that higher Fischer–Tropsch and agricultural waste polyethylene contents enhance the gas and gasoline formation (particularly 75% Fischer–Tropsch and 25% waste polyethylene containing materials for gasoline formation), while heavy residue of waste polyethylene thermal cracking allows the formation of JET fuel range hydrocarbons in higher amount in case when catalyst were placed in the 1st reaction zone.
Data availability
The data used and/or analyzed throughout the present study are available from the corresponding author on reasonable request.
Abbreviations
- FCC:
-
Fluid Catalytic Cracking
- FID:
-
Flame ionization detector
- FTS:
-
Fischer–Tropsch synthesis
- FTWAX:
-
Fischer–Tropsch paraffin wax
- GC:
-
Gas chromatography
- HPLC:
-
High-performance liquid chromatography
- HTFT:
-
High temperature Fischer–Tropsch synthesis
- HZSM-5:
-
H form of ZSM-5
- LTFT:
-
Low temperature Fischer–Tropsch synthesis
- MCM-41:
-
Mobil Composition of Matter No. 41 mesoporous silica
- PEWAX:
-
Residue wax from polyethylene pyrolysis
- TCD:
-
Thermal conductivity detector
- WPE:
-
Polyethylene waste
- ZSM-5:
-
Zeolite Socony Mobil–5 catalyst
References
Almeida D, Marques MF (2016) Thermal and catalytic pyrolysis of plastic waste. Polímeros 26(1):44–51
Ansari KB, Hassan SZ, Bhoi R, Ahmad E (2021) Co-pyrolysis of biomass and plastic wastes: a review on reactants synergy, catalyst impact, process parameter, hydrocarbon fuel potential, COVID-19. J Environ Chem Eng 9:106436
Ates F, Miskolczi N, Borsodi N (2013) Comparison of real waste (MSW and MPW) pyrolysis in batch reactor over different catalysts. Part I: product yields, gas and pyrolysis oil properties. Bioresour Technol 133:443–454
Buekens A (2006) Introduction to feedstock recycling of plastics. In: Scheirs J, Kaminsky W (eds) Feedstock recycling and pyrolysis of waste plastics. John Wiley & Sons Ltd, Brussels, pp 3–41
Burra KG, Gupta AK (2018) Kinetics of synergistic effects in co-pyrolysis of biomass with plastic wastes. Appl Energy 220:408–418
Cnudde P, De Wispelaere K, Vanduyfhuys L, Demuynck R, Van der Mynsbrugge J, Waroquier M, Van Speybroeck V (2018) How chain length and branching influence the alkene cracking reactivity on H-ZSM-5. ACS Catal 8(10):9579–9595
Daligaux V, Richard R, Manero M-H (2021) Deactivation and regeneration of zeolite catalysts used in pyrolysis of plastic wastes—a process and analytical review. Catalysts 11:770
de Klerk A (2016) Aviation turbine fuels through the Fischer–Tropsch process. In: Chuck C (ed) Biofuels for aviation, 1st edn. Academic Press, London, pp 241–259
de Klerk A (2007) Thermal cracking of Fischer–Tropsch waxes. Ind Eng Chem Res 46:5516–5521
ExxonMobil, Outlook for Energy: A perspective to 2040, October 2019. Available online: https://corporate.exxonmobil.com/-/media/Global/Files/outlook-for-energy/2019-Outlook-for-Energy_v4.pdf [Accessed: 28-April-2022]
Fischer F, Tropsch H (1923) Über die Herstellung synthetischer Ölgemische (Synthol) durch Aufbau aus Kohlenoxyd und Wasserstoff. Brennst Chem 4:276–285
Horváth D, Tomasek S, Miskolczi N (2022) Value-added pyrolysis of waste sourced high molecular weight hydrocarbon mixtures. Energies 15(3):997
Huang J, Vaksha A, Chan WP, Giannis A, Lisak G (2022) Chemical recycling of plastic waste for sustainable material management: a prospective review on catalysts and processes. Renew Sustain Energy Rev 154:111866
International Energy Agency, Global EV Outlook—Accelerating ambitions despite the pandemic, 2021. Available online: https://www.iea.org/reports/global-ev-outlook-2021 [Accessed: 28-April-2022]
Isaksson J, Asblad A, Berntsson T (2014) Pretreatment methods for gasification of biomass and Fischer–Tropsch crude production integrated with a pulp and paper mill. Clean Technol Environ Policy 16:1393–1402
Komvokis VG, Karakoulia S, Iliopoulou EF, Papapetrou MC, Vasalos IA, Lappas AA, Triantafyllidis KS (2012) Upgrading of Fischer–Tropsch synthesis bio-waxes via catalytic cracking: effect of acidity, porosity and metal modification of zeolitic and mesoporous aluminosilicate catalysts. Catalysis today 196(1):42–55
Liang C, Yin Z, Sun Y, Xu Y, Yao K, Liu Z, Zhu M (2022) Pyrolysis of waste Fischer–Tropsch wax: an experimental study. J Clean Prod 350:131529
Mantripraganda HC, Rubin ES (2011) CO2 reduction potential of coal-to-liquids (CTL) process: effect of gasification technology. Energy Procedia 4:2700–2707
Maqsood T, Dai J, Zhang Y, Guang M, Li B (2021) Pyrolysis of plastic species: a review of resources and products. J Anal Appl Pyrolysis 159:105295
Miandad R, Barakat MA, Aburiazaiza AS, Rehan M, Nizami AS (2016) Catalytic pyrolysis of plastic waste: a review. Process Saf Environ Prot 102:822–838
