Abstract
This study was conducted to report the richness of endophytic Penicillium and Talaromyces species isolated from Tillandsia catimbauensis, a bromeliad endemic in the Brazilian tropical dry forest (Caatinga), to verify their ability to produce the enzyme l-asparaginase and to partially optimise the production of biomass and l-asparaginase of the best enzyme producer. A total of 184 endophytes were isolated, of which 52 (29%) were identified through morphological and phylogenetic analysis using β-tubulin sequences into nine putative species, four in Penicillium and five in Talaromyces. Talaromyces diversus and T. cf. cecidicola were the most frequent taxa. Among the 20 endophytic isolates selected for l-asparaginase production, 10 had the potential to produce the enzyme (0.50–2.30 U/g), especially T. cf. cecidicola URM 7826 (2.30 U/g) and Penicillium sp. 4 URM 7827 (1.28 U/g). As T. cf. cecidicola URM 7826 exhibited significant ability to produce the enzyme, it was selected for the partial optimisation of biomass and l-asparaginase production. Results of the 23 factorial experimental design showed that the highest dry biomass (0.66 g) was obtained under pH 6.0, inoculum concentration of 1 × 108 and 1% l-proline. However, the inoculum concentration was found to be statistically significant, the pH was marginally significant and the concentration of l-proline was not statistically significant. l-Asparaginase production varied between 0.58 and 1.02 U/g and did not reach the optimal point for enzyme production. This study demonstrates that T. catimbauensis is colonised by different Penicillium and Talaromyces species, which are indicated for enzyme production studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Endophytes are microorganisms that colonise the host plant tissues at both intracellular and inter-cellular levels without causing disease symptoms (Tan and Zou 2001). Through this symbiotic interaction, microorganisms can produce compounds that provide resistance to phytopathogens, prevent being eaten by herbivores and enhance the development of the host plant (Arnold et al. 2003; Waqas et al. 2012; Zhou et al. 2016). Furthermore, the host provides an environment with less competition and greater nutritional source to the endophytic fungi (Peixoto Neto et al. 2004). Strobel and Daisy (2003) described that of about 300,000 existing plant species, each individual plant host harbours one or more endophytic microorganisms; however, only a few of these plants have been investigated for their endophytic microbiota. Therefore, there are tremendous possibilities of identifying new endophytes with biotechnological potential in plants from different environments.
Although only a few studies have confirmed the fungal endophytic associations of plants from desert, semi-arid, arid and dry tropical rainforest environments, these surveys have reported a remarkable diversity of endophytes (Fisher et al. 1994; Suryanarayanan et al. 2005; Khidir et al. 2010; Loro et al. 2012; Sun et al. 2012; Bezerra et al. 2012a, b, 2013). Few studies from Brazil have also contributed to the knowledge of the endophytic mycobiota of Caatinga plants, such as the studies on the cacti Opuntia ficus-indica (Bezerra et al. 2012a; Freire et al. 2015), Cereus jamacaru (Bezerra et al. 2013) and Tacinga inamoena (Bezerra et al. 2017); on Mandevilla catimbauensis (Apocynaceae) (Crous et al. 2017) and on the Fabaceae species Indigofera suffruticosa (Santos et al. 2015a).
Endophytic microorganisms have been considered as a major source of bioactive compounds as they occupy unique biological niches (Strobel and Daisy 2003). Chapla et al. (2013) reported that endophytic fungi have an excellent potential to produce yet unidentified bioactive substances that have been reported to have the potential to produce enzymes (Bischoff et al. 2009; Bezerra et al. 2012a, 2015), antimicrobials (Pinheiro et al. 2013; Kusari et al. 2013; Bezerra et al. 2015; Pires et al. 2015), plant growth hormones (Silva et al. 2006; Ting et al. 2008; Hwang et al. 2011) and other compounds of medicinal interest (Meng et al. 2011) such as the enzyme l-asparaginase (Theantana et al. 2007; Kalyanasundaram et al. 2015; Santos et al. 2015b).
Some studies on the diversity of endophytes in dry environments (Bezerra et al. 2012a, 2013, 2015; Freire et al. 2015) have reported the presence of the endophytic species of the genera Penicillium and Talaromyces, which were also proven to be promising sources for the production of l-asparaginase. For example, the endophyte Talaromyces pinophilus isolated from Curcuma amada can be considered as a potential candidate for industrial and clinical trials on the production of the enzyme l-asparaginase (Krishnapura and Belur 2016). Theantana et al. (2009) also verified that the endophytic Penicillium and Talaromyces species exhibited a high enzymatic activity when isolated from medicinal plants in Thailand. Another study by Chow and Ting (2015) also demonstrated that endophytes associated with plants have anticancer proprieties and further reported P. simplicissimum as one of the best producers of l-asparaginase. Another study from Brazil on the endophytes from the Caatinga cactus C. jamacaru also confirmed the enzymatic potential of Penicillium isolates, highlighting P. brevicompactum as the best l-asparaginase producer (Santos et al. 2015b).
l-asparaginase is an enzyme that catalyses the hydrolysis of the amino acid asparagine in ammonia and aspartic acid (Jain et al. 2012; Jha et al. 2012; Nomme et al. 2012). This enzyme has been used as an important drug in the treatment of several types of cancers (Devi and Azmi 2012; Guilleme et al. 2013). Its mechanism of action results in the extracellular reduction of the amino acid asparagine, which inhibits protein synthesis and induces apoptosis of neoplastic cells (Guilleme et al. 2013). The enzyme is mainly derived from bacteria, and its long-term use may cause hypersensitivity and lead to allergic reactions and anaphylaxis (Duval et al. 2002; Sarquis et al. 2004). In addition to its medicinal importance, this enzyme has been used in the food industry as an alternative for the reduction of acrylamide in foods (Hendriksen et al. 2009; Kornbrust et al. 2009). Acrylamide has been classified as a potentially carcinogenic compound for humans, which is formed in cooked and fried foods, especially in carbohydrate-rich foods that are heat-treated (Kumar et al. 2014; Zuo et al. 2015). l-asparaginase can reduce the level of free asparagine by hydrolysis, thus removing one of the essential compounds for the formation of acrylamide, but not affecting most of the amino acids (Hendriksen et al. 2009; Kornbrust et al. 2009). This enzyme has been commercially produced from two fungal sources, Aspergillus oryzae and A. niger (Krishnakumar and Visvanathan 2014). According to Jha et al. (2012), there is a need for a greater search by the food and pharmacological industries to meet the demands of this enzyme. However, no study has yet investigated the potential of l-asparaginase production by Penicillium and Talaromyces isolates from the bromeliad Tillandisia catimbauensis.
