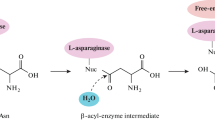
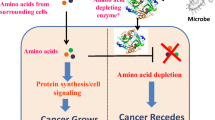
Abstract
Metabolic differences between normal and cancerous cells have been used as a point of view for developing anticancer drugs. Some degrading enzymes of certain amino acids have been regarded to kill cancerous cells. L-Asparaginase (ASNase) has shown an excellent therapeutic response to asparagine-auxotrophic cancers such as acute lymphoblastic leukemia (ALL). Some bacteria, yeasts, molds, plants, and animals produce ASNase. Bacterial ASNases from Escherichia coli and Erwinia chrysanthemi are the FDA-approved drugs for ALL treatment. Here, we review new natural prokaryotic and eukaryotic sources of ASNases, recent advances to introduce improvement strategies for the production of recombinant ASNases as well as their chemical modifications, immobilization, nanoencapsulation, and in silico studies to increase efficiency and decrease side effects. Recent studies for application of ASNases to treatment of asparagine-auxotrophic cancers, especially solid cancers, have been reviewed. Furthermore, challenges and future perspectives are discussed for this promising therapeutic enzyme.
Key points
• Review recent advances to introduce new sources of microbial L-asparaginases.
• Review improvement strategies for the development of stable and non-toxic L-asparaginases.
• Review microbial L-asparaginase application in various cancers’ treatment.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Enzymes are potent biocatalysts that are commercially utilized in various industrial functions, from clinical approaches to biofuels. Due to rapid growth in improvement techniques, expansion of enzyme application has attracted more attention over recent decades (Lukey et al. 2017). Tumor cells usually have unbalanced enzyme activity, which can be used as an effective strategy for therapeutic purposes. In other words, cancer is a consequence of perturbation of multiple different cellular pathways in which one or more enzymes lost their normal function (Baig et al. 2019). Cancer cells often have metabolically addictions to some extracellular essential substances because of either epigenetic silencing of enzyme genes or even genetic mutations in the enzyme’s coding sequence. Therefore, many enzymes with different functions could target cancer cells and exert an anticancer effect. As an example, some cancer cells are dependent on exterior arginine amino acid and cannot synthesize their required arginine. Therefore, arginase can act as an anticancer drug by limiting arginine usage in these cancer cells (Garcia-Bermudez et al. 2020). As another example, PEGylated kynureninase degrades kynurenine (as an immunosuppressive metabolite) in the tumor microenvironment (TME) leading to an increase in the number of cytotoxic T cells (CD8+ cells) and tumor cell death (Triplett et al. 2018). Enzyme therapy is a cost-effective and targeted approach that has fewer complications compared to conventional therapies such as surgery, radiotherapy, and chemotherapy (Baig et al. 2019). In addition, enzyme therapy approach offers several advantages relative to some novel treatments like DNA and mRNA cancer vaccines, which stimulate immune system against cancer cells. DNA and mRNA cancer vaccines need more various preparation laborious works such as the selection of target tumor antigen in comparison to enzyme therapy (Jahanafrooz et al. 2019). There have been many reported preclinical and clinical examples of enzyme application in cancer therapy. Some enzymes have been approved by the Food and Drug Administration (FDA); for instance, L-asparaginase (ASNase) or asparagine amidohydrolase (EC.3.5.1.1), as an anticancer drug (Dhankhar et al. 2020). In this review, recent information about various natural sources of ASNase and applied new improvement strategies, including chemical modifications, in silico studies, immobilization, and nanoencapsulation to provide more stable, less immunogenic, and more functional enzymes with high affinity to asparagine (Asn) are discussed. Then, we summarized the recent successes in the anticancer properties of ASNases in blood cancers. Furthermore, this review highlights the application of this enzyme as a promising bioactive molecule for the treatment of solid tumors.
Historical background of L-asparaginase as an anticancer agent
At the beginning of the 1900s, the capacity of beef liver suspension and pig and horse tissues to hydrolyze Asn was revealed (Geddes and Hunter 1928). In 1922, it was found that the presence of ASNase is a type of biochemical adaptation to a vegetarian diet. Other experiments determined the distribution of ASNase in bacteria, yeast, plants, and other animals (Geddes and Hunter 1928). Evidence of tumor inhibitory characteristics of ASNase goes back to 1953, in which, for the first time cytotoxic effect of guinea pig serum on the cells of three transplantable mice and rat lymphomas in vivo was indicated. Later in 1961–1963, it was described that ASNase is the efficient component of guinea pig serum (Krishnapura et al. 2016; Mashburn and WRISTON Jr 1964). Further investigation showed that ASNase from Escherichia coli has similar anti-neoplastic activity to one extracted from guinea pig serum. Then large-scale production of ASNase from bacterial sources increased the availability of enzymes for therapeutic application. Around the 1970s, ASNase was introduced against acute lymphoblastic leukemia (ALL), and its anticancer effect has been proven against ALL. FDA approved ASNases from E. coli and Erwinia chrysanthemi in 1978 for use in ALL treatments (Krishnapura et al. 2016). So far, ASNase has been extracted from various origins, including bacteria, yeast, fungi, algae, plants, and animals (Ghasemian et al. 2019; Mazloum-Ravasan et al. 2020). Each source has its advantages and disadvantage with different physicochemical and kinetic properties such as molecular mass, optimum temperature and pH, Vmax, and Km of the enzyme. Microorganisms are industrial sources for the production of ASNases (Mohideen 2020). There are two isoforms of bacterial ASNases with differences in structure and cellular localization. ASNase type I is a cytosolic enzyme with a lower affinity to Asn, and ASNase type II is a periplasmic enzyme with a higher affinity to Asn. ASNase type II is a promising enzyme in anticancer research (Mohideen 2020). Some recent studies about various microbial sources for ASNase production were summarized in Table 1. Compounds of culture media, fermentation, and purification processes are influencing factors on quality, stability, and activity of enzymes (Dias et al. 2016). ASNases from E. coli, Er. chrysanthemi, Er. aroideae, and Serratia marcescens were established and approved for cancer treatment. Immunogenicity of Er. chrysanthemi ASNase is lower than purified ASNase from E. coli (Duval et al. 2002). Recombinant ASNases have been investigated widely as a solution to decrease immunogenicity and glutaminase activity as well as increase stability and substrate affinity. Recombinant ASNases have been produced in safer and high-yield hosts (Table 2). The amino acid sequences can improve in recombinant ASNases via genetic engineering techniques such as site-directed mutagenesis (Brumano et al. 2019).