Motawie M, Hanafi SA, Elmelawy MS, Ahmed SM, Mansour NA, Darwish MSA, Abulyazied DE (2015) Wax co-cracking synergism of high density polyethylene to alternative fuels. Egypt J Pet 24:353–361
Neuner P, Graf D, Mild H, Rauch R (2021) Catalytic hydroisomerisation of Fischer–Tropsch waxes to lubricating oil and investigation of the correlation between its physical properties and the chemical composition of the corresponding fuel fractions. Energies 14:4202
Noureldin MMB, Bao B, Elbashir NO, El-Halwagi MM (2014) Benchmarking, insights, and potential for improvement of Fischer–Tropsch-based biomass-to-liquid technology. Clean Technol Environ Policy 16:37–44
Panahi M, Yasari E, Rafiee A (2018) Multi-objective optimization of a gas-to-liquids (GTL) process with staged Fischer-Tropsch reactor. Energy Convers Manage 163:239–249
Paradela F, Pinto F, Gulyurtlu I, Cabrita I, Lapa N (2009) Study of the co-pyrolysis of biomass and plastic wastes. Clean Technol Environ Policy 11:115–122
Ratan S, Farnad S, Li J (2014) Hydrogen perspectives for 21st century refineries. Hydrocarbon Process. pp 71–75
Ryu HW, Kim DH, Jae J, Lam SS, Park ED, Park YK (2020) Recent advances in catalytic co-pyrolysis of biomass and plastic waste for the production of petroleum-like hydrocarbons. Bioresour Technol 310:123473
Sakata Y, Uddin MdA, Muto A (1999) Degradation of polyethylene and polypropylene into fuel oil by using solid acid and non-acid catalysts. J Anal Appl Pyrolysis 51(1–2):135–155
Santos E, Rijo B, Lemos F, Lemos MANDA (2019) A catalytic reactive distillation approach to high density polyethylene pyrolysis—part 1—light olefin production. Chem Eng J 378:122077
Sharuddin ASA, Abnisa F, Daud WMAW, Aroua MK (2016) A review on pyrolysis of plastic wastes. Energy Convers Manage 115:308–326
Sotoudehnia F, Orji B, Mengistie E, Alayat AM, McDonald AG (2021) Catalytic upgrading of pyrolysis wax oil obtained from waxed corrugated cardboard using zeolite Y catalyst. Energ Fuel 35(11):9450–9461
Tomasek S, Varga Z, Hancsók J (2020) Production of jet fuel from cracked fractions of waste polypropylene and polyethylene. Fuel Process Technol 197:106197
U.S. Department of Energy, Office of Energy Efficiency & Renewable Energy, Sustainable Aviation Fuel—Review of Technical Pathways, September 2020. Available online: https://www.energy.gov/eere/bioenergy/downloads/sustainable-aviation-fuel-review-technical-pathways-report [Accessed: 28-April-2022]
Vlasenko NV, Kochkin YN, Telbiz GM, Shvets OV, Strizhak PE (2019) Insight into the active site nature of zeolite H-BEA for liquid phase etherification of isobutylene with ethanol. RSC Adv 9:35957–35968
World Economic Forum, Ellen MacArthur Foundation and McKinsey & Company, The New Plastics Economy Rethinking the future of plastics, 2016. Available online: http://www.ellenmacarthurfoundation.org/publications [Accessed: 28-April-2022].
Wong SL, Armenise S, Nyakuma BB, Bogush A, Towers S, Lee CH, Wong KY, Lee TH, Rebrov E, Muñoz M (2023) Plastic pyrolysis over HZSM-5 zeolite and fluid catalytic cracking catalyst under ultra-fast heating. J Anal Appl Pyrolysis 169:105793
Wu F, Ben H, Yang Y, Jia H, Wang R, Han G (2020) Effects of different conditions on co-pyrolysis behavior of corn stover and polypropylene. Polymers 12:973
Yang M, Zhang L, Wang G, Chen Z, Han J, Gao C (2021) Fischer–Tropsch wax catalytic cracking for the production of low olefin and high octane number gasoline: experiment and molecular level kinetic modeling study. Fuel 303:121226
Yansaneh OY, Zein SH (2022) Recent advances on waste plastic thermal pyrolysis: a critical overview. Processes 10:332
Acknowledgements
This work has been implemented by the TKP2021-NKTA-21 project with the support provided by the Ministry for Innovation and Technology of Hungary from the National Research, Development and Innovation Fund, financed under the 2021 Thematic Excellence Programme funding scheme and the National Research, Development and Innovation Office through the project nr. 2019-1.3.1-KK-2019-00015, titled “Establishment of a circular economy-based sustainability competence centre at the University of Pannonia”.
Author information
Authors and Affiliations
Contributions
Conceptualization: N.M.; Formal analysis: D.H.; Investigation: D.H. and S.T.; Methodology: D.H., S.T. and N.M.; Supervision: S.T. and N.M.; Validation: S.T.; Visualization, D.H.; Writing—original draft, D.H., S.T. and N.M. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Horváth, D., Tomasek, S. & Miskolczi, N. Thermo-catalytic co-pyrolysis of waste plastic and hydrocarbon by-products using β-zeolite. Clean Techn Environ Policy 26, 235–244 (2024). https://doi.org/10.1007/s10098-023-02699-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10098-023-02699-6