Abstract
L-asparaginase is a tetrameric enzyme from the amidohydrolases family, that catalyzes the breakdown of L-asparagine into L-aspartic acid and ammonia. Since its discovery as an anticancer drug, it is used as one of the prime chemotherapeutic agents to treat acute lymphoblastic leukemia. Apart from its use in the biopharmaceutical industry, it is also used to reduce the formation of a carcinogenic substance called acrylamide in fried, baked, and roasted foods. L-asparaginase is derived from many organisms including plants, bacteria, fungi, and actinomycetes. Currently, L-asparaginase preparations from Escherichia coli and Erwinia chrysanthemi are used in the clinical treatment of acute lymphoblastic leukemia. However, they are associated with low yield and immunogenicity problems. At this juncture, endophytic fungi from medicinal plants have gained much attention as they have several advantages over the available bacterial preparations. Many medicinal plants have been screened for L-asparaginase producing endophytic fungi and several studies have reported potent L-asparaginase producing strains. This review provides insights into fungal endophytes from medicinal plants and their significance as probable alternatives for bacterial L-asparaginase.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer is one of the most dreaded health diseases worldwide. Globally, about 1 in 6 deaths is due to cancer [1]. Among various types of cancer, lung cancer is the most commonly identified, with 11.6% of the total cases reported and acute lymphoblastic leukemia (ALL) is the most common childhood cancer, which accounts for 28% of total cases reported [2]. One of the chemotherapeutic drugs used for the treatment of ALL is L-asparaginase, an amidohydrolase class of enzymes that catalyzes the hydrolysis of L-asparagine into L-aspartic acid and ammonia [3]. It has gained vital prominence in the field of scientific research because of its antineoplastic property. L-asparaginase is also used for the treatment of acute myeloblastic leukemia, Hodgkin’s disease, chronic lymphocytic leukemia, pancreatic carcinoma, non-Hodgkin’s lymphoma, and bovine lymphosarcoma [4]. Besides, L-asparaginase is also employed in food industries as it reduces the formation of carcinogenic acrylamide in fried, baked, and roasted foods [5].
Two main types of L-asparaginase have been identified based on the homology in the sequence, function, and structure, namely, type I and type II. Type I L-asparaginases are cytoplasmic and possess similar enzymatic activity towards both L-glutamine and L-asparagine. Type II L-asparaginases are periplasmic and display higher specific activity towards L-asparagine than L-glutamine [6]. Type II L-asparaginase has shown potential antitumor activity and is used in the treatment of ALL.
Industrial production of L-asparaginase is often carried out using Escherichia coli and Erwinia chrysanthemi (Previously known as Dickeya dadanti) [7]. However, L-asparaginase from prokaryotic sources possesses many complications including hypersensitivity. Besides this, contamination with glutaminase, and short half-life are the major problems that make bacterial L-asparaginase an inefficient anticancer agent [8]. Whereas, L-asparaginase from fungi has lesser side effects as they are eukaryotes and thus, it has been studied in many fungal species [9]. Production of L-asparaginase is reported from several fungi such as Aspergillus niger, A. terreus, Penicillium cyclopium, Rhizomucor miehei, Flammulina velutipes and Ganoderma australe [10, 11].
Among these, endophytic fungi have gained much attention because of their ubiquity and diversity. Endophytic fungi are a group of diverse fungi that reside inside the internal tissues of living plants without causing any noticeable infections to the host. The rationale behind studying endophytic fungi is, as a result of their mutualistic association they can produce compounds found in the host plant. The discovery of taxol, an anticancer compound from the endophytic fungi Taxomyces andreanae inhabiting Taxus brevifolia promoted the research on endophytic fungi [12]. Subsequently, several anticancer compounds have been isolated from endophytic fungi, such as campthothecin from Nothapodytes foetida, podophyllotoxin from Fusarium oxysporum, and cajanol from Hypocrea lixii [13]. Similarly, many important secondary metabolites like terpenoids, alkaloids, steroids, phenols, quinines, and flavonoids with various bioactivities have been isolated from several species of endophytic fungi. Some of them are sterigmatocystin from A. nidulans, Huperzine A from F. verticillioides and Corynesidone D from Corynespora cassiicola [14]. They also serve as a reservoir of many important commercial enzymes including tannase, laccase, chitinase, chitin deacetylase, acidic protease, alkaline protease, and several other enzymes [15]. L-asparaginase is one of such enzymes which can be produced by endophytic fungi. Several studies have reported L-asparaginase producing endophytic fungi from different medicinal plants. This review focuses on the significance of L-asparaginases from endophytic fungi.
Mechanism of Action of L-Asparaginase
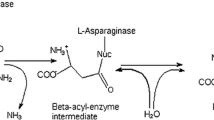
L-asparagine is necessary for the synthesis of ribonucleic acid (RNA) and protein in both normal and lukemic cells. In normal cells, it is synthesized by the enzyme asparagine synthetase. On the other hand, lukemic cells are unable to synthesize L-asparagine due to the absence of asparagine synthetase. As a result, these cells depend upon L-asparagine present in surrounding cells and tissues. Thus, when L-asparaginase is administered into the bloodstream, it makes them deprived of L-asparagine. This leads to the inhibition of RNA and protein synthesis followed by cell cycle arrest and apoptosis, eventually leading to the death of leukemic cells [16]. The mechanism of action of L-asparaginase is described in Fig. 1.
Commercial L-Asparaginases
Four main types of L-asparaginase under different trade names have been used to date including the native L-asparaginase derived from E. coli, a PEGylated form of this enzyme (PEG-L-asparaginase), L-asparaginase derived from E. chrysanthemi, and a recombinant E. coli L-asparaginase preparation [17]. Native E. coli L-asparaginase is available as Kidrolase® (EUSA Pharma); Elspar® (Ovation Pharmaceuticals); Leunase® (Sanofi-aventis); PEGylated E. coli L-asparaginase is available as Oncaspar® (Baxalta Incorporated, Deerfield, IL; formerly Sigma‑Tau Pharmaceuticals, Inc., Gaithersburg, MD); and Erwinia L-asparaginase is available as Erwinaze® (Jazz Pharmaceuticals, Palo Alto, CA) [3].