Tillandisia catimbauensis is an endemic bromeliad in the Brazilian tropical dry forest (Caatinga). However, due to its restricted distribution and conservation status of protected areas of the Caatinga in the conservation units, this species is under a critical risk of extinction (Santos et al. 2011; Fabricante et al. 2014; Ferreira et al. 2015). The Catimbau National Park is the only Caatinga region that has records of T. catimbauensis in its natural environment, protecting about 62,000 ha of the forest that occupies 54% of the northeast region and 11% of the country, presenting a remarkable diversity of flora and fauna with several endemic species (Leal et al. 2003; Alves et al. 2009; Fabricante et al. 2014; Ferreira et al. 2015; ICMBio 2018).
Considering the uniqueness of the bromeliad T. catimbauensis in the Brazilian tropical dry forest (Caatinga) and the significance of the verification of the biotechnological capacity of l-asparaginase production by the endophytes, this study was conducted with the following aims: (a) to report the richness of the endophytic Penicillium and Talaromyces isolates from T. catimbauensis, (b) to investigate the potential of the isolates for the production of l-asparaginase and (c) to use the most promising endophytic fungus in terms of the enzymatic activity in the partial optimisation stage of fungal biomass and l-asparaginase production.
Materials and methods
Plant material collection
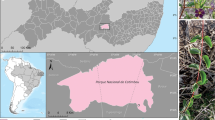
The plant material was collected from the areas of the Caatinga forest in the Catimbau National Park, Buíque, Pernambuco, Brazil (8°36ʹ35ʺ S, 37°14ʹ40ʺ W). The Caatinga forest is primarily characterised by a shrub, spiny and branched vegetation, consisting of several bromeliads, cacti and euphorbiaceous plants (Leal et al. 2005). This forest has a warm and semi-arid climate, with rainfall < 1000 mm/year distributed within a range of 3–6 months (Velloso et al. 2002) and an average annual temperature between 20 and 28 °C (Maracajá and Benevides 2006). The leaves of the bromeliad T. catimbauensis (Fig. 1) were collected from 15 individual plants during the dry season in May 2015. The collections were authorised by the Ministério do Meio Ambiente (MMA)/Instituto Chico Mendes para Conservação da Biodiversidade (ICMBio), the approval being issued on 10 April 2015; permission number: 48641-1 and authentication code: 17827693.
Endophytic fungi isolation
The plant material was used within 48 h of collection, and the endophytic fungi were isolated as described by Bezerra et al. (2015). Briefly, the leaves of the bromeliad were cut into fragments measuring about 10 cm and washed with water containing neutral detergent. Subsequently, the asepsis of the leaves was achieved by soaking them in 70% ethanol for 60 s, sodium hypochlorite (2–2.5% active chlorine) for 180 s and 70% ethanol for 30 s, followed by washing three times with distilled and sterilised water. Under aseptic conditions, 28 fragments measuring about 1 cm2 were cut from the plant tissue of each individual (totalling 420 fragments), transferred to Petri dishes containing potato dextrose agar (PDA) supplemented with the antibiotics chloramphenicol (100 mg/L) and tetracycline (50 mg/L) and then incubated at 28 °C ± 2 °C for up to 30 days. To verify the effectiveness of asepsis, 1-mL aliquots of the last water wash were transferred to Petri dishes containing the same culture medium and incubated under the same conditions.
Endophytic fungi identification
All the endophytic fungi that were morphologically identified as Penicillium sp. were cultured on malt extract agar (MEA) for 7 days at 25 °C. Slides were prepared using lactic acid, and the fungal microstructures (conidiophores, phialides, conidia, etc.) were analysed. Representative endophytic strains are deposited in the URM culture collection (Micoteca URM Prof. Maria Auxiliadora Cavalcanti) at the Federal University of Pernambuco, Recife, Brazil.
Genomic DNA was extracted for molecular analysis from all the endophytic fungi that were morphologically grouped as Penicillium spp. The genomic DNA was obtained from the colonies grown on MEA for 7 days at 25 °C using the Wizard® Genomic DNA Purification Kit (Promega, Madison, WI) according to the manufacturer’s instructions. DNA amplification of a part of the gene β-tubulin was performed using the primers Bt2a and Bt2b (Glass and Donaldson 1995), and PCR was carried out as described by Visagie et al. (2014). Sequencing and sequence analyses were performed as described by Bezerra et al. (2017).
The sequences obtained in this study were used for conducting searches using the tool BLASTn in the GenBank database at NCBI for verifying the previous identity with sequences deposited in the database. After these searches, an alignment was constructed using the sequences from the type material or the reference strains according to Houbraken and Samson (2011), Yilmaz et al. (2014) and Visagie et al. (2014, 2016). The online MAFFT interface (Katoh and Standley 2013) was used to perform the alignment, and MEGA v. 7.0 (Kumar et al. 2016) was used for sequence adjustments. The maximum likelihood (ML) analysis was performed using MEGA v. 7.0 with 1000 bootstrap replicates, and gaps were treated as missing data. The general time-reversible (GTR) nucleotide substitution model was estimated using the online tool Findmodel (http://www.hiv.lanl.gov/content/sequence/findmodel/findmodel.html). The tree that was obtained was printed using TreeView v. 1.6.6 (Page 1996). Newly generated β-tubulin sequences were deposited in the GenBank database (MG906521–MG906572).
l-Asparaginase production in liquid medium
A total of 20 endophytes belonging to the genera Penicillium and Talaromyces (10 for each genus) were randomly selected for evaluating the production of l-asparaginase. First, the biomass production was carried out using Czapex Dox’s medium (CDM) modified by adding l-asparagine (Saxena and Sinha 1981), modified by Gulati et al. (1997). Erlenmeyer flasks (250 mL) containing 50 mL of CDM were inoculated with 1 mL of spore suspension (1 × 108). These flasks were incubated at 30 °C for 96 h at 120 rpm. Then, the cultures were filtered using Whatman no. 1 filter paper, and the biomass thus obtained was used for analysing enzyme production. The biomass obtained in the first step was inoculated into the modified CDM as described previously, but with two differences, i.e. the glucose concentration was adjusted from 14.0 to 2.0 g/L, and no (NH4)2SO4 (2.0 g/L) was added. The inoculated media were incubated at 30 °C for 96 h at 120 rpm. Finally, the cultures were filtered using Whatman no. 1 filter paper, and the biomass thus obtained was used for quantification of the enzymatic activity (Loureiro et al. 2012, modified).