Underlying anticancer mechanism of L-asparaginase
Asn auxotrophic state or low/no expression of ASNS is a required feature for a cancer cell to be sensitive to ASNase (Garcia-Bermudez et al. 2020). According to studies, increased expression of ASNS promotes cancer cell invasion and metastasis (Chiu et al. 2019). In addition to ASNS activity, the amount of glutamine (Gln) is another influencing host factor in ALL and other cancer (such as prostate cancer) cells response to ASNase, though, more resistance to ASNase is reported in cells with a normal Gln synthase, a high expression of glutamic acid (Glu) transporters, and a residual ASNS protein expression (Fig. 1) (Chiu et al. 2019). ASNase exerts anticancer effects at last in two perspectives. At first view, it hydrolyses non-essential Asn amino acid to aspartic acid and ammonium ions (NH4+) that cause depletion of Asn in the plasma. Cancer cells mostly lose/reduce their asparagine synthetase (asparagine synthase, ASNS) and are dependent on plasma Asn to provide this amino acid for their protein, DNA, and RNA synthesis and metabolism; moreover, because of the negative charge of aspartic acid and its impermeability to the plasma membrane, internal Asn is also the main precursor for aspartic acid in tumor cells. Therefore, Asn depletion automatically leads to aspartic acid depletion in cancer cells which results in decreased protein synthesis and possibly cell death. At the second view or non-canonical route, ASNase influences the cancer cells directly; it was shown that ASNase affects the level of reactive oxygen species (ROS), cell cycle progression, autophagy, and apoptotic cell death (Song et al. 2015 and 2017; Costa-Silva et al. 2020). Inhibition of Akt/mTOR and Erk signaling pathways under ASNase treatment is another anticancer mechanism. Akt/mTOR and Erk signaling pathways are essential in cell growth and survival (Dhankhar et al. 2020). Akt/mTOR signaling pathway is also involved in autophagy induction. Administration of autophagy inhibitors in K562 chronic myeloid leukemia (CML) cells caused an increase in apoptotic cell death under ASNase treatment (Song et al. 2015). In addition, diffusion of ammonium ions into the cytosol by modifying pH leads to the activating of an apoptotic signaling pathway (Krishnapura et al. 2016).
Effect of L-asparaginase (ASNase) therapy on cancer cell context. As indicated here, ASNase treatment in cancer cells with no asparagine synthase (ASNS) drives apoptosis. However, the absence or presence of glutamine (Gln), as an amine group donor, is a crucial factor in driving apoptosis or survival/rescue, respectively, in cancer cells with fewer ASNS
Chemically modified L-asparaginases
Like many other peptide drugs, various formulation strategies have been applied to reach more extended bioavailability or stability and decreased immunogenicity of ASNase. PEGylated E. coli ASNase pegaspargase (Oncaspar®) was approved by the FDA in 1994 and applied for the first-line treatment of ALL in 2006. In pegaspargase, 69–82 molecules of monomethoxy polyethylene glycol (PEG) chains are covalently attached to the cysteine amino acids side chains in ASNase (Lima et al. 2020). According to a comparative study, patients who received pegaspargase showed fewer side effects, less allergic response, and required fewer medical care visits which caused similar treatment overall cost to a native enzyme (Brumano et al. 2019). The half-life of pegaspargase (i.e., 5.5–7 days) is significantly higher than the native one (i.e., 26–30 h). Further, the required dosage of pegaspargase is reported as 2000–2500 IU/m2 every 2 or 4 weeks that is less than for native ASNase, which is 6000 IU/m2 thrice per week times/week (Dhankhar et al. 2020). Compared to native enzymes, PEGylated ASNase shows a decreased drug immunogenicity, increased water solubility, less peptide aggregation, increased pH and temperature resistance, and improved drug stability and efficiency. However, random attachment of chemical substances like PEG to ASNase causes batch-to-batch variation (Brumano et al. 2019). A novel more stable bioconjugate of ASNase and PEG named calaspargase pegol-mknl (CALASP) was approved in 2018 in which a recombinant E. coli ASNase conjugated with monomethoxy-PEG with a succinimidyl carbonate (SC) linker (Li et al. 2020). Intravenous injection of CALASP showed significantly longer serum asparaginase activity compared to pegaspargase which is critical to drug efficacy and successful treatment (Li et al. 2020). PASylation is an alternative to PEGylation in which proline/alanine-rich sequences (PAS) covalently bond with peptide drugs via genetic fusion or chemical coupling (Binder and Skerra 2017). PASylated ASNase, similar to PEGylated ASNase, introduced a stable form of ASNase with less/no immunogenicity in vivo (Brumano et al. 2019). Lactosylation and coupling with dextran are other chemical modifications that lead to a prolonged half-life of ASNase and increased resistance to thermal and protease cleavage (Muneer et al. 2020). It was reported that the involvement of some adjuvants such as sucrose and sorbitol in ASNase formulation could lead to an increase in the specific activity and stability, and an increase enzyme aggregation (Wlodarczyk et al. 2019).
Immobilization and encapsulation of L-asparaginase
Different delivery methods have been applied to provide stability of peptide drugs under body temperature and pH. A promising approach for the cost-effective delivery of ASNase is its immobilization on various support materials, either physically or in a covalent attachment. In physical attachment, hydrogen bonds, van der Waals forces, and ionic interactions cause adsorption between enzymes and supports, which usually have a more negligible effect on the natural 3D structure of enzymes than covalent attachment (Brumano et al. 2019). According to one study, immobilization of ASNase on magnetic (i.e., Fe3O4) poly 2-hydroxyethyl methacrylate (HEMA), glycidyl methacrylate (GMA) nanoparticles decreased the kcat value of enzyme, which results in an increased affinity to a substrate. Also, the thermal stability and operational stability of immobilized ASNase on magnetic poly (HEMA-GMA) nanoparticles were significantly higher than Free-ASNase (Orhan and Aktaş Uygun 2020). Moreover, nanoparticles can be used for encapsulation of ASNase, which provides numerous advantages such as drug stability, reduced concentration of the drug, enhanced circulating time in body fluids, and decreased unwanted interaction between host proteins such as antibodies and drugs (Mu et al. 2020). Various biodegradable and biocompatible materials used in noncarrier forms, including liposomes, cationic polymers, cationic peptides, carbon nanotubes, and hollow nanospheres, are used widely to deliver peptide drugs such as ASNase (Brumano et al. 2019). Blackman et al. designed a polymeric vesicle known as polymersome or “nanobioreactor” encapsulated ASNase, which had some advantages compared to other nanocapsule-based ASNase because not only it could exert an anticancer effect without entering the cells but also protect enzyme against proteases and antibody recognition (Blackman et al. 2018). Both encapsulation and immobilization techniques not only increase enzyme stability and selectivity but also decrease the side effect of ASNase, including allergic response, blood coagulation, hepatic, pancreatitis, and central nervous system toxicity. Moreover, the incorporation of bio-conjugate with cell surface-specific monoclonal cancer-specific antibodies could demonstrate an even more targetable function of ASNase in tumor cells (Poznansky et al. 1982). There is no nanoencapsulated FDA-approved ASNase so far. Size or mass heterogeneity of prepared nanostructures, less optimal biocompatibility, and a requirement to be decorated with targetable agents to reach specific binding to cancer cells are among the suggested drawbacks for the application of nanostructure-based ASNase, which can be solved by time (Brumano et al. 2019).