Usually, patients showing susceptibility to one formulation of L-asparaginase are administered with another formulation. E. coli L-asparaginase and PEG-asparaginase are used for first-line treatment of ALL, whereas Erwinia L-asparaginase is used as second or third-line treatment [18]. The clinical use of these L-asparaginases still faces many problems as they cause many types of allergic reactions and toxicities. Despite their universal acceptance in the treatment of ALL, there is much debate regarding the optimal formulation and dosage of these agents [3]. The details are summarized in Table 1.
Sources of L-Asparaginase
The occurrence of L-asparaginase has been described in several organisms, such as animals, plants, and microbes. However, L-asparaginase from microbes is studied extensively as they confer several advantages over other sources, such as easy optimization of culture conditions, simplified extraction, and purification, and can be grown using simple substrates [6]. Also, the enzymes obtained from microorganisms are comparatively more stable when compared to enzymes acquired from plants or animals [19]. Besides, microbes can be genetically modified to obtain a high yield.
Bacterial Sources
The currently used L-asparaginase formulations are produced industrially from E. coli and E. chrysanthemi. L-asparaginase from both sources is similar in terms of the mechanism of action. However, their pharmacokinetic properties and toxicities are different [20]. In Gram-negative bacteria, the enzyme produced is intracellular in nature. Whereas, Gram-positive bacteria usually secrete enzymes into an external medium as they lack periplasmic space. For large scale production, extracellular secretion is more beneficial as it aids in downstream processing. Thus, Gram-positive bacteria are advantageous over Gram-negative bacteria [21]. Most of the L-asparaginase producing isolates are Enterobacteriaceae members. Some of the major L-asparaginase producing bacteria are E. coli [22], E. chrysanthemi [23], Corynebacterium glutamicum [24], Pseudomonas aeruginosa [25], Helicobacter pylori [26], Pyrococcus furiosus [17], Bacillus licheniformis [27], Serratia marcescens [28] and Pectobacterium carotovorum [29].
Fungal Sources
Fungi are the second largest sources of L-asparaginase and are estimated to overtake bacterial sources because of their efficiency. L-asparaginase obtained from fungi is easy to purify as it is released into an external medium [30]. Further, fungi can be grown easily using low-cost culture mediums like industrial wastes [31]. Some of the important L-asparaginase producing fungi are A. flavus [32], A. fumigatus [33], Trichoderma viride [34], Cladosporium sp. [35], F. equiseti [36], Flammulina velutipes [10] and Ganoderma australe [11].
Actinomycetes Sources
Actinomycetes also produce L-asparaginase. Among actinomycetes, the genera of streptomyces are an important source of L-asparaginase. Some of the examples are Streptomyces ginsengisoli [37], S. gulbargensis [38], S. noursei [39], S. thermoluteus [40], S. albidoflavus [41], and S. griseus [42].
Yeast Sources
L-asparaginase production is reported from several yeasts such as Candida utilis [43], Candida bombicola [44], Pichia polymorpha [45], Rhodosporidium toruloides [46] and Saccharomyces cerevisiae [47].
Algal Sources
Some of the algae such as Chlamydomonas sp. [48], Chlorella vulgaris [49], and Spirulina maxima [50] are also known to produce L-asparaginase.
Endophytic Fungal Sources
Endophytic fungi are one of the richest sources of L-asparaginase. Many plants have been screened for L-asparaginase producing endophytic fungi and several isolates are known to produce L-asparaginase. Chow et al., screened eighty-nine endophytic fungi from Pereskia bleo, Cymbopogon citratus, Oldenlandia diffusa, and Murraya koenigii for L-asparaginase production. Among them, twenty-five isolates exhibited positive results by showing pink zones around the colonies on modified Czapek dox (MCD) agar in primary screening. P. simplicissimum from Pereskia bleo grown in MCD broth under shaking condition showed the highest activity of 0.019 µM/ml/min in secondary screening by the nesslerization method [51]. Thus, showing medicinal plants are a potential source of L-asparaginase producing endophytic fungi. Especially, medicinal plants harbor thousands of fungal endophytes with potential bioactivities including their ability to produce L-asparaginase. Some of the endophytic Diaporthe sp. have shown great potential to produce L-asparaginase. Pádua et al., isolated sixteen L-asparaginase producing isolates from leaves of Myracrodruon urundeuva, and among the most prevailing fungal members from the genus Diaporthe and Colletotrichum, they identified Diaporthe sp. URM 7793 is the best producer of L-asparaginase with an enzyme activity of 2.41 IU/g in the secondary quantitative spectrometric method [52]. Diaporthe is generally considered multi-host fungal endophytes as they are found to occur frequently in varied tropical tree species of diverse environmental locations [53]. Many species of Diaporthe are reported to produce several enzymes including L-asparaginase [54].
Manasa and Nalini reported F. verticillioides a potential producer of L-asparaginase from leaves of Tabernaemontana heyneana with the enzyme activity of 1.136 IU/ml [55]. Another Fusarium sp. with an enzyme activity of 111.07 ± 1.53 IU/ml was isolated from the Carica papaya leaves [56]. The genera of Fusarium are considered to be a potential source of L-asparaginase. Previously, the production of L-asparaginase is reported from many Fusarium species such as F. culmorum [57], F. solani [58] and F. proliferatum [59]. In another study F. solani with an enzyme activity of 1.459 IU/ml was recovered from Withania somnifera [60]. Bhosale and As-Suhbani, reported the production of glutaminase free L-asparaginase by F. solani with the activity of 619.102 IU/ml and specific activity of 8.807 IU/mg isolated from Curcuma longa [61]. The enzyme activity reported is higher than those reported for L-asparaginase from several bacteria such as Bacillus subtilis [62], Ocimum tenuiflorum [63] and, Mesoflavibacter zeaxanthinifaciens [64].