l-Asparaginase activity
l-asparaginase activity was determined according to Drainas et al. (1977) with the following modifications: 1.5 mL Tris–HCl buffer (20 mM, pH 8.6) and 0.1 g of mycelium from each culture obtained during the fermentation step were macerated and vortexed. To the samples, 0.2 mL of l-asparagine solution (100 mM) and 0.2 mL of stock hydroxylamine solution (1 M, pH 7.0) were added and incubated at 37 °C at 150 rpm. After 30 min, the reaction was stopped by adding 0.5 mL of ferric chloride reagent [10% (w/v) FeCl3 plus 5% (w/v) trichloroacetic acid in 0.66 mol/L HCl] to all the samples and the blank samples (Tris–HCl and mycelium). The reaction mixture was centrifuged at 6000 rpm for 15 min at 4 °C to remove the precipitates. Absorbance was measured at 500 nm against the blank samples that received l-asparagine and hydroxylamine solutions after 30 min of incubation. One unit of l-asparaginase was defined as the amount of enzyme that releases 1 µmol of β-hydroxamic aspartic acid per minute.
Partial optimisation of fungal biomass and l-asparaginase production
For determining the best producer of l-asparaginase, a statistical experimental design was used to optimise the fungal biomass production. In this study, the 23 factorial design (Myers and Montgomery 1995; Box et al. 2005) was selected to evaluate the influence of the variables on biomass production. This experimental design comprised 12 trials, three variables with two levels for each variable (23) and four replications at the central point. The variables that were analysed in this study were l-proline concentration, pH and inoculum (spore) concentration (Suppl. Table 1). l-proline was chosen as the substrate based on analyses of previous experiments (unpublished data), which had demonstrated it as the best inducer of fungal biomass production. At this stage, 100 mL of CDM was added to the Erlenmeyer flasks (250 mL), and the culture media were adjusted according to the specific conditions for each trial design. These flasks were incubated under the same pre-fermentation conditions as mentioned above. After the incubation period, the fungal biomass was filtered, dried at 60 °C and the weight was checked.
The 23 factorial design was also used to partial optimise l-asparaginase production. The initial experimental process was given by the conditions established during the pre-fermentation stage, which provided higher biomass production. This experimental design and the corresponding dependent variables described above, except the inoculum concentration (spores mL−1), which was replaced by fungal biomass in grams, are presented in Table 2 of the Supplementary Material. The fungus was inoculated as described above, and the flasks were incubated at 120 rpm for 120 h at 30 °C. After the incubation period, the fungal biomass was filtered and used to determine enzyme activity (Drainas et al. 1977).
Data analyses: richness of endophytes and l-asparaginase production
The absolute (fa) and the relative (fr) frequencies of the endophytes were calculated. The absolute frequency was expressed by the number of times that each taxon was isolated from the plant, and the relative frequency was defined as the absolute frequency divided by the total number (tn) of endophytes isolated (fr = fa/tn*100).
All the results obtained during l-asparaginase production were subjected to the non-parametric Kruskal–Wallis test to verify whether any statistically significant difference existed (p < 0.05) between the enzymatic activities produced by the endophytes isolated from T. catimbauensis. The results obtained in the partial optimisation of fungal biomass and l-asparaginase production were analysed by the F-test (ANOVA) to verify and evaluate the relationship between the selected independent variables and the biomass. Finally, the response surface theory was used to visualise trends among the variables. All statistical analyses were conducted using the R software (R Development Core Team 2015).
Results
A total of 184 endophytic fungi were isolated from the leaves of the bromeliad T. catimbauensis, of which 52 endophytes were identified as Penicillium and Talaromyces spp. (Table 1). Phylogenetic analysis using β-tubulin sequences from these isolates recognised a total of nine putative species. These data indicated the richness of four Penicillium species, and the other five species were grouped in the genus Talaromyces, both in the Trichocomaceae (Fig. 2). The most frequently isolated species in Talaromyces was T. diversus (20 isolates), and Penicillium sp. 4 (8 isolates) was the more frequently recovered Penicillium putative new species. Other species such P. decaturense were isolated once or twice and were reported as rare isolation. Other isolates were identified up to the genus level, and they were considered as putative new species in both genera.
Maximum Likehood (ML) tree obtained by phylogenetic analysis using β-tubulin sequences from 52 endophytic Penicillium and Talaromyces putative species isolated from leaves of the bromeliad Tillandsia catimbauensis in the Brazilian tropical dry forest (Caatinga). ML bootstrap values above 70% are showed at nodes. Endophytic fungi obtained in this study are in blue colour. Trichocoma paradoxa CBS 247.57 was used as outgroup
Of the total 20 endophytic fungi tested in the liquid medium, 10 exhibited the capacity to produce the enzyme l-asparaginase, with the enzymatic activity varying between 0.50 and 2.30 U/g. l-Asparaginase production was statistically analysed using the non-parametric Kruskal–Wallis test to determine whether any statistically significant difference existed between the isolates. This test resulted in a p value of 0.00483, based on which we can confirm that at least one value of the enzymatic activity of one isolate was statistically different from the others (Table 2). In addition, we can observe that the following five groups can be formed between the endophytes used for enzyme production: A (URM 7826 and URM 7827), B (URM 7828, URM 7829 and URM 7830), C (URM 7831 and URM 7667), D (URM 7832, URM 7833 and URM 7665) and E (T106, T114, T12, T82, T20, T63, T95B, T95A, T10A and T124 isolates). There was no statistically significant difference between the isolates from the same group; however, a statistically significant difference was observed between the isolates from different groups. The best results were obtained with the isolates T. cf. cecidicola URM 7826 and Penicillium sp. 4 URM 7827 from the group A, which produced 2.30 and 1.28 U/g of the intracellular enzyme, respectively. Based on the results obtained in the l-asparaginase activity, the endophyte T. cf. cecidicola URM 7826, which demonstrated the best enzymatic activity (2.30 U/g), was selected for the partial optimisation of fungal biomass and l-asparaginase production.