In silico and molecular docking studies for improvement of L-asparaginase
Computer-aided techniques such as in silico studies have been used to analyze a collection of the previously reported experimental database to screen high-performance enzymes (Darvishi et al. 2019). Molecular docking, as more useful in silico studies, has been used for analyzing the interaction between both enzyme–substrate and receptor-ligand (Baral et al. 2020; Mohideen 2020). Indeed, docking has been used to select the better ASNase from a large group of ASNases from various sources. Baral et al. analyzed ASNases produced by different bacteria and archaea in a phylogenetic tree by homology modeling and bioinformatics tools to find the genus that synthesizes ASNase with a similar function and structure to E. coli ASNase as a template (Baral et al. 2020). It was proposed that enzymes with a different amino acid sequence are less immunogenic. The selected ASNase producers were at the most phylogenetic distance from the two commercially available genus producers ASNase type II (E. coli and Erwinia). Then, they evaluated the Km, kcat, binding energy, and active site interaction for selected ASNases by docking software and found ASNase from Streptomyces griseus, Streptomyces collinus, and Streptomyces venezuelae have better kinetics rather than currently commercially available ASNase (Baral et al. 2020). Another in silico study demonstrated that ASNase type I from Vibrio campbellii is a stable dimeric enzyme with a molecular weight of 36.9 kDa, which has a higher binding affinity to Asn and can be regarded as an alternative for commercially ASNase for treatment of ALL (Mohideen 2020). In addition, molecular docking studies have been used to predict the kinetic characteristics and glutaminase activity of various mutant versions of ASNase. The V27T mutant version of ASNase showed more stability and less glutaminase activity than the wild type with 100% retained activity (Ardalan et al. 2018). Also, N24S mutation was proposed as a protease-resistant and more stable ASNase by in silico studies (Maggi et al. 2017). Overall, molecular docking studies can suggest required modifications to improve ASNase for cancer treatment.
L-Asparaginase as a potent anticancer agent
Unbalanced enzymes not only can be regarded as cancer biomarkers for cancer detection and validation but also can provide therapeutic targets in various approaches (Baig et al. 2019). The anti-proliferative effects of many natural agents are because of inhibiting enzymes or modulation of their expression. For instance, rice callus suspension culture by targeting and inhibiting lactate dehydrogenase leads to an increase in reactive oxygen species (ROS) and apoptosis induction in cancer cells (Baig et al. 2019). In addition, enzymes themselves have been used as therapeutic agents in cancer treatment. As normal cells can synthesize their required amino acids through their normal pathways, the application of depleting enzymes along with chemotherapy in auxotrophic tumors is reported as a targeted therapy in various cancers. Arginine deiminase, ANSase, methionase, lysine oxidase, glutaminase, and phenylalanine ammonia lyase are some microbial-depleting enzymes under investigation for cancer treatment (Dhankhar et al. 2020). ANSase causes depletion of Asn in TME, resulting in cancer cell death or growth arrest. ASNases from E. coli and Er. chrysanthemi have been approved and used globally in different brand names for ALL treatments. Further, its anticancer capacity in other blood cancers and solid cancers is promising, as discussed in the following (Ghasemian et al. 2019; Song et al. 2015).
L-Asparaginase in treatment of hematological cancers
Blood cancers originate from blood cells or their precursors inside the bone marrow in various stages of differentiation. According to Fig. 2, blood cancers are divided based on their start location, subsequent tumor cell behavior, and originated cells; for example, acute myeloid leukemia (AML) originates from myeloid cells, which proliferate quickly (acute), or ALL starts in lymphocytes; if the cancerous state of lymphocytes begins in the lymph system, they will create lymphoma which according to their origin (T or B cells) and behavior are classified into a few subtypes. Radiation therapy, stem cell transplantation, chemotherapy, and targeted therapy are frequently used treatments for blood cancers (Chu et al. 2020). Some novel therapeutic approaches such as chimeric antigen receptor (CAR) T cell therapy have been also applied for the treatment of some leukemias (ALL and CLL) and large B cells non-Hodgkin’s lymphoma. CAR T cell therapy is an exceptional therapy in which the patient’s T cells are modified in the laboratory, so they can efficiently recognize and react against cancer cells. Today, researchers are working on expanding CAR therapy to cancers other than blood cancers (Lemal and Tournilhac 2019). CAR T cell therapy has some drawbacks, namely, a lack of determining cancer antigens approach, cytokine-derived toxicities, and immunosuppressive media of TME (Liu et al. 2017). Hematopoietic stem cell transplantation (HSCT), the oldest immunotherapy, is another cell therapy applied in pediatric ALL. Although HSCT can rescue children with high-risk ALL, scarcity of HLA-matched siblings or unrelated donors and conditioning regimens are some of the main obstacles to this type of therapy (Merli et al. 2019; Shem-Tov et al. 2020). Because of some severe side effects, CAR T cell therapy and HSCT, or both of them are primarily used for the treatment of relapsed ALL. Despite various sophisticated therapeutic approaches, easy, safe, and simple treatments are constantly attracting more attention, so enzymes, as a growing class of peptide drugs, are valuable agents for medicinal purposes.
Various blood cancer groups and L-asparaginase (ASNase) therapy. As the hematopoietic stem cell divisions go through in the bone marrow, the progenitors become progressively more specialized in the range of cell types that they can give rise to, as indicated in this diagram. However, abnormalities in genetic, epigenist, and environmental factors mislead the normal differentiation of blood cells and convert them to malignant cells (red arrows), which are classified according to their starting cells and place (i.e., lymphoma starts in lymph nodes). ASNase has been approved for the treatment of acute lymphocytic leukemia (ALL) and has shown potential antineoplastic characteristics against chronic lymphocytic leukemia (CLL), adult acute myeloid leukemia (AML), chronic myeloid leukemia (CML), and lymphoma
ALL treatment response to ASNase has the most improved outcomes among blood cancers. Thus ALL cells are the most dependent cells to blood Asn for survival (Costa-Silva et al. 2020). T cell-derived ALL (T-ALL) showed more response to ASNase therapy among the hematological cancers (Dhankhar et al. 2020). As discussed in previous sections, investigation to find an improved form of E. coli and Er. chrysanthemi ASNase (e.g., recombinant, chemically modified, encapsulated, immobilized form) or finding alternative sources of ASNase is still ongoing. As an example, we reported that ASNase purified from Yarrowia lipolytica DSM3286 as a eukaryotic source because of its higher anticancer effects, no glutaminase activity is a promising alternative enzyme for ALL and Burkitt’s lymphoma treatment (Mazloum-Ravasan et al. 2020). ASNase has shown anticancer characteristics in other types of blood cancer; for instance, because of low expression of ASNS in M0, M1, M4, and M5 subgroups of AML, they also showed sensitivity to ASNase. Moreover, the frequency of chromosome 7 monosomy is noticeable in AML patients because the gene encoding ASNS is located on chromosome 7 (Chiu et al. 2019).