Pleospora alli with an enzyme activity of 1.98 ± 0.16 IU/ml was recovered from Withania somnifera [65]. W. somnifera is a well-known medicinal plant used in the treatment of several clinical conditions including cancer. Withaferin-A and withanone from W. somnifera have demonstrated potential anticancer activity [66]. Further, Prihanto et al., reported L-asparaginase producing Aspergillus sp. and Trichoderma sp. from Avicennia germinans and Sonneratia alba, respectively [67, 68]. Aspergillus sp. is also an important source of L-asparaginase. L-asparaginase obtained from Aspergillus oryzae and A. niger, commercially known as PreventASe® (DSM) and Acrylaway® (Novozymes) are used in the food industry for the reduction of acrylamide, a carcinogenic substance formed in foods containing L-asparagine and reducing sugars, such as glucose and fructose when heated at high temperature [69]. Glutaminase free L-asparaginase was produced from the novel endophyte Chaetomium sp. [70]. Most of the toxic effects of L-asparaginase are related to its glutaminase activity. Therefore it is necessary to decrease glutaminase activity for the effective treatment of ALL. Further, L-asparaginase producing endophytic fungi L. theobromae with an enzyme activity of 31.5 µM/mL/min was recovered from Teucrium polium [71] and Talaromyces cf. cecidicola was isolated from Tillandsia catimbauensis with the enzyme activity of 2.30 U/g [72]. The production of L-asparaginase has been reported in several endophytic fungi. The L-asparaginase producing endophytic fungi isolated from different plants are presented in Table 2.
Production of L-Asparaginase by Endophytic Fungi
The production of L-asparaginase differs with the organism, the process of production, and the fermentation media used for production. Different methods are reported for the production of L-asparaginase, the two major methods employed are solid state fermentation (SSF) and submerged fermentation (SmF). SmF process is well-established but has certain limitations like very less net yield and high production cost [73]. In recent times, the production of L-asparaginase using agricultural raw materials by SSF fermentation has gained importance. SSF is economical and product yield is much higher as compared to the SmF [74]. It uses low cost agricultural by-products such as rice straw, wheat straw, orange peel, sugarcane bagasse, etc. as a substrate. Thus, it is environmentally friendly when compared to SmF. Ruma et al., based on ease of availability and cost effectiveness screened different substrates (corn flour, coconut oil cake, groundnut oil cake, rice bran, wheat bran, orange peel and tea waste) for the production of L-asparaginase, by endophytic fungus F. solani isolated from W. sominifera. They obtained maximum enzyme activity using orange peel as substrate [60]. The seven agricultural substrates displayed different degrees of fermentation abilities, showing that the choice of the right substrate is critical for the production of L-asparaginase. The substrate plays a vital role in the supply of nutrients to the growth of the cells and has a major effect on the anchoring of the growing cells [60]. In another study, Silva et al., screened seventeen endophytic fungi for L-asparaginase production using Opuntia ficus-indica and Nopalea cochenillifera as substrate and they suggested O. ficus-indica flour as the best inexpensive substrate for the production of L-asparaginase by the endophytic fungus D. ueckerae URM 8321 [75].
Many factors have a prominent effect on the SSF process. The major factors are pH, temperature, substrate, carbon source, nitrogen source, agitation rate, and incubation period. Among these, the most important constituents in the fermentation medium are carbon and nitrogen source. The effect of several carbon sources such as glucose, sucrose, fructose, lactose, maltose, xylose, and starch on L-asparaginase production has been studied and many studies have shown that glucose is the ideal carbon source for the production of L-asparaginase by endophytic fungi [35, 59, 76]. Uzma et al., screened five carbon sources (glucose, sucrose, maltose, lactose, and starch) for maximizing the production of L-asparaginase by the endophytic fungus F. solani isolated from Tinospora cordifolia under submerged fermentation conditions. However, in contrast to the general understanding, they recorded maximum enzyme activity with sucrose as a carbon source [77]. It is reported that the production of L-asparaginase needs a low amount of carbon as it is under catabolic repression [78]. In another study by Nagarajan et al., they found that high glucose concentration in the media as a carbon source inhibited colony growth and enzyme production of endophytic Alternaria sp. isolated from W. somnifera [79].
Nitrogen is a significant nutrient source for the growth of microorganisms. Reports have indicated that ammonium sulphate is the best nitrogen source to obtain a high yield of L-asparaginase by endophytic fungi [80,81,82]. Jenila et al., screened two inorganic (ammonium sulphate, ammonium nitrate) and two organic nitrogen (peptone, yeast extract) sources for the production of L-asparaginase by endophytic Fusarium sp. isolated from Adhatoda vasica. Maximum L-asparaginase production of 10.21 U/mL was obtained when ammonium sulphate was used as a nitrogen source. The influence of various concentrations of ammonium sulphate (5 to 25 g/L) was further studied for increasing the production of L-asparaginase and the highest enzyme activity of 13.69 U/mL was obtained at 20 g/L concentration of ammonium sulphate [80]. Additionally, a study was conducted to know the effect of two nitrogen sources i.e., L-asparagine and sodium nitrate on L-asparaginase production by endophytic Fusarium sp. isolated from the roots of Andrographis paniculata. The results revealed that sodium nitrate induced less L-asparaginase production when compared to L-asparagine [83]. This shows nitrogen source impacts fungal biomass and growth which subsequently influences L-asparaginase production.