In the experimental 23 factorial design, the biomass production ranged from 0.16 to 0.66 g after 96 h of incubation, which demonstrated the significance of the variables used for the partial optimisation process of biomass production (Table 3). The results of the experimental design showed that the concentration of the inoculum was statistically significant (p < 0.05), whereas the variable pH was marginally significant (p < 0.1), and the concentration of l-proline was not statistically significant (p > 0.1) (Suppl. Table 3). The model adequately adjusts the data, since the independent variables explained 92.74% of biomass production variation, the lack of fit was not statistically significant and the predicted values were close to the observed values (Table 3 and Suppl. Table 3). Based on the experimental design used in this study, the best conditions for biomass production were pH 6.0, inoculum concentration of 1 × 108 and 1% of l-proline concentration, which produced 0.66 g of biomass. The analysis based on the experimental design showed that the maximum point has possibly not yet reached; however, there are clearly marked trends (Fig. 3).
l-Asparaginase production using the experimental 23 factorial design varied between 0.58 and 1.02 U/g (Table 4). The adjusted statistical model did not explain the production of l-asparaginase, as the p value for the lack of fit was significant (p < 0.05) (Suppl. Table 4). The optimal point for enzyme production was not reached, demonstrating that further studies and detailed analyses are required to verify the influence of the variables on l-asparaginase production.
Discussion
According to Strobel and Daisy (2003), plants of unique environments, endemic and occupying areas of great biodiversity must be collected for the purpose of isolating endophytes and discovering natural products. The present study demonstrated a high frequency of Penicillium and Talaromyces isolates when exploring the fungal endophytic richness associated with the bromeliad T. catimbauensis. Similar studies using Caatinga plants have reported the presence of Penicillium isolates as endophytes. For example, Freire et al. (2015) reported the presence of P. funiculosum, P. citrinum and P. janthinellum in healthy Opuntia ficus-indica and infested by Dactylopius opuntiae. Other studies on cacti species from the Caatinga forest have also reported the isolation of P. aurantiogriseum and P. glandicola from O. ficus-indica (Bezerra et al. 2012a) and nine endophytic Penicillium species from Cereus jamacaru, with the latter isolates corresponding to 5% of the endophytic community (Bezerra et al. 2013). In contrast, the study of Santos et al. (2015a) on Indigofera suffruticosa in the Caatinga forest did not report Penicillium and Talaromyces species as endophytes.
Endophytic Penicillium species have also been isolated from plants from warm and dry environments (Loro et al. 2012; Sun et al. 2012), but there was no association of Talaromyces species (Suryanarayanan et al. 2005; Khidir et al. 2010). In an interesting study on Cannabis sativa in the Netherlands, > 90% of the endophytic fungi isolated belonged to the genus Penicillium (Kusari et al. 2013). Talaromyces species have been described as endophytes of plants from different environments, such as Amomum siamense in Thailand (Bussaban et al. 2001), medicinal plants in Thailand (Theantana et al. 2009), Dactylis glomerata in Spain (Márquez et al. 2007), Cupressus sempervirens in Iran (Soltani and Moghaddam 2015) and Bauhinia forficata in Brazil (Bezerra et al. 2015). These studies may also help in understanding the relationship between these microorganisms and their hosts and the protection of plants living in stressful environments.
The endophytic fungi isolated from T. catimbauensis possess the biotechnological potential for the production of the enzyme l-asparaginase. Endophytic microorganisms are considered as the major sources of bioactive natural products with potential use in agriculture, medicine, pharmaceuticals and industries (Jalgaonwala et al. 2011), and among these microorganisms, fungi have been reported as potential producers of novel secondary metabolites (Schulz et al. 2002). Some studies have demonstrated the potential of endophytic fungi for the production of the enzyme l-asparaginase (Chow and Ting 2017; Kalyanasundaram et al. 2015; Manasa and Nalini 2014; Theantana et al. 2007, 2009; Thirunavukkarasu et al. 2011).
The genera Penicillium and Talaromyces have been emphasised in the production of l-asparaginase. For example, Theantana et al. (2009) using fungi isolated from medicinal plants reported an enzymatic activity varying between 0.014 and 1.530 U/mL from 53 endophytes, highlighting the high activity of Penicillium and Talaromyces isolates. Similarly, Santos et al. (2015b) investigated the potential of endophytic fungi isolated from C. jamacaru in Brazil and found that of nine Penicillium isolates tested, four showed activity for l-asparaginase, with P. brevicompactum being considered as one of the largest producers of the enzyme (2.54 U/mL). Chow and Ting (2015) confirmed the enzymatic activity of 25 endophytic fungi, reporting P. simplicissimum as the third largest producer of l-asparaginase. Similar results were obtained by Krishnapura and Belur (2016) who investigated the endophytic fungus T. pinophilus isolated from the rhizomes of Curcuma amada and partially purified and characterised the enzyme produced by the endophyte, which was considered as a potential candidate for industrial and clinical trials because of its biochemical properties and high efficiency. However, these studies used different methodologies to verify the enzymatic activity (e.g. the use of the culture filtrate and the nesslerisation technique to evaluate the enzymatic production), due to which the results may not be comparable with the results obtained in the present study, wherein fungal biomass and quantification of β-hydroxamic aspartic acid were used to determine the enzymatic activity. Similar to this study, Drainas et al. (1977) used the biomass of A. nidulans to quantify the enzymatic activity based on the formation of β-hydroxamic aspartic acid. Based on the studies of Drainas et al. (1977), Kumar and Manonmani (2013) and Kumar et al. (2013), using the extracellular enzyme, the fungal enzymatic production can be verified through the quantification of β-hydroxamic aspartic acid (see Suppl. Tables 5 and 6).