L-Asparaginase in treatment of non-hematological cancers
ASNase is a potent anticancer agent for ALL and Hodgkin’s lymphoma. Given that, about 80% of human cancer belongs to carcinoma, the effectiveness of ASNase in this large group would be valuable. Carcinoma starts in epithelial cells throughout the body, which cover the outside and inside layers of the body (Lee-Six et al. 2019). Sarcoma cancers are another group of human cancers originating from connective tissues other than blood. Most successful anticancer agents are used for a few types of cancer, and one of the suggested reasons for their limited usage is the different nature of tumor cells and their strange TME (Jahanafrooz et al. 2020). As well as other anticancer agents, the anticancer activity of ASNase is also evaluated on several human cancer cell lines; for instance, the cervical cancer HeLa cell line showed reduced proliferation under the ASNase treatment isolated from Pseudomonas aeruginosa in a dose-depended manner (Fatima et al. 2019). In one study, ASNase from Helicobacter pylori inhibited cell cycle progression in fibroblasts and gastric cancer cell lines (Scotti et al. 2010). Interestingly, ASNase can disrupt several forms of peptide glycosylation in the endoplasmic reticulum (ER), including sialylation. Oligosaccharide is transferred to the side-chain NH2 group of an Asn in the newly synthesized peptide. It was shown that distortion of the glycosylation pattern under ASNase treatment could inhibit the binding of ovarian cancer cells to the endothelial cell surface, thus inhibiting heterotypic cell–cell adhesion, which is needed for cancer cell dissemination (Yu et al. 2012).
Furthermore, phase I and II clinical trials have been performed to evaluate the effect of ASNase from E. coli in patients with pancreatic adenocarcinoma with null/low ASNS expression. In order to reduce toxicity, ASNase was applied in the form of erythrocyte-encapsulated ASNase (eryaspase) (ERYTECH Pharma, Lyon, France) or “cellular microbioreactor” in the phase I trial. In phase II clinical study in 2019, a combination of eryaspase with chemotherapy showed more improvements in patients’ overall survival without progression. Notably, it was mentioned that a phase III clinical trial is underway (Bachet et al. 2015; Hammel et al. 2020). Table 3 summarizes the other examples of ASNase application for treating non-hematological cancers.
Challenges and future perspectives in L-asparaginase application
ASNase is the intrinsic targetable drug for asparagine-auxotroph cancer cells. However, some undesirable aspects need to be considered to reach the best version of ASNase. Incorporation of non-myelosuppressive agents such as PEG with the enzyme has yielded better Asn depletion in adult ALL patients (Patel et al. 2017). The emergence of anti-PEG immunity in some patients, as well as the different number and architecture of attached PEG to peptide drugs like ASNase, are unsolved issues in the pharmaceutical market (Lima et al. 2020). It is worth mentioning that immunogenicity against ASNase itself is another possible undesirable effect of ASNase therapy, especially in adult patients. Therefore, recombinant glycosylated ASNase is somewhat preferred to PEGylated ASNase and non-glycosylated one (Lima et al. 2020). In addition, there are some other simple considerations to overcoming the previous barrier, including the amount of injected ASNase, administration route of a drug (intravenous or IV injection has more risk of allergic reaction than intramuscular or IM), injection times, and overall health/other health problems of cancer patients (Hasan et al. 2016). Moreover, the incorporation of ASNase with nano-carriers could palliate its immunogenicity or hypersensitivity (Hasan et al. 2016). In one in vitro study, selenium nanobiocomposites including fungal ASNases were synthesized, and its anti-proliferative effect on human colon cancer, liver cancer, and osteosarcoma cell lines was evaluated (Baskar et al. 2019). Selenium nanoparticles selectively induce intrinsic apoptosis only in cancer cells and these nanobiocomposites of ASNase are suggested as a promising anticancer agent for colon cancer (Baskar et al. 2019).
The engineered mesenchymal stem cells (MSCs) involving the gene encoding ASNase could be used as a novel version of enzyme therapy (Lin et al. 2019). Furthermore, vectors carrying a gene encoding ASNase could be another way for enzyme therapy. Still, low transfection efficiency of the plasmids to cancer cells as well as a possible unnecessary response of the immune system to vectors are regarded as the main drawbacks of this strategy (Johansson and Ward 2017; Martino and Markusic 2020). The two mentioned methods have not been evaluated so far.
Stem cell-based cancer therapies are interesting and recommended for cancer treatment. Furthermore, MSC-derived membrane microvesicles (MVs) are also considered a drug delivery system. MVs are in a size range of 0.1–1 μm and loaded MVs with anticancer drugs demonstrated their promising potential in successful drug delivery (Chulpanova et al. 2018). Hence, MSC-derived MVs can be loaded with ASNase for effective cancer therapy.
Another caution in ASNase therapy is because of the side products that are produced during asparagine breakdown; agitation, confusion, and disorientation are some reported neurologic side effects as the result of a higher amount of circulating aspartic acid and ammonia (Vimal and Kumar 2017). For amelioration of the impact of an increasing amount of aspartic acid and ammonia, some combination therapy for purgation of aspartic acid and ammonia may be helpful. Liver and pancreas damage are also other reported toxicities following ASNase administration; in this regard, preclinical studies showed that application of glutaminase-free ASNase decreased its hepatotoxic effect because Gln is one of the ASNS substrates and the mentioned form of ASNase is not capable of inhibiting Asn synthesis in normal cells (Sahoo and Hart 2003). Other depleting strategies such as blocking Asn uptake by usually overexpressed transporters, for example, SLC transporters, on cancer cells is one of the alternatives suggested therapy to exploiting this goal; however, so far, they have not received FDA approval (Bhutia et al. 2014; Wang et al. 2015).
Finally, the production of ASNase in the eukaryotic host such as S. cerevisiae and Y. lipolytica could be a good substitution for the prokaryotic host because of the deletion of prokaryotic immunogenic epitopes and production of a glycosylated and more stable enzyme (Varsha et al. 2015; Darvishi 2014; Darvishi et al. 2018; Liu et al. 2021). ASNase from a human source is another alternative eukaryotic enzyme and would avoid the problems caused by the bacterial ASNase. Although the human ASNase did not have any glutaminase activity, its Km is significantly higher than bacterial ASNase. Therefore, studies to increase the affinity of the human ASNase to Asn and provide an effective anticancer enzyme are moving on it (Belviso et al. 2017). On the other hand, humanizing pig ASNase by generating chimeras with the human ASNase is another suggested solution to reach a low Km human-like enzyme. Human-like ASNases were a combination of the N-terminal domain of pig ASNase and the C-terminal domain of the human ASNase, which were cloned and expressed in E. coli and exerted in vitro ALL killing potential (Rigouin et al. 2017).