The pH and temperature of the fermentation medium play an important role in the production of L-asparaginase. Several studies reported that the optimum pH for endophytic fungal L-asparaginase production ranges from 6.0 to 9.0 [72, 76, 77]. Similarly, temperature ranging from 25 and 40 °C is stated as the optimum temperature for L-asparaginase production by endophytic fungi [80, 84, 85]. Jalgaonwala and Mahajan carried out production of L-asparaginase under different pH and temperatures by endophytic Eurotium sp. isolated from rhizomes of C. longa and they observed maximum enzyme activity at a temperature of 40 °C and a pH of 8.0 [86]. In another study, a pH of 6.0 and a temperature of 30 °C were found to be optimum for L-asparaginase production by endophytic Aspergillus sp. isolated from Cassia fistula [82]. Further, a pH of 6.0 and incubation temperature of 30 °C was reported to be optimum for another Aspergillus sp. isolated from leaves of salt marsh Sueada monoica [81]
Every organism requires different conditions for high yield. Therefore, optimization of cultural conditions and media components is necessary. Different optimization methods are used to maximize the production of L-asparaginase. The conventional method of one factor at a time (OFAT) used for optimization leads to mistakes in understanding of results as it disregards the effects of interactions between the factors [87]. Currently, many statistical tools such as surface response methodology (RSM), Plackett–Burman design (PBD), and Box-Behnken design (BBD) have been used for optimization studies [10]. These methods have many benefits such as they require a smaller number of experiments, several factors can be easily studied and response can be predicted initially, thus, aiding in finding the most suitable conditions for the production [88]. Yap et al., applied both OFAT and RSM to increase the production of L-asparaginase from the endophytic fungus F. proliferatum isolated from C. citratus. They obtained similar results in both OFAT and RSM. The optimum conditions were 0.20% of glucose, 0.99% of L-asparagine, and 5.34 days of incubation at 30.50 °C. They also found that the L-asparaginase production increased from 16.75 ± 0.76 IU/mL to 22.42 ± 0.20 IU/mL after optimization [58]. In another study, six factors (carbon, nitrogen source, and concentrations, incubation time, incubation temperature, pH, and agitation rate) were screened using the traditional OFAT method, and four significant variables were further optimized using Central Composite Rotatable Design (CCRD) for L-asparaginase production by Colletotrichum gloeosporioides. The optimum conditions shown by the OFAT and CCRD results were, 0.2% w/v glucose, 1% w/v L-asparagine, and 4 days incubation period at a temperature of 25 °C. Under the optimized conditions, the production of L-asparaginase significantly increased from 15.14 ± 0.25 IU/mL to 23.51 ± 0.13 IU/mL [89].
Araújo-Magalhães et al., used a 23 factorial design with three variables (pH, concentration of L-asparagine, and inoculum concentration) to optimize the production of L-asparaginase by endophytic fungus Phyllosticta catimbauensis isolated from Mandevilla catimbauensis. The production of L-asparaginase varied between 0.61 and 2.25 U/g and the maximum enzyme production (2.25 U/g) was achieved at pH 5.0, 1.5% L-asparagine, and 1.5% inoculum concentration. Further, based on the effect of independent variables an experiment was carried out to obtain a high yield by adjusting variables. In this experiment, 3.5 U/g of L-asparaginase production was achieved at pH 4.2, 3.5% L-asparagine, and 1.0% inoculum concentration. They found that through optimization the production of L-asparaginase was increased by 36.02% [90]. In another study Silva et al. used a similar 23 factorial design to study the effect of different variables on enzyme production from the endophytic fungus T. cf. cecidicola isolated from Tillandsia catimbauensis. They analyzed three variables that were pH, the concentration of L-proline, and spore concentration. Maximum dry biomass (0.66 g) production was achieved under pH 6.0, 1% L-proline, and 1 × 108 spore concentration and L-asparaginase production varied between 0.58 and 1.02 U/g. However, the analysis showed that the optimal point for the production of L-asparaginase was not achieved [72]. Further, the production of L-asparaginase by L. theobromae was optimized using the Taguchi model under four variables i.e., temperature, L-asparagine, glucose, and pH. The enzyme activity ranged from 10 to 175 IU/mL. However, after induction, the enzyme activity was found to be 315 IU/mL [85].
The incubation period and aeration also influence L-asparaginase production. El-Said et al., studied the effect of the incubation period on the production of L-asparaginase from endophytic fungi A. niger isolated from leaves of Datura innoxia and the highest production of L-asparaginase was achieved at shorter incubation period of 5 days [76]. Krishnapura and Belur studied the impact of agitation rate (100–150 rpm) on L-asparaginase production by the endophytic fungus T. pinophilus isolated from the rhizome of C. amada. Although an agitation rate of 100 rpm favoured the growth of the fungus it reduced the production of L-asparaginase. They recorded that an agitation rate of 120 rpm was found to be most appropriate for both biomass and enzyme production [84]. Most of the reports have indicated that at an agitation rate of around 120 rpm and an incubation period of 5 to 6 days, maximum production of L-asparaginase is easily achieved [59, 81, 89].
The above studies confirm that L-asparaginase production by endophytic fungi is affected by several parameters mainly pH, temperature, carbon source, nitrogen source, and substrate. These parameters vary from one organism to another. The study of the effect of process parameters and their optimization helps to increase the yield of the enzyme. A schematic representation of L-asparaginase production from the endophytic fungi is illustrated in Fig. 2.
Conclusion
L-asparaginase has become one of the most important drugs in anticancer therapy, specifically in the treatment of ALL. Although it is can be obtained from many sources, microbes are preferred owing to several advantages, mainly bacteria. However, it is found that the commercially available L-asparaginases from bacteria are coupled with many side effects and also the yield of an enzyme is not enough to fulfill the demand. Thus, there is a necessity for potent L-asparaginase producer strains with improved activity and reduced immunogenicity. Endophytic fungi from medicinal plants have shown great ability to produce L-asparaginase and some have exhibited prominent L-asparaginase activity. Further, optimization of cultural conditions of endophytic fungi have resulted in increasing the yield of L-asparaginase. Thus, endophytic fungi from medicinal plants can be potent source of L-asparaginae. However, biochemical characterization of these L-asparaginases are required to establish them as potential alternatives for bacterial L-asparaginases.
Data Availability
Not applicable.
Code Availability
Not applicable.