According to Kumar et al. (2010), most of the microorganisms accumulate the enzyme l-asparaginase as an intracellular product. Similar to this study, other studies have also verified the production of intracellular l-asparaginase by fungi from the genus Penicillium. Using the nesslerisation technique, Elshafei et al. (2012) evaluated the intracellular and extracellular enzymatic activity of filamentous fungi and verified that the isolates had the highest activities at the intracellular level, highlighting P. brevicompactum (1.7 U/mg) and P. purpurescens (1.24 U/mg). They further demonstrated l-asparaginase as an intracellular enzyme. Other researchers such as Gupta et al. (2009) who analysed fungi from the mangrove ecosystem reported that 85 isolates had intracellular enzymatic activity and only 20 other isolates had extracellular enzymatic activity, especially the intracellular production of Penicillium sp. PF 52 and Penicillium sp. RF2, with activities of 16.71 and 5.41 U/g, respectively. In addition, Patro and Gupta (2012) using the nesslerisation method to verify l-asparaginase activity demonstrated enzymatic activity in the cellular biomass of Penicillium sp.
In the present study, the best biomass production conditions were identified as inoculum concentration of 1 × 108 spores, pH 6.0 and 1% of l-proline. According to Amena et al. (2010), optimisation of the inoculum concentration is essential because few spores can lead to insufficient biomass, whereas numerous spores can result in an increased biomass production, leading to rapid nutrient depletion. In addition, the growth of microorganisms can be drastically affected by pH (Niharika and Supriya 2014). Similar to this study, Niharika and Supriya (2014) analysed the influence of the variable proline on the growth of Fusarium oxysporum and found that proline (1%) was the best among all nitrogen sources for mycelial growth. However, Sarquis et al. (2004) reported that proline (2%) was the second largest source of nitrogen for A. tamarii. Using soil samples from Egypt, Bedaiwy et al. (2016) demonstrated that pH 7.0 was ideal for the growth of A. tamarii and for the production of l-asparaginase. Gbolagade et al. (2006) using submerged conditions to optimise the biomass production of Pleurotus florida, an edible fungus, reported that the fungus produced the highest biomass at pH 6.5 and at a temperature of 30 °C.
According to Thakur et al. (2013), the initial pH of the culture medium can affect enzyme production, as it affects nutrient availability. According to Hosamani and Kaliwal (2011), it is necessary to optimise the inoculum because an excess biomass can lead to nutrient depletion of the substrate or the accumulation of substances that inhibit formation of the product. Sarquis et al. (2004) suggested that the production of l-asparaginase is regulated by nitrogen; whereas Elshafei et al. (2012) reported that pH 6.0 was ideal to produce intracellular l-asparaginase from P. brevicompactum. Similarly, Kumar et al. (2013) discovered that pH 5.8 was optimal for the production of l-asparaginase by Cladosporium sp. As shown in the present study, Thakur et al. (2013) found that pH 7.0 was the best condition for enzymatic production by Mucor hiemalis. In this study, the optimal concentrations of l-proline and the inoculum were 0.5% and 3.5 g, respectively. However, Baskar and Renganathan (2012) optimised the production of l-asparaginase and observed that a concentration of 1.7% l-proline was ideal for enzymatic production by A. terreus. Dias and Sato (2016) optimised l-asparaginase production by A. oryzae and obtained optimum conditions of pH 8.0, 2% l-proline, and 3 × 107 spores/mL as the inoculum concentration. In this study, the experimental design provided the guidelines to reach optimal enzyme production. Our results suggest that future experiments should fix one of the variables (inoculum or pH) and vary the others, as the range of inoculum and l-proline concentrations in some assays resulted in different effects on enzymatic production.
The results of the present study show that the bromeliad T. catimbauensis, which is endemic in the Brazilian tropical dry forest, is an important host for endophytic species belonging to the genera Penicillium and Talaromyces. To our knowledge, this is the first record of the production of the enzyme l-asparaginase by endophytic fungi isolated from T. catimbauensis, which demonstrates the importance of the study on the richness of endophytes in arid environments. Among the 10 isolates that exhibited enzymatic activity (0.50–2.30 U/g), the endophytes T. cf. cecidicola URM 7826 (2.30 U/g) and Penicillium sp. 4 URM 7827 (1.28 U/g) were found to be the most promising. In the partial optimisation stage of the biomass production of T. cf. cecidicola URM 7826, pH (6.0) was found to be marginally significant and the inoculum concentration was found to be significant (1 × 108), whereas the concentration of l-proline (1%) was not significant. Furthermore, l-asparaginase production by T. cf. cecidicola URM 7826 varied between 0.58 and 1.02 U/g when using the experimental 23 factorial design and did not reach the optimal point for enzyme production. Considering the industrial importance of the enzyme l-asparaginase, it is necessary to search for new sources of enzyme production, as there is a growing demand for this enzyme in Brazil, and the endophyte T. cf. cecidicola URM 7826 is indicated for further optimisation studies to produce l-asparaginase.