Conclusions
Natural products have always played a crucial role in discovering new therapeutics. ASNase is a nature-provided enzyme and, as a bioactive molecule, influences only Asn-dependent cancer cells with no cytotoxic effect on normal cells; therefore, ASNase has the main characteristic of being a successful anticancer drug. In addition, this approach does not depend on prodrug conversion to be effective. As discussed above, not only ASNase considered a powerful chemotherapeutic anti-leukemic and anti-lymphoma drug, but also an excellent prospective drug for usage in the treatment of other cancers. Therefore, by minimizing its side effects and combination with commercial and novel strategies, this promising bio-compound could be a widespread anticancer drug in the future. As an essential point, the dependency content of cancer cells to Asn is a determinant factor in the responsiveness of cancer cells to ASNase therapy.
References
Abbas Ahmed MM, Nageh Abo Dahab F, Taher Taha M, Fareed Hassan SM (2015) Production, purification and characterization of L-asparaginase from marine endophytic Aspergillus sp. ALAA-2000 under submerged and solid state fermentation. J Microb Biochem Technol 7:165–172. https://doi.org/10.4172/1948-5948.1000199
Al-Dulimi AG, Al-Saffar AZ, Sulaiman GM, Khalil KA, Khashan KS, Al-Shmgani HS, Ahmed EM (2020) Immobilization of L-asparaginase on gold nanoparticles for novel drug delivery approach as anti-cancer agent against human breast carcinoma cells. J Mater Res Technol 9(6):15394–15411. https://doi.org/10.1016/j.jmrt.2020.10.021
Ardalan N, Mirzaie S, Sepahi AA, Khavari-Nejad RA (2018) Novel mutant of Escherichia coli asparaginase II to reduction of the glutaminase activity in treatment of acute lymphocytic leukemia by molecular dynamics simulations and QM-MM studies. Med Hypotheses 112:7–17. https://doi.org/10.1016/j.mehy.2018.01.004
Ashok A, Doriya K, Rao JV, Qureshi A, Tiwari AK, Kumar DS (2019) Microbes producing L-asparaginase free of glutaminase and urease isolated from extreme locations of antarctic soil and moss. Sci Rep 9(1):1–10. https://doi.org/10.1038/s41598-018-38094-1
Bachet JB, Gay F, Maréchal R, Galais MP, Adenis A, David Salako M, Cros J, Demetter P, Svrcek M, Bardier-Dupas A, Emile JF (2015) Asparagine synthetase expression and phase I study with L-asparaginase encapsulated in red blood cells in patients with pancreatic adenocarcinoma. Pancreas 44(7):1141–1147. https://doi.org/10.1097/MPA.0000000000000394
Baig MH, Adil M, Khan R, Dhadi S, Ahmad K, Rabbani G, Bashir T, Imran MA, Husain FM, Lee EJ, Kamal MA (2019) Enzyme targeting strategies for prevention and treatment of cancer: implications for cancer therapy. Semin Cancer Biol 56:1–11. https://doi.org/10.1016/j.semcancer.2017.12.003
Baral A, Gorkhali R, Basnet A, Koirala S, Bhattarai HK (2020) In-silico development of a method for the selection of optimal enzymes using L-asparaginase II against acute lymphoblastic leukemia as an example. Jmirx Med 2(3):e29844. https://doi.org/10.2196/29844
Baskar G, Lalitha K, Bikku George G (2019) Synthesis, characterization and anticancer activity of selenium nanobiocomposite of L-asparaginase. Bull Mate Sci 42(1):4. https://doi.org/10.1007/s12034-018-1686-z
Belviso S, Iuliano R, Amato R, Perrotti N, Menniti M (2017) The human asparaginase enzyme (ASPG) inhibits growth in leukemic cells. PLoS ONE 12(5):e0178174. https://doi.org/10.1371/journal.pone.0178174
Binder U, Skerra A (2017) PASylation®: a versatile technology to extend drug delivery. Curr Opin Colloid Interface Sci 31:10–17. https://doi.org/10.1016/j.cocis.2017.06.004
Blackman LD, Varlas S, Arno MC, Houston ZH, Fletcher NL, Thurecht KJ, Hasan M, Gibson MI, O’Reilly RK (2018) Confinement of therapeutic enzymes in selectively permeable polymer vesicles by polymerization-induced self-assembly (PISA) reduces antibody binding and proteolytic susceptibility. ACS Cent Sci 4(6):718–723. https://doi.org/10.1021/acscentsci.8b00168
Brumano LP, da Silva FV, Costa-Silva TA, Apolinário AC, Santos JH, Kleingesinds EK, Monteiro G, Rangel-Yagui CD, Benyahia B, Junior AP (2019) Development of L-asparaginase biobetters: current research status and review of the desirable quality profiles. Front Bioeng Biotechnol 6:212. https://doi.org/10.3389/fbioe.2018.00212
Chiu M, Taurino G, Bianchi MG, Kilberg MS, Bussolati O (2019) Asparagine synthetase in cancer: beyond acute lymphoblastic leukemia. Front Oncol 9:1480. https://doi.org/10.3389/fonc.2019.01480
Chu DT, Nguyen TT, Tien NL, Tran DK, Jeong JH, Anh PG, Thanh VV, Truong DT, Dinh TC (2020) Recent progress of stem cell therapy in cancer treatment: molecular mechanisms and potential applications. Cells 9(3):563. https://doi.org/10.3390/cells9030563
Chulpanova DS, Kitaeva KV, Tazetdinova LG, James V, Rizvanov AA, Solovyeva VV (2018) Application of mesenchymal stem cells for therapeutic agent delivery in anti-tumor treatment. Front Pharmacol 9:259. https://doi.org/10.3389/fphar.2018.00259
Costa-Silva TA, Costa IM, Biasoto HP, Lima GM, Silva C, Pessoa A, Monteiro G (2020) Critical overview of the main features and techniques used for the evaluation of the clinical applicability of L-asparaginase as a biopharmaceutical to treat blood cancer. Blood Rev 43:100651. https://doi.org/10.1016/j.blre.2020.100651
Darvishi F (2014) Biotechnological applications of the yeast Yarrowia lipolytica. Springer, New York. https://doi.org/10.1007/978-3-319-06437-6
Darvishi F, Ariana M, Marella ER, Borodina I (2018) Advances in synthetic biology of oleaginous yeast Yarrowia lipolytica for producing non-native chemicals. Appl Microbiol Biotechnol 102(14):5925–5938. https://doi.org/10.1007/s00253-018-9099-x
Darvishi F, Faraji N, Shamsi F (2019) Production and structural modeling of a novel asparaginase in Yarrowia lipolytica. Int J Biol Macromol 125:955–961. https://doi.org/10.1016/j.ijbiomac.2018.12.162