References
Siegel RL, Miller KD, Jemal A (2019) Cancer statistics, 2019. Cancer J Clin 69:7–34. https://doi.org/10.3322/caac.21551
Bray F, Ferlay J, Soerjomataram I et al (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J Clin 68:394–424. https://doi.org/10.3322/caac.21492
Egler RA, Ahuja SP, Matloub Y (2016) L-asparaginase in the treatment of patients with acute lymphoblastic leukemia. J Pharmacol Pharmacother 7:62–71. https://doi.org/10.4103/0976-500X.184769
Darvishi F, Jahanafrooz Z, Mokhtarzadeh A (2022) Microbial L-asparaginase as a promising enzyme for treatment of various cancers. Appl Microbiol Biotechnol 106:5335–5347. https://doi.org/10.1007/s00253-022-12086-8
Dange VU, Sakhale BK, Giri NA (2018) Enzyme application for reduction of acrylamide formation in fried potato chips. Curr Res Nutr Food Sci 6:222–226. https://doi.org/10.12944/CRNFSJ.6.1.25
Krishnapura PR, Belur PD, Subramanya S (2016) A critical review on properties and applications of microbial l-asparaginases. Crit Rev Microbiol 42:720–737. https://doi.org/10.3109/1040841X.2015.1022505
Cachumba JJM, Antunes FAF, Peres GFD et al (2016) Current applications and different approaches for microbial L-asparaginase production. Braz J Microbiol 47:77–85. https://doi.org/10.1016/j.bjm.2016.10.004
El-Naggar NEA, El-Ewasy SM, El-Shweihy NM (2014) Microbial L-asparaginase as a potential therapeutic agent for the treatment of acute lymphoblastic leukemia: the pros and cons. Int J Pharmacol 10:182–199. https://doi.org/10.3923/ijp.2014.182.199
de Sarquis MIM, Oliveira EMM, Santos AS, da Costa GL (2004) Production of L-asparaginase by filamentous fungi. Mem Inst Oswaldo Cruz 99:489–492. https://doi.org/10.1590/S0074-02762004000500005
Souza PM, de Freitas MM, Cardoso SL et al (2017) Optimization and purification of L-asparaginase from fungi: a systematic review. Crit Rev Oncol Hematol 120:194–202. https://doi.org/10.1016/j.critrevonc.2017.11.006
Chakraborty M, Shivakumar S (2021) Bioprospecting of the agaricomycete Ganoderma australe GPC191 as novel source for l-asparaginase production. Sci Rep 11:6192. https://doi.org/10.1038/s41598-021-84949-5
Stierle A, Strobel G, Stierle D (1993) Taxol and taxane production by Taxomyces andreanae, an endophytic fungus of Pacific yew. Science 260:214–216. https://doi.org/10.1126/science.8097061
Hridoy M, Gorapi MZH, Noor S et al (2022) Putative anticancer compounds from plant-derived endophytic fungi: a review. Molecules 27:296. https://doi.org/10.3390/molecules27010296
Wen J, Okyere SK, Wang S et al (2022) Endophytic fungi: an effective alternative source of plant-derived bioactive compounds for pharmacological studies. J Fungi 8:205. https://doi.org/10.3390/jof8020205
Bhadra F, Gupta A, Vasundhara M, Reddy MS (2022) Endophytic fungi: a potential source of industrial enzyme producers. 3 Biotech 12:86. https://doi.org/10.1007/s13205-022-03145-y
Shrivastava A, Khan AA, Khurshid M et al (2016) Recent developments in l-asparaginase discovery and its potential as anticancer agent. Crit Rev Oncol Hematol 100:1–10. https://doi.org/10.1016/j.critrevonc.2015.01.002
Saeed H, Hemida A, El-Nikhely N et al (2020) Highly efficient Pyrococcus furiosus recombinant L-asparaginase with no glutaminase activity: expression, purification, functional characterization, and cytotoxicity on THP-1, A549 and Caco-2 cell lines. Int J Biol Macromol 156:812–828. https://doi.org/10.1016/j.ijbiomac.2020.04.080
Michael Rytting M (2012) Role of L-asparaginase in acute lymphoblastic leukemia: focus on adult patients. Blood Lymphat Cancer. https://doi.org/10.2147/blctt.s18699
Singh R, Kumar M, Mittal A, Mehta PK (2016) Microbial enzymes: industrial progress in 21st century. 3 Biotech 6:1–15. https://doi.org/10.1007/s13205-016-0485-8
Sanches M, Krauchenco S, Polikarpov I (2012) Structure, substrate complexation and reaction mechanism of bacterial asparaginases. Curr Chem Biol 1:5–86. https://doi.org/10.2174/2212796810701010075
Vimal A, Kumar A (2017) Biotechnological production and practical application of L-asparaginase enzyme. Biotechnol Genet Eng Rev 33:40–61. https://doi.org/10.1080/02648725.2017.1357294
Cedar H, Schwartz JH (1968) Production of L-asparaginase II by Escherichia coli. J Bacteriol 96:2043–2048. https://doi.org/10.1128/jb.96.6.2043-2048.1968
Kotzia GA, Labrou NE (2007) l-Asparaginase from Erwinia Chrysanthemi 3937: cloning, expression and characterization. J Biotechnol 127:657–669. https://doi.org/10.1016/j.jbiotec.2006.07.037
Mesas JM, Gil JA, Martin JF (1990) Characterization and partial purification of L-asparaginase from Corynebacterium glutamicum. J Gen Microbiol 136:515–519. https://doi.org/10.1099/00221287-136-3-515
Badoei-Dalfard A (2015) Purification and characterization of l-asparaginase from Pseudomonas aeruginosa strain SN004: production optimization by statistical methods. Biocatal Agric Biotechnol 4:388–397. https://doi.org/10.1016/j.bcab.2015.06.007
Belén LH, Beltrán JF, Pessoa A et al (2022) Helicobacter pylori l-asparaginase: a study of immunogenicity from an in silico approach. 3 Biotech 12:286. https://doi.org/10.1007/s13205-022-03359-0
Alrumman SA, Mostafa YS, Al-izran KA et al (2019) Production and anticancer activity of an L-Asparaginase from Bacillus licheniformis isolated from the Red Sea, Saudi Arabia. Sci Rep 9:1–14. https://doi.org/10.1038/s41598-019-40512-x
Ghosh S, Murthy S, Govindasamy S, Chandrasekaran M (2013) Optimization of L-asparaginase production by Serratia marcescens (NCIM 2919) under solid state fermentation using coconut oil cake. Sustain Chem Process 1:1–8. https://doi.org/10.1186/2043-7129-1-9