References
Alves JJA, Araújo MA, Nascimento SS (2009) Degradação da Caatinga: uma investigação ecogeográfica. Rev Caatinga 22(3):126–135
Amena S, Vishalakshi N, Prabhakar M, Dayanand A, Lingappa K (2010) Production, purification and characterization of L-asparaginase from Streptomyces gulbargensis. Braz J Microbiol 41(1):173–178
Arnold AE, Mejía LC, Kyllo D, Rojas EI, Maynard Z, Robbins N, Herre EA (2003) Fungal endophytes limit pathogen damage in a tropical tree. Proc Natl Acad Sci USA 100(26):15649–15654
Baskar G, Renganathan S (2012) Optimization of L-asparaginase production by Aspergillus terreus MTCC 1782 using response surface methodology and artificial neural network-linked genetic algorithm. Asia-Pac J Chem Eng 7(2):212–220
Bedaiwy MY, Awadalla OA, Abou-Zeid AM, Hamada HT (2016) Optimal conditions for production of L-asparaginase from Aspergillus tamarii. Egypt J Exp Biol (Bot) 12(2):229–237
Bezerra JDP, Santos MGS, Svedese VM, Lima DMM, Fernandes MJS, Paiva LM, Souza-Motta CM (2012a) Richness of endophytic fungi isolated from Opuntia ficus-indica. Mill. (Cactaceae) and preliminary screening for enzyme production. World J Microbiol Biotechnol 28:1989–1995
Bezerra JDP, Lopes DHG, Santos MGS, Svedese VM, Paiva LM, Almeida-Cortez JS, Souza-Motta CM (2012b) Riqueza de micro-organismos endofíticos em espécies da família Cactaceae. Bol Soc Latin Carib Cact Suc 9(2):19–23
Bezerra JDP, Santos MGS, Barbosa RN, Svedese VM, Lima DMM, Fernandes MJS, Gomes BS, Paiva LM, Almeida-Cortez JS, Souza-Motta CM (2013) Fungal endophytes from cactus Cereus jamacaru in Brazilian tropical dry forest: a first study. Symbiosis 60(2):53–63
Bezerra JDP, Nascimento CCF, Barbosa RN, Silva DCV, Svedese VM, Silva-Nogueira EB, Gomes BS, Paiva LM, Souza-Motta CM (2015) Endophytic fungi from medicinal plant Bauhinia forficata: diversity and biotechnological potential. Braz J Microbiol 46(1):49–57
Bezerra JDP, Oliveira RJV, Paiva LM, Silva GA, Groenewald JZ, Crous PW, Souza-Motta CM (2017) Bezerromycetales and Wiesneriomycetales ord. nov. (class Dothideomycetes), with two novel genera to accommodate endophytic fungi from Brazilian cactus. Mycol Prog 16(4):297–309
Bischoff KM, Wicklow DT, Jordan DB, Rezende ST, Liu S, Hughes SR, Rich JO (2009) Extracellular hemicellulolytic enzymes from the maize endophyte Acremonium zeae. Curr Microbiol 58(5):499–503
Box GEP, Hunter JS, Hunter WG (2005) Statistics for experimenters: design, Innovation, and Discovery. Wiley, New Jersey
Bussaban B, Lumyong S, Lumyong P, McKenzie EH, Hyde KD (2001) Endophytic fungi from Amomum siamense. Can J Microbiol 47(10):943–948
Chapla VM, Biasetto CR, Araujo AR (2013) Fungos endofíticos: uma fonte inexplorada e sustentável de novos e bioativos produtos naturais. Rev Virtual Quim 5(3):421–437
Chow YY, Ting ASY (2015) Endophytic L-asparaginase-producing fungi from plants associated with anticancer properties. J Adv Res 6:869–876
Chow YY, Ting ASY (2017) Influence of glucose and L-asparagine concentrations on L-asparaginase production by endophytic fungi. J Microbiol Biotechnol Food Sci 7(2):186
Crous PW, Wingfield MJ, Burgess TI et al (2017) Fungal Planet description sheets: 625–715. Persoonia 39:270–467
Devi S, Azmi W (2012) One step purification of glutaminase free L-asparaginase from Erwinia carotovora with anticancerous activty. Int J Life Sci Pharma Res 2(3):36–45
Dias FFG, Sato HH (2016) Sequential optimization strategy for maximum L-asparaginase production from Aspergillus oryzae CCT 3940. Biocatal Agric Biotechnol 6:33–39
Drainas C, Kinghorn JR, Pateman JA (1977) Aspartic hydroxamate resistance and asparaginase regulation in the fungus Aspergillus nidulans. Microbiology 98(2):493–501
Duval M, Suciu S, Ferster A, Rialland X, Nelken B, Lutz P, Benoit Y, Robert A, Manel AM, Vilmer E, Otten J, Philippe N (2002) Comparison of Escherichia coli–asparaginase with Erwinia-asparaginase in the treatment of childhood lymphoid malignancies: results of a randomized European Organisation for Research and Treatment of Cancer—Children’s Leukemia Group phase 3 trial. Blood 99(8):2734–2739
Elshafei AM, Hassan MM, Ali MA-E, Mahmoud DA, Elghonemy DH (2012) Screening and optimization of L-asparaginase and L-glutaminase production by some filamentous fungi. Adv Food Sci 34(3):150–158
Fabricante JR, Araújo KCT, Ferreira JVA, Castro RA, Silva ACCP, Siqueira-Filho JA (2014) Categorização do risco de extinção de Dyckia limae LB Sm. e Tillandsia catimbauensis Leme. W. T. & JA S. por meio de critérios de distribuição geográfica. Biotemas 27(2):203–207
Ferreira JVA, Fabricante JR, Siqueira-Filho JA (2015) Checklist preliminar de Bromeliaceae do Parque Nacional do Catimbau, Pernambuco, Brasil. Natureza on line 13(2):92–97
Fischer PJ, Sutton BC, Petrini LE, Petrini O (1994) Fungal endophytes from Opuntia stricta: a first report. Nova Hedwigia 59:195–200
Freire KTLS, Araújo GR, Bezerra JDP, Barbosa RN, Silva DC, Svedese VM, Souza-Motta CM (2015) Fungos endofíticos de Opuntia ficus-indica. (L.) Mill.(Cactaceae) sadia e infestada por Dactylopius opuntiae (Cockerell, 1896) (Hemiptera: Dactylopiidae). Gaia Scientia 9(2):104–110
Gbolagade J, Sobowale A, Adejoye D (2006) Optimization of sub-merged culture conditions for biomass production in Pleurotus florida (mont.) Singer, a Nigerian edible fungus. Afr J Biotechnol 5(16):1464–1469
Glass NL, Donaldson GC (1995) Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl Environ Microbiol 61(4):1323–1330
Guilleme CM, Delgado RF, Navarro JS, Aguirre IA, Solà SR, Codina JST et al (2013) Actualización del tratamiento con L-asparraginasa en Pediatría. An Pediatr 79(5):329.e1–329.e11
Gulati R, Saxena RK, Gupta R (1997) A rapid plate assay for screening L-asparaginase producing micro-organisms. Lett Appl Microbiol 24(1):23–26