Darvishi F, Shamsi F (2018) Investigating the L-asparaginase production in the yeast Yarrowia lipolytica. Biological J Microorganisms 7(27):73–79. https://doi.org/10.22108/BJM.2018.111339.1133
Dhankhar R, Gupta V, Kumar S, Kapoor RK, Gulati P (2020) Microbial enzymes for deprivation of amino acid metabolism in malignant cells: biological strategy for cancer treatment. Appl Microbiol Biotechnol 104(7):2857–2869. https://doi.org/10.1007/s00253-020-10432-2
Dias FF, Ruiz AL, Della Torre A, Sato HH (2016) Purification, characterization and antiproliferative activity of L-asparaginase from Aspergillus oryzae CCT 3940 with no glutaminase activity. Asian Pac J Trop Biomed 6(9):785–794. https://doi.org/10.1016/j.apjtb.2016.07.007
Do TT, Do TP, Nguyen TN, Nguyen TC, Vu TT, Nguyen TG (2019) Nanoliposomal L-asparaginase and its antitumor activities in lewis lung carcinoma tumor-induced BALB/c mice. Adv Mater Sci Eng 2019:3534807. https://doi.org/10.1155/2019/3534807
Doriya K, Kumar DS (2016) Isolation and screening of L-asparaginase free of glutaminase and urease from fungal sp 3. Biotech 6(2):1–10. https://doi.org/10.1007/s13205-016-0544-1
Duval M, Suciu S, Ferster A, Rialland X, Nelken B, Lutz P, Benoit Y, Robert A, Manel AM, Vilmer E, Otten J (2002) Comparison of Escherichia coli–asparaginase with Erwinia-asparaginase in the treatment of childhood lymphoid malignancies: results of a randomized European Organisation for Research and Treatment of Cancer—children’s leukemia group phase 3 trial. Blood 99(8):2734–2739. https://doi.org/10.1182/blood.v99.8.2734
El-Gendy MM, Awad MF, El-Shenawy FS, El-Bondkly AM (2021) Production, purification, characterization, antioxidant and antiproliferative activities of extracellular L-asparaginase produced by Fusarium equiseti AHMF4. Saudi J Biol Sci 28:2540–2548. https://doi.org/10.1016/j.sjbs.2021.01.058
El-Naggar NE, Deraz SF, Soliman HM, El-Deeb NM, El-Ewasy SM (2016) Purification, characterization, cytotoxicity and anticancer activities of L-asparaginase, anti-colon cancer protein, from the newly isolated alkaliphilic Streptomyces fradiae NEAE-82. Sci Rep 6(1):1–16. https://doi.org/10.1038/srep32926
El-Naggar NE, El-Shweihy NM (2020) Bioprocess development for L-asparaginase production by Streptomyces rochei, purification and in-vitro efficacy against various human carcinoma cell lines. Sci Rep 10:7942. https://doi.org/10.1038/s41598-020-64052-x
Emadi A, Law JY, Strovel ET, Lapidus RG, Jeng LJ, Lee M, Blitzer MG, Carter-Cooper BA, Sewell D, Van Der Merwe I, Philip S (2018) Asparaginase Erwinia chrysanthemi effectively depletes plasma glutamine in adult patients with relapsed/refractory acute myeloid leukemia. Cancer Chemother Pharmacol 81(1):217–222. https://doi.org/10.1007/s00280-017-3459-6
Fatima N, Khan MM, Khan IA (2019) L-Asparaginase produced from soil isolates of Pseudomonas aeruginosa shows potent anti-cancer activity on HeLa cells. Saudi J Biol Sci 26:1146–1153. https://doi.org/10.1016/j.sjbs.2019.05.001
Garcia-Bermudez J, Williams RT, Guarecuco R, Birsoy K (2020) Targeting extracellular nutrient dependencies of cancer cells. Mol Metab 33:67–82. https://doi.org/10.1016/j.molmet.2019.11.011
Geddes WF, Hunter A (1928) Observations upon the enzyme asparaginase. J Biol Chem 77(1):197–229. https://doi.org/10.1016/S0021-9258(18)84052-2
Ghasemian A, Al-marzoqi AH, Al-abodi HR, Alghanimi YK, Kadhum SA, Shokouhi Mostafavi SK, Fattahi A (2019) Bacterial L-asparaginases for cancer therapy: current knowledge and future perspectives. J Cell Physiol 234(11):1–9. https://doi.org/10.1002/jcp.28563
Hammel P, Fabienne P, Mineur L, Metges JP, Andre T, De La Fouchardiere C, Louvet C, El Hajbi F, Faroux R, Guimbaud R, Tougeron D (2020) Erythrocyte-encapsulated asparaginase (eryaspase) combined with chemotherapy in second-line treatment of advanced pancreatic cancer: an open-label, randomized phase IIb trial. Eur J Cancer 124:91–101. https://doi.org/10.1016/j.ejca.2019.10.020
Hasan H, Shaikh OM, Rassekh SR, Howard AF, Goddard K (2016) Comparison of hypersensitivity rates to intravenous and intramuscular PEG-asparaginase in children with acute lymphoblastic leukemia: a meta-analysis and systematic review. Pediatr Blood Cancer 64(1):81–88. https://doi.org/10.1002/pbc.26200
Isaac GS, Abu-Tahon MA (2016) Production of extracellular anti-leukemic enzyme L-asparaginase from Fusarium solani AUMC 8615 grown under solid-state fermentation conditions: purification and characterization of the free and immobilized enzyme. Egypt J Bot 56:799–816. https://doi.org/10.21608/EJBO.2016.3776
Jahanafrooz Z, Baradaran B, Mosafer J, Hashemzaei M, Rezaei T, Mokhtarzadeh A, Hamblin MR (2019) Comparison of DNA and mRNA vaccines against cancer. Drug Discov Today 25(3):552–560. https://doi.org/10.1016/j.drudis.2019.12.003
Jahanafrooz Z, Mosafer J, Akbari M, Hashemzaei M, Mokhtarzadeh A, Baradaran B (2020) Colon cancer therapy by focusing on colon cancer stem cells and their tumor microenvironment. J Cell Physiol 235(5):4153–4166. https://doi.org/10.1002/jcp.29337
Jiao L, Chi H, Lu Z, Zhang C, Chia SR, Show PL, Tao Y, Lu F (2020) Characterization of a novel type I l-asparaginase from Acinetobacter soli and its ability to inhibit acrylamide formation in potato chips. J Biosci Bioeng 129(6):672–678. https://doi.org/10.1016/j.jbiosc.2020.01.007
Johansson O, Ward M (2017) The human immune system’s response to carcinogenic and other infectious agents transmitted by mosquito vectors. Parasitol Res 116:1–9. https://doi.org/10.1007/s00436-016-5272-2
Krishnapura PR, Belur PD, Subramanya S (2016) A critical review on properties and applications of microbial L-asparaginases. Crit Rev Microbiol 42(5):720–737. https://doi.org/10.3109/1040841X.2015.1022505