Chityala S, Venkata Dasu V, Ahmad J, Prakasham RS (2015) High yield expression of novel glutaminase free l-asparaginase II of Pectobacterium carotovorum MTCC 1428 in Bacillus subtilis WB800N. Bioprocess Biosyst Eng 38:2271–2284. https://doi.org/10.1007/s00449-015-1464-x
Costa-Silva TA, Flores-Santos JC, Freire RKB et al (2018) Microbial cell disruption methods for efficient release of enzyme L-asparaginase. Prep Biochem Biotechnol 48:707–717. https://doi.org/10.1080/10826068.2018.1487850
Mishra A (2006) Production of L-asparaginase, an anticancer agent, from Aspergillus niger using agricultural waste in solid state fermentation. Appl Biochem Biotechnol 135:33–42. https://doi.org/10.1385/ABAB:135:1:33
Patro KR, Basak UC, Mohapatra AK, Gupta N (2014) Development of new medium composition for enhanced production of L-asparaginase by Aspergillus flavus. J Environ Biol 35:295–300
Benchamin D, Roshan JF, Kurup BS, Dani Benchamin C (2019) Production and characterization of L-Asparaginase isolated from Aspergillus fumigatus. Pharm Innov J 8:220–223
Lincoln L, Niyonzima F, More S (2019) Purification and properties of a fungal L-asparaginase from Trichoderma Viride Pers: Sf grey. J Microbiol Biotechnol Food Sci 9:310–316. https://doi.org/10.15414/jmbfs.2014.4.4.310-316
Mohan Kumar NS, Ramasamy R, Manonmani HK (2013) Production and optimization of l-asparaginase from Cladosporium sp. using agricultural residues in solid state fermentation. Ind Crops Prod 43:150–158. https://doi.org/10.1016/j.indcrop.2012.07.023
El-Gendy MMAA, Awad MF, El-Shenawy FS, El-Bondkly AMA (2021) Production, purification, characterization, antioxidant and antiproliferative activities of extracellular L-asparaginase produced by Fusarium equiseti AHMF4. Saudi J Biol Sci 28:2540–2548. https://doi.org/10.1016/j.sjbs.2021.01.058
Deshpande N, Choubey P, Agashe M (2014) Studies on optimization of growth parameters for L-asparaginase production by streptomyces ginsengisoli. Sci World J. https://doi.org/10.1155/2014/895167
Amena S, Vishalakshi N, Prabhakar M et al (2010) Production, purification and characterization of L-asparaginase from Streptomyces gulbargensis. Braz J Microbiol 41:173–178. https://doi.org/10.1590/S1517-83822010000100025
Dharmaraj S (2011) Study of L-asparaginase production by Streptomyces noursei MTCC 10469, isolated from marine sponge Callyspongia diffusa. Iran J Biotechnol 9:102–108
Hatanaka T, Usuki H, Arima J et al (2011) Extracellular production and characterization of two Streptomyces L-asparaginases. Appl Biochem Biotechnol 163:836–844. https://doi.org/10.1007/s12010-010-9087-9
Narayana KJP, Kumar KG, Vijayalakshmi M (2008) L-asparaginase production by Streptomyces albidoflavus. Indian J Microbiol 48:331–336. https://doi.org/10.1007/s12088-008-0018-1
DeJong PJ (1972) L-asparaginase production by Streptomyces griseus. Appl Microbiol 23:1163–1164. https://doi.org/10.1128/aem.23.6.1163-1164.1972
Kil JO, Kim GN, Park I (1995) Extraction of extracellular L-asparaginase from Candida utilis. Biosci Biotechnol Biochem 59:749–750. https://doi.org/10.1271/bbb.59.749
Daverey A, Pakshirajan K (2010) Kinetics of growth and enhanced sophorolipids production by candida bombicola using a low-cost fermentative medium. Appl Biochem Biotechnol 160:2090–2101. https://doi.org/10.1007/s12010-009-8797-3
Salah Foda M, Zedan HH, Abd El-Megeed S, Hashem A (1980) Formation and properties of L-glutaminase and L-asparaginase activities in Pichia polymorpha. Acta Microbiol Pol 29:343–352
Ramakrishnan MS, Joseph R (1996) Characterization of an extracellular asparaginase of Rhodosporidium toruloides CBS14 exhibiting unique physicochemical properties. Can J Microbiol 42:316–325. https://doi.org/10.1139/m96-047
Costa IM, Schultz L, Pedra DAB, B, et al (2016) Recombinant L-asparaginase 1 from Saccharomyces cerevisiae: an allosteric enzyme with antineoplastic activity. Sci Rep 6:36239. https://doi.org/10.1038/srep36239
Paul JH (1982) Isolation and characterization of a Chlamydomonas L-asparaginase. Biochem J 203:109–115. https://doi.org/10.1042/bj2030109
Ebrahiminezhad A, Rasoul-Amini S, Ghoshoon MB, Ghasemi Y (2014) Chlorella vulgaris, a novel microalgal source for L-asparaginase production. Biocatal Agric Biotechnol 3:214–217. https://doi.org/10.1016/j.bcab.2013.10.005
Abd El Baky HH, El Baroty GS (2016) Optimization of growth conditions for purification and production of L-asparaginase by Spirulina maxima. Evid Based Complementary Altern Med. https://doi.org/10.1155/2016/1785938
Chow YY, Ting ASY (2015) Endophytic l-asparaginase-producing fungi from plants associated with anticancer properties. J Adv Res 6:869–876. https://doi.org/10.1016/j.jare.2014.07.005
de Pádua APSL, de Freire KTL, S, de Oliveira TGL, et al (2019) Fungal endophyte diversity in the leaves of the medicinal plant myracrodruon urundeuva in a Brazilian dry tropical forest and their capacity to produce L-asparaginase. Acta Bot Brasilica 33:39–49. https://doi.org/10.1590/0102-33062018abb0108
Del Frari G, Gobbi A, Aggerbeck MR et al (2019) Characterization of the wood mycobiome of Vitis vinifera in a vineyard affected by esca. Spatial distribution of fungal communities and their putative relation with leaf symptoms. Front Plant Sci 10:910. https://doi.org/10.3389/fpls.2019.00910