Gupta N, Dash SJ, Basak UC (2009) L-asparaginases from fungi of Bhitarkanika mangrove ecosystem. Asia-Pac J Mol Biol Biotechnol 17(1):27–30
Hendriksen HV, Kornbrust BA, Østergaard PR, Stringer MA (2009) Evaluating the potential for enzymatic acrylamide mitigation in a range of food products using an asparaginase from Aspergillus oryzae. J Agric Food Chem 57(10):4168–4176
Hosamani R, Kaliwal BB (2011) L-asparaginase-an anti tumor agent production by Fusarium equiseti using solid state fermentation. Int J Drug Discov 3(2):88–99
Houbraken J, Samson RA (2011) Phylogeny of Penicillium and the segregation of Trichocomaceae into three families. Stud Mycol 70:1–51
Hwang JS, You YH, Bae JJ, Khan SA, Kim JG, Choo YS (2011) Effects of endophytic fungal secondary metabolites on the growth and physiological response of Carex kobomugi Ohwi. J Coast Res 27(3):544–548
Instituto Chico Mendes de Conservação da Biodiversidade—ICMBio (2018) Parna do Catimbau. http://www.icmbio.gov.br/portal/unidadesdeconservacao/biomas-brasileiros/caatinga/unidades-de-conservacao-caatinga/2135-parna-do-catimbau. Accessed 27 Jan 2018
Jain R, Zaidi KU, Verma Y, Saxena P (2012) L-asparaginase: A promising enzyme for treatment of acute lymphoblastic leukiemia. People’s J Sci Res 5(1):29–35
Jalgaonwala RE, Mohite BV, Mahajan RT (2011) A review: natural products from plant associated endophytic fungi. J Microbiol Biotechnol Res 1(2):21–32
Jha SK, Pasrija D, Sinha RK, Singh HR, Nigam VK, Vidyarthi AS (2012) Microbial L-asparaginase: a review on current scenario and future prospects. Int J Pharm Sci Res 3(9):3076–3090
Kalyanasundaram I, Nagamuthu J, Srinivasan B, Pachayappan A, Muthukumarasamy S (2015) Production, purification and characterisation of extracellular L-asparaginase from salt marsh fungal endophytes. World J Pharm Pharm Sci 4(3):663–677
Katoh K, Standley DM (2013) MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol 30:772–780
Khidir HH, Eudy DM, Porras-Alfaro A, Herrera J, Natvig DO, Sinsabaugh RL (2010) A general suite of fungal endophytes dominate the roots of two dominant grasses in a semiarid grassland. J Arid Environ 74(1):35–42
Kornbrust BA, Stringer MA, Lange NEK, Hendriksen HV (2009) Asparaginase—an enzyme for acrylamide reduction in food products. In: Whitehurst RJ, Oort MV (eds) Enzymes in food technology, 2nd edn. Wiley-Blackwell, Hoboken, pp 59–87
Krishnakumar T, Visvanathan R (2014) Acrylamide in food products: a review. J Food Process Technol 5(7):1–9
Krishnapura PR, Belur PD (2016) Partial purification and characterization of L-asparaginase from an endophytic Talaromyces pinophilus isolated from the rhizomes of Curcuma amada. J Mol Catal B 124:83–91
Kumar NSM, Manonmani HK (2013) Purification, characterization and kinetic properties of extracellular L-asparaginase produced by Cladosporium sp. World J Microbiol Biotechnol 29(4):577–587
Kumar S, Dasu VV, Pakshirajan K (2010) Localization and production of novel L-asparaginase from Pectobacterium carotovorum MTCC 1428. Process Biochem 45(2):223–229
Kumar NSM, Ramasamy R, Manonmani HK (2013) Production and optimization of L-asparaginase from Cladosporium sp. using agricultural residues in solid state fermentation. Ind Crops Prod 43:150–158
Kumar NSM, Shimray CA, Indrani D, Manonmani HK (2014) Reduction of acrylamide formation in sweet bread with L-asparaginase treatment. Food Bioprocess Technol 7(3):741–748
Kumar S, Stecher G, Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33:1870–1874
Kusari P, Kusari S, Spiteller M, Kayser O (2013) Endophytic fungi harbored in Cannabis sativa L.: diversity and potential as biocontrol agents against host plant-specific phytopathogens. Fungal Divers 60(1):137–151
Leal IR, Tabarelli M, Silva JMC (2003) Ecologia e conservação da Caatinga. Editora Universitária UFPE, Recife
Leal IR, Silva JD, Tabarelli M, Lacher JTE (2005) Mudando o curso da conservação da biodiversidade na Caatinga do Nordeste do Brasil. Megadiversidade 1(1):139–146
Loro M, Valero-Jiménez CA, Nozawa S, Márquez LM (2012) Diversity and composition of fungal endophytes in semiarid Northwest Venezuela. J Arid Environ 85:46–55
Loureiro CB, Borges KS, Andrade AF, Tone LG, Said S (2012) Purification and biochemical characterization of native and pegylated form of L-Asparaginase from Aspergillus terreus and evaluation of its antiproliferative activity. Adv Microbiol 2:138–145
Manasa C, Nalini MS (2014) L-Asparaginase activity of fungal endophytes from Tabernaemontana heyneana wall (Apocynaceae), endemic to the Western Ghats (India). Int Sch Res Not. https://doi.org/10.1155/2014/925131
Maracajá PB, Benevides DS (2006) Estudo da flora herbácea da Caatinga no município de Caraúbas no Estado do Rio Grande do Norte. Rev Biol Ciênc Terra 6(1):165–175
Márquez SS, Bills GF, Zabalgogeazcoa I (2007) The endophytic mycobiota of the grass Dactylis glomerata. Fungal Divers 27:171–195
Meng L, Sun P, Tang H, Li L, Draeger S, Schulz B, Yi Y (2011) Endophytic fungus Penicillium chrysogenum, a new source of hypocrellins. Biochem Syst Ecol 39(2):163–165
Myers RH, Montgomery DC (1995) Response surface methodology: process and product optimization using designed experiments. Wiley, Hoboken
Niharika YC, Supriya S (2014) Production of L-asparaginase by Fusarium oxysporum using submerged fermentation. Int J Pharm Sci Invent 3:32–39
Nomme J, Su Y, Konrad M, Lavie A (2012) Structures of apo and product-bound human L-asparaginase: insights into the mechanism of autoproteolysis and substrate hydrolysis. Biochemistry 51(34):6816–6826
Page RDM (1996) TreeView: an application to display phylogenetic trees on personal computers. Comput Appl Biosci 12:357–358
Patro KR, Gupta N (2012) Extraction, purification and characterization of L-asparaginase from Penicillium sp. by submerged fermentation. Int J Biotechnol Mol Biol Res 3(3):30–34
Peixoto Neto PAS, Azevedo JL, Caetano LC (2004) Microrganismos endofíticos em plantas: status atual e perspectivas. BLACPMA 3(4):69–72