Lee-Six H, Olafsson S, Ellis P, Osborne RJ, Sanders MA, Moore L, Georgakopoulos N, Torrente F, Noorani A, Goddard M, Robinson P (2019) The landscape of somatic mutation in normal colorectal epithelial cells. Nature 574(7779):532–537. https://doi.org/10.1038/s41586-019-1672-7
Lemal R, Tournilhac O (2019) State-of-the-art for CAR T-cell therapy for chronic lymphocytic leukemia in 2019. J Immunother Cancer 7:202. https://doi.org/10.1186/s40425-019-0686-x
Li RJ, Jin R, Liu C, Cao X, Manning ML, Di XM, Przepiorka D, Namuswe F, Deisseroth A, Goldberg KB, Blumenthal GM (2020) FDA approval summary: calaspargase pegol-mknl for treatment of acute lymphoblastic leukemia in children and young adults. Clin Cancer Res 26(2):328–331. https://doi.org/10.1158/1078-0432.CCR-19-1255
Lima GM, Effer B, Biasoto HP, Feijoli V, Pessoa A, Palmisano G, Monteiro G (2020) Glycosylation of L-asparaginase from E. coli through yeast expression and site-directed mutagenesis. Biochem Eng J 156:10751. https://doi.org/10.1016/j.bej.2020.107516
Lin W, Huang L, Li Y, Fang B, Li G, Chen L, Xu L (2019) Mesenchymal stem cells and cancer: clinical challenges and opportunities. Biomed Res Int 2019:2820853. https://doi.org/10.1155/2019/2820853
Liu J, Zhang X, Zhong JF, Zhang C (2017) CAR-T cells and allogeneic hematopoietic stem cell transplantation for relapsed/refractory B-cell acute lymphoblastic leukemia. Immunotherapy 9(13):1115–1125. https://doi.org/10.2217/imt-2017-0072
Liu Z, Moradi H, Shi S, Darvishi F (2021) Yeasts as microbial cell factories for sustainable production of biofuels. Renew Sustain Energy Rev 143:110907. https://doi.org/10.1016/j.rser.2021.110907
Lukey MJ, Katt WP, Cerione RA (2017) Targeting amino acid metabolism for cancer therapy. Drug Discov Today 22(5):796–804. https://doi.org/10.1016/j.drudis.2016.12.003
Maggi M, Mittelman SD, Parmentier JH, Colombo G, Meli M, Whitmire JM, Merrell DS, Whitelegge J, Scotti C (2017) A protease-resistant Escherichia coli asparaginase with outstanding stability and enhanced anti-leukaemic activity in vitro. Sci Rep 7:14479. https://doi.org/10.1038/s41598-017-15075-4
Mahajan RV, Kumar V, Rajendran V, Saran S, Ghosh PC, Saxena RK (2014) Purification and characterization of a novel and robust L-asparaginase having low-glutaminase activity from Bacillus licheniformis: in vitro evaluation of anticancerous properties. PLoS ONE 9(6):e99037. https://doi.org/10.1371/journal.pone.0099037
Martino AT, Markusic DM (2020) Immune response mechanisms against AAV vectors in animal models. Mol Ther - Methods Clin Dev 17:198–208. https://doi.org/10.1016/j.omtm.2019.12.008
Mashburn LT, Wriston JC (1964) Tumor inhibitory effect of L-asparaginase from Escherichia coli. Arch Biochem Biophys 105:450–452. https://doi.org/10.1016/0003-9861(64)90032-3
Mazloum-Ravasan S, Madadi E, Fathi Z, Mohammadi A, Mosafer J, Mansoori B, Mokhtarzadeh A, Baradaran B, Darvishi F (2020) The effect of Yarrowia lipolytica L-asparaginase on apoptosis induction and inhibition of growth in Burkitt’s lymphoma Raji and acute lymphoblastic leukemia MOLT-4 cells. Int J Biol Macromol 146:193–201. https://doi.org/10.1016/j.ijbiomac.2019.12.156
Mazloum-Ravasan S, Madadi E, Mohammadi A, Mansoori B, Amini M, Mokhtarzadeh A, Baradaran B, Darvishi F (2021) Yarrowia lipolytica L-asparaginase inhibits the growth and migration of lung (A549) and breast (MCF7) cancer cells. Int J Biol Macromol 170:406–414. https://doi.org/10.1016/j.ijbiomac.2020.12.141
Meena B, Anburajan L, Dheenan PS, Begum M, Vinithkumar NV, Dharani G, Kirubagaran R (2015) Novel glutaminase free L-asparaginase from Nocardiopsis alba NIOT-VKMA08: production, optimization, functional and molecular characterization. Bioproc Biosystems Eng 38(2):373–388. https://doi.org/10.1007/s00449-014-1277-3
Meena B, Anburajan L, Sathish T, Vijaya Raghavan R, Dharani G, Valsalan Vinithkumar N, Kirubagaran R (2015) L-Asparaginase from Streptomyces griseus NIOT-VKMA29: optimization of process variables using factorial designs and molecular characterization of L-asparaginase gene. Sci Rep 5(1):1–12. https://doi.org/10.1038/srep12404
Merli P, Algeri M, Del Bufalo F, Locatelli F (2019) Hematopoietic stem cell transplantation in pediatric acute lymphoblastic leukemia. Curr Hematol Malig Rep 14(2):94–105. https://doi.org/10.1007/s11899-019-00502-2
Moguel IS, Yamakawa CK, Pessoa A Jr, Mussatto SI (2020) L-Asparaginase production by Leucosporidium scottii in a bench-scale bioreactor with co-production of lipids. Front Bioeng Biotechnol 8:576511. https://doi.org/10.3389/fbioe.2020.576511
Mohideen AKS (2020) Molecular docking study of L-asparaginase I from Vibrio campbellii in the treatment of acute lymphoblastic leukemia (ALL). EuroBiotech J 4(1):8–16. https://doi.org/10.2478/ebtj-2020-0002
Mu W, Chu Q, Liu Y, Zhang N (2020) A review on nano-based drug delivery system for cancer chemoimmunotherapy. Nano-Micro Lett 12:142. https://doi.org/10.1007/s40820-020-00482-6
Muneer F, Siddique MH, Azeem F, Rasul I, Muzammil S, Zubair M, Afzal M, Nadeem H (2020) Microbial l-asparaginase: purification, characterization and applications. Arch Microbiol 202(5):967–981. https://doi.org/10.1007/s00203-020-01814-1
Nguyen H, Su AY, Lavie A (2016) Design and characterization of Erwinia chrysanthemi L-asparaginase variants with diminished l-glutaminase activity. J Biol Chem 291(34):17664–17676. https://doi.org/10.1074/jbc.M116.728485
Orhan H, Aktaş Uygun D (2020) Immobilization of L-asparaginase on magnetic nanoparticles for cancer treatment. Appl Biochem Biotechnol 191:1432–1443. https://doi.org/10.1007/s12010-020-03276-z
Patel B, Kirkwood AA, Dey A, Marks DI, McMillan AK, Menne TF, Micklewright L, Patrick P, Purnell S, Rowntree CJ, Smith P (2017) Pegylated-asparaginase during induction therapy for adult acute lymphoblastic leukaemia: toxicity data from the UKALL14 trial. Leukemia 3(1):58–64. https://doi.org/10.1038/leu.2016.219