Baluyot JC, Santos HK, Batoctoy DCR et al (2022) Diaporthe/Phomopsis longicolla degrades an array of bisphenol analogues with secreted laccase. Microbiol Res 257:126973. https://doi.org/10.1016/j.micres.2022.126973
Manasa C, Nalini MS (2014) L-asparaginase activity of fungal endophytes from Tabernaemontana heyneana Wall. (Apocynaceae), endemic to the Western Ghats (India). Int Sch Res Notices. https://doi.org/10.1155/2014/925131
Kumar R, Sedolkar VK, Triveni AG et al (2016) Isolation, screening and characterization of L-asparaginase producing fungi from medicinal plants. Int J Pharm Pharm Sci 8:281–283
Meghavarnam AK, Janakiraman S (2017) Solid state fermentation: an effective fermentation strategy for the production of L-asparaginase by Fusarium culmorum (ASP-87). Biocatal Agric Biotechnol 11:124–130. https://doi.org/10.1016/j.bcab.2017.06.001
El-Hadi A, El-Refai H, Shafei M et al (2017) Statistical optimization of L-asparaginase production by using Fusarium solani. Egypt Pharm J 16:16. https://doi.org/10.4103/1687-4315.205825
Yap LS, Lee WL, Ting ASY (2021) Optimization of L-asparaginase production from endophytic Fusarium proliferatum using OFAT and RSM and its cytotoxic evaluation. J Microbiol Methods 191:106358. https://doi.org/10.1016/j.mimet.2021.106358
Ruma K, George TK, Aswani P, Jisha MS (2017) Production and optimization of extra cellular L-asparaginase by Fusarium solani isolated from Withania sominifera. J Biol Act Prod Nate 7:81–88. https://doi.org/10.1080/22311866.2017.1325007
Bhosale H, As-Suhbani AE (2019) Screening of fungal endophytes isolated from medicinal plants for glutaminase free L-asparaginase activity. J Exp Biol Agric Sci 7:396–402. https://doi.org/10.18006/2019.7(4).396.402
Pradhan B, Dash SK, Sahoo S (2013) Screening and characterization of extracelluar L-asparaginase producing Bacillus subtilis strain hswx88, isolated from Taptapani hotspring of Odisha, India. Asian Pac J Trop Biomed 3:936–941. https://doi.org/10.1016/S2221-1691(13)60182-3
Pola M, Rajulapati SB, Potla Durthi C et al (2018) In silico modelling and molecular dynamics simulation studies on L-asparaginase isolated from bacterial endophyte of Ocimum tenuiflorum. Enzyme Microb Technol 117:32–40. https://doi.org/10.1016/j.enzmictec.2018.06.005
Lee SJ, Lee Y, Park GH et al (2016) A newly identified glutaminase-free L-asparaginase (L-ASPG86) from the marine bacterium mesoflavibacter zeaxanthinifaciens. J Microbiol Biotechnol 26:1115–1123. https://doi.org/10.4014/jmb.1510.10092
Moharram A, Zohri A, Of NS-SIJ (2016) L-Asparaginase production by endophytic fungi isolated from Withania Somnifera in Egypt. SS Int j multidiscip res 2:30–40
Vaishnavi K, Saxena N, Shah N et al (2012) Differential activities of the two closely related withanolides, withaferin A and withanone: bioinformatics and experimental evidences. PLoS ONE 7:e44419. https://doi.org/10.1371/journal.pone.0044419
Prihanto AA, Caisariyo IO, Pradarameswari KA (2019) Aspergillus sp. as a potential producer for L-asparaginase from mangrove (Avicennia germinans). IOP Conf Ser: Earth Environ Sci 230:012101. https://doi.org/10.1088/1755-1315/230/1/012101
Prihanto AA, Swardhika G, Pradarameswari KA (2019) Trichoderma sp., a potential producer for L-asparaginase isolated from Sonneratia alba inAeng Sareh Beach, Madura. IOP Conf Ser: Earth Environ Sci 239:012033. https://doi.org/10.1088/1755-1315/239/1/012033
Xu F, Oruna-Concha MJ, Elmore JS (2016) The use of asparaginase to reduce acrylamide levels in cooked food. Food Chem 210:163–171. https://doi.org/10.1016/j.foodchem.2016.04.105
Arumugam N, Thangavelu P (2022) Purification and anticancer activity of glutaminase and urease free intracellular L-asparaginase from Chaetomium sp. Protein Expr Purif 190:106006. https://doi.org/10.1016/j.pep.2021.106006
Balbool BA, Abdel-Azeem AM, Moubasher MH, Helmy EA (2018) Production of L-Asparaginase (L-ASN) from endophytic Lasiodiplodia theobromae hosted Teucrium polium in Egypt. Microb biosyst 3:46–55. https://doi.org/10.21608/mb.2018.26276
Silva LF, Freire KTLS, Araújo-Magalhães GR et al (2018) Penicillium and Talaromyces endophytes from Tillandsia catimbauensis, a bromeliad endemic in the Brazilian tropical dry forest, and their potential for l-asparaginase production. World J Microbiol Biotechnol 34:1–12. https://doi.org/10.1007/s11274-018-2547-z
Barrios-González J (2012) Solid-state fermentation: physiology of solid medium, its molecular basis and applications. Process Biochem 47:175–185. https://doi.org/10.1016/j.procbio.2011.11.016
Viniegra-González G, Favela-Torres E, Aguilar CN et al (2003) Advantages of fungal enzyme production in solid state over liquid fermentation systems. Biochem Eng J 13:157–167. https://doi.org/10.1016/S1369-703X(02)00128-6
da Silva LF, de Pádua APSL, de Oliveira FL et al (2022) Cacti as low-cost substrates to produce L-asparaginase by endophytic fungi. World J Microbiol Biotechnol 38:247. https://doi.org/10.1007/s11274-022-03420-3
El-said AHM, Shebany YM, Hussein MA, El-dawy EGA (2016) Antimicrobial and L-asparaginase activities of endophytic fungi isolated from Datura innoxia and Hyoscyamus muticus medicinal plants. Eur J Biol Res 6:135–144. https://doi.org/10.5281/zenodo.56056
Uzma F, Murthy N, Srinivas C (2016) Optimization of physiological conditions for L-asparaginase production by endophytic fungi (Fusarium solani) isolated from Tinospora cordifolia (Willd.) Hook. F & Thomson. Eur J Exp Biol 6:37–45
Freitas M, Souza P, Cardoso S et al (2021) Filamentous fungi producing l-asparaginase with low glutaminase activity isolated from brazilian savanna soil. Pharmaceutics 13:1268. https://doi.org/10.3390/pharmaceutics13081268