Pinheiro EAA, Carvalho JM, Santos DCP, Feitosa ADO, Marinho PSB, Guilhon GMSP, Marinho AMDR (2013) Antibacterial activity of alkaloids produced by endophytic fungus Aspergillus sp. EJC08 isolated from medical plant Bauhinia guianensis. Nat Prod Res 27(18):1633–1638
Pires IM, Silva AV, Santos MGS, Bezerra JDP, Barbosa RN, Silva DCV et al (2015) Potencial antibacteriano de fungos endofíticos de cactos da Caatinga, uma Floresta Tropical Seca no Nordeste do Brasil. Gaia Scientia 9(2):155–161
R Development Core Team (2015) R: a language, environment for statistical computing. The R Foundation for Statistical Computing, Vienna
Santos JC, Almeida-Cortez JS, Fernandes GW (2011) Richness of gall-inducing insects in the tropical dry forest (caatinga) of Pernambuco. Rev Bras Entomol 55(1):45–54
Santos IP, Bezerra JDP, Souza-Motta CM, Cavalcanti MS, Lima VLM (2015a) Endophytic mycobiota from leaves of Indigofera suffruticosa Miller (Fabaceae): the relationship between seasonal change in Atlantic Coastal Forest and tropical dry forest (Caatinga), Brazil. Afr J Microbiol Res 9(18):1227–1235
Santos MGS, Bezerra JDP, Svedese VM, Sousa MA, Silva DCV, Maciel MDHC, Paiva LM, Porto ALF, Souza-Motta CM (2015b) Screening of endophytic fungi from cactus of the Brazilian tropical dry forest according to their L-asparaginase activity. Sydowia 67:147–156
Sarquis MIM, Oliveira EMM, Santos AS, Costa GLD (2004) Production of L-asparaginase by filamentous fungi. Mem Inst Oswaldo Cruz 99(5):489–492
Saxena RK, Sinha U (1981) L-Asparaginase and glutaminase activities in the cultures filtrates of Aspergillus nidulans. Curr Sci 50:218–219
Schulz B, Boyle C, Draeger S, Römmert AK, Krohn K (2002) Endophytic fungi: a source of novel biologically active secondary metabolites. Mycol Res 106(9):996–1004
Silva RLO, Luz JS, Silveira EB, Cavalcante UMT (2006) Fungos endofíticos em Annona spp.: isolamento, caracterização enzimática e promoção do crescimento em mudas de pinha (Annona squamosa L.). Acta Bot Bras 20(3):649–655
Soltani J, Moghaddam MSH (2015) Fungal endophyte diversity and bioactivity in the mediterranean cypress Cupressus sempervirens. Curr Microbiol 70(4):580–586
Strobel G, Daisy B (2003) Bioprospecting for microbial endophytes and their natural products. Microbiol Mol Biol Rev 67(4):491–502
Sun Y, Wang Q, Lu X, Okane I, Kakishima M (2012) Endophytic fungal community in stems and leaves of plants from desert areas in China. Mycol Prog 11(3):781–790
Suryanarayanan TS, Wittlinger SK, Faeth SH (2005) Endophytic fungi associated with cacti in Arizona. Mycol Res 109(5):635–639
Tan RX, Zou WX (2001) Endophytes: a rich source of functional metabolites. Nat Prod Rep 18(4):448–459
Thakur M, Lincoln L, Niyonzima FN, More SS (2013) Isolation, Purification and characterization of fungal extracellular L-Asparaginase from Mucor Hiemalis. J Biocatal Biotransform 2(2):1–9
Theantana T, Hyde KD, Lumyong S (2007) Asparaginase production by endophytic fungi isolated from some Thai medicinal plants. KMITL Sci Technol J 7(S1):13–18
Theantana T, Hyde KD, Lumyong S (2009) Asparaginase production by endophytic fungi from Thai medicinal plants: citoxicity properties. IJIB 7(1):1–8
Thirunavukkarasu N. Suryanarayanan TS, Murali TS, Ravishankar JP, Gummadi SN (2011) L-asparaginase from marine derived fungal endophytes of seaweeds. Mycosphere 2(2):147–155
Ting ASY, Meon S, Kadir J, Radu S, Singh G (2008) Endophytic microorganisms as potential growth promoters of banana. Biocontrol 53(3):541–553
Velloso AL, Sampaio EVSB, Pareyn FGC (2002) Ecorregiões propostas para o bioma Caatinga. Associação Plantas do Nordeste; Instituto de Conservação Ambiental. The Nature Conservancy do Brasil, Recife
Visagie CM, Houbraken J, Frisvad JC, Hong SB, Klaassen CHW, Perrone G, Samson RA (2014) Identification and nomenclature of the genus Penicillium. Stud Mycol 78:343–371
Visagie CM, Renaud JB, Burgess KMN, Malloch DW, Clark D, Ketch L, Seifert KA (2016) Fifteen new species of Penicillium. Persoonia 36:247–280
Waqas M, Khan AL, Kamran M, Hamayun M, Kang SM, Kim YH, Lee IJ (2012) Endophytic fungi produce gibberellins and indoleacetic acid and promotes host-plant growth during stress. Molecules 17(9):10754–10773
Yilmaz N, Visagie CM, Houbraken J, Frisvad JC, Samson RA (2014) Polyphasic taxonomy of the genus Talaromyces. Stud Mycol 78:175–341
Zhou W, Starr JL, Krumm JL, Sword GA (2016) The fungal endophyte Chaetomium globosum negatively affects both above-and belowground herbivores in cotton. FEMS Microbiol Ecol 92(10):1–15
Zuo S, Zhang T, Jiang B, Mu W (2015) Reduction of acrylamide level through blanching with treatment by an extremely thermostable L-asparaginase during French fries processing. Extremophiles 19(4):841–851
Acknowledgements
We would like to thank the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for financial support and scholarships. J.D.P. Bezerra also thanks the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Finance code 001) and Fundação de Amparo à Ciência e Tecnologia de Pernambuco (FACEPE) for the postdoctoral fellowships. We are also grateful for the suggestions made by two anonymous reviewers. We extend our thanks to Aline Barboza, Ana P. Pádua, Tamara Caldas, Dr. Marília Maciel, and the students of the Laboratório de Micologia Ambiental/UFPE for their technical help and processing of samples.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Silva, L.F., Freire, K.T.L.S., Araújo-Magalhães, G.R. et al. Penicillium and Talaromyces endophytes from Tillandsia catimbauensis, a bromeliad endemic in the Brazilian tropical dry forest, and their potential for l-asparaginase production. World J Microbiol Biotechnol 34, 162 (2018). https://doi.org/10.1007/s11274-018-2547-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-018-2547-z