Pillaca-Pullo O, Rodrigues D, Sánchez-Moguel I, Lopes A, Pimenta M, Basi T, Feitosa V, Iris Zavaleta A, Monteiro G, Pessoa A Jr, Vitolo M (2021) Recombinant L-asparaginase production using Pichia pastoris (MUT s strain): establishment of conditions for growth and induction phases. J Chem Technol Biotechnol 96(1):283–292. https://doi.org/10.1002/jctb.6540
Poznansky MJ, Shandling M, Salkie MA, Elliott J, Lau E (1982) Advantages in the use of L-asparaginase-albumin polymer as an antitumor agent. Cancer Res 42(3):1020–1025
Rigouin C, Nguyen HA, Schalk AM, Lavie A (2017) Discovery of human-like L-asparaginases with potential clinical use by directed evolution. Sci Rep 7(1):1–13. https://doi.org/10.1038/s41598-017-10758-4
Rodrigues D, Pillaca-Pullo O, Torres-Obreque K, Flores-Santos J, Sánchez-Moguel I, Pimenta MV, Basi T, Converti A, Lopes AM, Monteiro G, Fonseca LP (2019) Fed-batch production of Saccharomyces cerevisiae L-asparaginase II by recombinant Pichia pastoris MUTs strain. Front Bioeng Biotechnol 7:16. https://doi.org/10.3389/fbioe.2019.00016
Roth G, Nunes JE, Rosado LA, Bizarro CV, Volpato G, Nunes CP, Renard G, Basso LA, Santos DS, Chies JM (2013) Recombinant Erwinia carotovora l-asparaginase II production in Escherichia coli fed-batch cultures. Braz J Chem Eng 30(2):245–256. https://doi.org/10.1590/S0104-66322013000200003
Saeed H, Ali H, Soudan H, Embaby A, El-Sharkawy A, Farag A, Hussein A, Ataya F (2018) Molecular cloning, structural modeling and production of recombinant Aspergillus terreus L-asparaginase in Escherichia coli. Int J Biol Macromol 106:1041–1051. https://doi.org/10.1016/j.ijbiomac.2017.08.110
Sahoo S, Hart J (2003) Histopathological features of L-asparaginase–induced liver disease. Semin Liver Dis 23(3):295–299. https://doi.org/10.1055/s-2003-42647
Sajitha S, Vidya J, Binod P, Pandey A (2015) Cloning and expression of l-asparaginase from E. coli in eukaryotic expression system. Biochem Eng J 102:14–17. https://doi.org/10.1016/j.bej.2015.02.027
Scotti C, Sommi P, Pasquetto MV, Cappelletti D, Stivala S, Mignosi P, Savio M, Chiarelli LR, Valentini G, Bolanos-Garcia VM, Merrell DS (2010) Cell-cycle inhibition by Helicobacter pylori L-asparaginase. PLoS ONE 5(11):e13892. https://doi.org/10.1371/journal.pone.0013892
Shem-Tov N, Peczynski C, Labopin M, Itälä-Remes M, Blaise D, Labussière-Wallet H, Socié G, Kröger N, Mielke S, Afanasyev B, Chevallier P (2020) Haploidentical vs unrelated allogeneic stem cell transplantation for acute lymphoblastic leukemia in first complete remission: on behalf of the ALWP of the EBMT. Leukemia 3(1):283–292. https://doi.org/10.1038/s41375-019-0544-3
Shiromizu S, Kusunose N, Matsunaga N, Koyanagi S, Ohdo S (2018) Optimizing the dosing schedule of L-asparaginase improves its antitumor activity in breast tumor-bearing mice. J Pharmacol Sci 136:228e233. https://doi.org/10.1016/j.jphs.2018.01.008
Sindhu R, Manonmani HK (2018) l-Asparaginase induces intrinsic mitochondrial-mediated apoptosis in human gastric adenocarcinoma cells and impedes tumor progression. Biochem Biophys Res Commun 503(4):2393–2399. https://doi.org/10.1016/j.bbrc.2018.06.167
Song P, Wang Z, Zhang X, Fan J, Li Y, Chen Q, Wang S, Liu P, Luan J, Ye L, Ju D (2017) The role of autophagy in asparaginase-induced immune suppression of macrophages. Cell Death Dis 8(3):e2721. https://doi.org/10.1038/cddis.2017.144
Song P, Ye L, Fan J, Li Y, Zeng X, Wang Z, Wang S, Zhang G, Yang P, Cao Z, Ju D (2015) Asparaginase induces apoptosis and cytoprotective autophagy in chronic myeloid leukemia cells. Oncotarget 6(6):3861. https://doi.org/10.18632/oncotarget.2869
Triplett T, Garrison K, Marshall N (2018) Reversal of indoleamine 2,3-dioxygenase–mediated cancer immune suppression by systemic kynurenine depletion with a therapeutic enzyme. Nat Biotechnol 36:758–764. https://doi.org/10.1038/nbt.4180
Vimal A, Kumar A (2017) Biotechnological production and practical application of L-asparaginase enzyme. Biotechnol Genet Eng Rev 33(1):40–61. https://doi.org/10.1080/02648725.2017.1357294
Wang Q, Hardie RA, Hoy AJ, Van Geldermalsen M, Gao D, Fazli L, Sadowski MC, Balaban S, Schreuder M, Nagarajah R, Wong JJ (2015) Targeting ASCT2-mediated glutamine uptake blocks prostate cancer growth and tumour development. J Pathol 236(3):278–289. https://doi.org/10.1002/path.4518
Wlodarczyk SR, Costa-Silva TA, Pessoa-Jr A, Madeira P, Monteiro G (2019) Effect of osmolytes on the activity of anti-cancer enzyme L-asparaginase II from Erwinia chrysanthemi. Process Biochem 81:123–131. https://doi.org/10.1016/j.procbio.2019.03.009
Yari M, Eslami M, Ghoshoon MB, Nezafat N, Ghasemi Y (2019) Decreasing the immunogenicity of Erwinia chrysanthemi asparaginase via protein engineering: computational approach. Mol Biol Rep 46(5):4751–4761. https://doi.org/10.1007/s11033-019-04921-5
Yu M, Henning R, Walker A, Kim G, Perroy A, Alessandro R, Virador V, Kohn EC (2012) L-Asparaginase inhibits invasive and angiogenic activity and induces autophagy in ovarian cancer. J Cell Mol Med 16(10):2369–2378. https://doi.org/10.1111/j.1582-4934.2012.01547.x
Author information
Authors and Affiliations
Contributions
FD provided conception of this review, wrote and edited the manuscript. ZJ wrote and edited the manuscript. AM edited the manuscript. All authors contributed to the manuscript and approved the submitted version.
Corresponding authors
Ethics declarations
Ethics approval
This article does not contain any studies with human participants or animals.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Darvishi, F., Jahanafrooz, Z. & Mokhtarzadeh, A. Microbial L-asparaginase as a promising enzyme for treatment of various cancers. Appl Microbiol Biotechnol 106, 5335–5347 (2022). https://doi.org/10.1007/s00253-022-12086-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-022-12086-8