Nagarajan A, Thirunavukkarasu N, Suryanarayanan TS, Gummadi SN (2014) Screening and isolation of novel glutaminase free L-asparaginase from fungal endophytes. Res J Microbiol 9:163
Jenila VA, Gnanadoss JJ (2018) Formulation of a suitable medium and its optimization for maximizing L-asparaginase production from endophytic fungi Fusarium sp. LCJ273. Biosci Biotechnol Res Asia 15:887–898. https://doi.org/10.13005/bbra/2699
Kalyanasundaram I, Nagamuthu J, Srinivasan B et al (2015) Production, purification and characterisation of extracellular L-asparaginase from salt marsh fungal endophytes. World J Pharm Pharm Sci 4:663–677
Pundir RK, Yadav D, Jain P (2020) Production, optimization and partial purification of l-asparaginase from endophytic fungus Aspergillus sp., isolated from Cassia fistula. Appl Biol Res 22:26–33. https://doi.org/10.5958/0974-4517.2020.00008.7
Hermanto A, Ting ASY (2016) Comparative effect of L-asparagine and sodium nitrate in inducing L-asparaginase production by endophytic Fusarium sp. Acta Biol Szeged 60:145–150
Krishnapura PR, Belur PD (2016) Partial purification and characterization of L-asparaginase from an endophytic Talaromyces pinophilus isolated from the rhizomes of Curcuma amada. J Mol Catal B 124:83–91. https://doi.org/10.1016/j.molcatb.2015.12.007
Moubasher HA, Balbool BA, Helmy YA et al (2022) Insights into asparaginase from endophytic fungus Lasiodiplodia theobromae: purification, characterization and antileukemic activity. Int J Environ Res Public Health 19:680. https://doi.org/10.3390/ijerph19020680
Jalgaonwala RE, Mahajan RT (2014) Production of anticancer enzyme asparaginase from endophytic Eurotium sp. isolated from rhizomes of Curcuma longa. Eur J Exp Biol 4:36–43
Singh V, Haque S, Niwas R et al (2017) Strategies for fermentation medium optimization: an in-depth review. Front Microbiol 7:2087. https://doi.org/10.3389/fmicb.2016.02087
Gilman J, Walls L, Bandiera L, Menolascina F (2021) Statistical design of experiments for synthetic biology. ACS Synth Biol 10:1–18. https://doi.org/10.1021/acssynbio.0c00385
Yap LS, Lee WL, Ting ASY (2022) Bioprocessing and purification of extracellular L-asparaginase produced by endophytic Colletotrichum gloeosporioides and its anticancer activity. Prep Biochem Biotechnol. https://doi.org/10.1080/10826068.2022.2122064
Araújo-Magalhães GR, Maciel MHC, da Silva LF et al (2021) Fungal endophytes from leaves of Mandevilla catimbauensis (Apocynaceae): diversity and potential for L-asparaginase production. Braz J Microbiol 52:1431–1441. https://doi.org/10.1007/s42770-021-00505-3
Heo YA, Syed YY, Keam SJ (2019) Pegaspargase: a review in acute lymphoblastic leukaemia. Drugs 79:767–777. https://doi.org/10.1007/s40265-019-01120-1
Salzer WL, Asselin BL, Plourde PV et al (2014) Development of asparaginase Erwinia chrysanthemi for the treatment of acute lymphoblastic leukemia. Ann NY Acad Sci 1329:81–92. https://doi.org/10.1111/nyas.12496
Völler S, Pichlmeier U, Zens A, Hempel G (2018) Pharmacokinetics of recombinant asparaginase in children with acute lymphoblastic leukemia. Cancer Chemother Pharmacol 81:305–314. https://doi.org/10.1007/s00280-017-3492-5
Da Silva Santos MG, Pereira Bezerra JD, Svedese VM et al (2015) Screening of endophytic fungi from cactus of the Brazilian tropical dry forest according to their L-asparaginase activity. Sydowia 67:147–156. https://doi.org/10.12905/0380.sydowia67-2015-0147
Krishnapura PR, Belur PD (2016) Isolation and screening of endophytes from the rhizomes of some Zingiberaceae plants for L-asparaginase production. Prep Biochem Biotechnol 46:281–287. https://doi.org/10.1080/10826068.2015.1031385
Bhavana NS, Prakash HS, Nalini MS (2019) Antioxidative and L-asparaginase potentials of fungal endophytes from Rauvolfia densiflora (Apocynaceae), an ethnomedicinal species of the Western Ghats. Czech Mycol 71:187–203. https://doi.org/10.33585/cmy.71205
Bhavana NS, Prakash HS, Nalini MS (2020) Fungal endophytes from Tabernaemontana heyneana Wall. (Apocynaceae), their molecular characterization, L-asparaginase and antioxidant activities. Jordan J Biol Sci 13:543–550
Hatamzadeh S, Rahnama K, Nasrollahnejad S et al (2020) Isolation and identification of L-asparaginase-producing endophytic fungi from the asteraceae family plant species of Iran. PeerJ 2020:e8309. https://doi.org/10.7717/peerj.8309
Prabavathy D (2020) Cytotoxic activity of L-asparaginase isolated from endophytic Aspergillus nomius of Justicia adhatoda on A549 cell lines. Int J Green Pharm 14:195–200. https://doi.org/10.22377/ijgp.v14i02.2884
Acknowledgements
The authors are thankful to the Council of Scientific and Industrial Research (CSIR) for providing a Junior Research Fellowship (JRF) and DOS in Microbiology, University of Mysore for providing facilities.
Funding
Not applicable.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethical Approval
Not applicable. As the submitted manuscript is a review article, it does not contain any kind of experiments that includes animals or clinical samples; hence it does not require any Ethical Clearance.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Parashiva, J., Nuthan, B.R., Rakshith, D. et al. Endophytic Fungi as a Promising Source of Anticancer L-Asparaginase: A Review. Curr Microbiol 80, 282 (2023). https://doi.org/10.1007/s00284-023-03392-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00284-023-03392-z