Abstract
Purpose
Taxane monotherapy is widely used for advanced gastric cancer (AGC) after failure of standard first-line chemotherapy with fluoropyrimidine and cisplatin. Triplet chemotherapy with docetaxel, cisplatin, and S-1 (DCS) is a promising regimen for first-line chemotherapy of AGC. The aim of this study was to evaluate the efficacy of taxane monotherapy in patients refractory to DCS.
Methods
We retrospectively evaluated the efficacy and safety of taxane monotherapy in patients with AGC refractory to first-line therapy with DCS between January 2010 and April 2015. Selection criteria were as follows: ECOG PS of 0–2, treatment with taxane monotherapy in second-line or third-line therapy after failure of second-line irinotecan, absence of massive ascites, and adequate organ function.
Results
A total of 30 patients were included in this study. Of these, 15 patients received paclitaxel while another 15 received nanoparticle albumin-bound paclitaxel in either second- or third-line treatment. Median age for the second/third-line group was 64.0/62.0 (range 27–75/42–75); 14/13 (93.3/86.7%) had ECOG PS of 0 or 1. No patients achieved complete or partial response and stable disease was observed in 37.5/35.7% of the patients in the second/third line. Median progression-free survival and overall survival were 3.4 and 5.8 months in the second-line group, and 2.0 and 4.5 months in the third-line group, respectively. The incidences of any grade ≥3 adverse events in the second-line group and the third-line group were 60.0 and 33.3%, respectively. There was no treatment-related death.
Conclusions
Taxane monotherapy after DCS failure had acceptable toxicities but was ineffective in AGC patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer is the fifth most common cancer, and the third leading cause of cancer death in the world [1]. Although early gastric cancer is curable with endoscopic or surgical resection, treatment for unresectable and metastatic gastric cancer is mainly palliative chemotherapy. In relation to first-line chemotherapy for advanced gastric cancer (AGC), the platinum-fluoropyrimidine doublet with or without trastuzumab according to human epidermal growth factor receptor 2 (HER2) status is the standard treatment worldwide.
In Japan, cisplatin plus S-1 (CS) is most widely used as the standard first-line chemotherapy. However, as its efficacy is not satisfactory, showing a response rate (RR) of 54%, median progression-free survival (PFS) of 6.0 months, and median overall survival (OS) of 13.0 months [2], a more effective regimen is warranted. One of the promising regimens for first-line chemotherapy is triplet chemotherapy based on the V-325 trial demonstrating that the addition of docetaxel (DTX) to the platinum doublet (cisplatin/5-fluorouracil) improves PFS and OS [3]. However, the triplet regimen is not commonly used for first-line chemotherapy because of its severe toxicity. The Gastric Cancer Group of the Japan Clinical Oncology Group (JCOG) is currently conducting a phase III study (JCOG1013) comparing DTX/cisplatin/S-1 (DCS) with CS (UMIN000007652).
It is also important to consider the optimal treatment strategy relating to second- and third-line chemotherapy. Second-line chemotherapy can be considered for patients with good performance status (PS). Since clinical trials showed a survival benefit with second-line chemotherapy over best supportive care, taxanes [i.e., DTX, paclitaxel (PTX), and nanoparticle albumin-bound paclitaxel (Nab-PTX)] and irinotecan have been widely used in this setting. Also, ramucirumab has been shown to be effective in second-line chemotherapy either as monotherapy or in combination with PTX [4, 5]. In Japan, PTX is most widely used not only for second-line chemotherapy based on the results of WJOG4007 [6] but also for third-line treatment in which PTX appears to show similar efficacy to second-line use in terms of RR and PFS [7].
In consideration of subsequent chemotherapy after triplet chemotherapy, while irinotecan may be a reasonable candidate, rechallenge with another taxane, e.g., PTX after DTX failure, may be another option, an approach which is used for breast cancer [8, 9]. There are three reports on rechallenge of taxanes showing conflicting results. Ando et al. reported that the efficacy of weekly PTX was similar in AGC patients regardless of prior chemotherapy with DTX [10]. Another study suggested that DTX was modestly effective in patients with AGC refractory to PTX [11]. On the other hand, Shimura et al. reported that the rechallenge of taxane in patients with AGC refractory to the other taxane had little efficacy [12]. Thus, it remains unclear whether cross-resistance between the different taxanes is incomplete and whether taxane rechallenge is effective.
In this study, we retrospectively assessed the efficacy and safety of taxane monotherapy after failure of first-line chemotherapy with DCS.
Patients and methods
Patients
We retrospectively reviewed medical records of patients with AGC fulfilling the selection criteria described below at the following four cancer centers in Japan: National Cancer Center Hospital, Aichi Cancer Center Hospital, Shizuoka Cancer Center, and Cancer Institute Hospital of Japanese Foundation for Cancer Research. This study was approved by the institutional review board of each participating hospital.
The selection criteria were: (1) histologically proven, unresectable, or recurrent gastric adenocarcinoma; (2) disease progression confirmed by image diagnosis during first-line chemotherapy with DCS; (3) receiving taxane monotherapy between January 2010 and April 2015 either in the second-line immediately after DCS or third-line therapy after failure of second-line irinotecan; (5) Eastern Cooperative Oncology Group (ECOG) PS of 0–2 at the initiation of taxane monotherapy; (6) absence of massive ascites extending beyond the pelvic cavity to the upper abdomen on a CT scan or requiring drainage; (7) liver and bone marrow function satisfying absolute neutrophil count ≥1000/mm3, platelet ≥7.5 × 104/mm3, total bilirubin ≤1.5 mg/dL, aspartate transaminase ≤100 IU/L, alanine transaminase ≤100 IU/L (total bilirubin ≤2.0 mg/dL, aspartate transaminase ≤200 IU/L, alanine transaminase ≤200 IU/L in patients with liver metastasis).
Treatment
For the first-line therapy, patients received intravenous infusions of DTX 40 mg/m2 (day 1) and cisplatin 60 mg/m2 (day 1), and oral S-1 40 mg/m2 twice daily (day 1–14), repeated every 4 weeks. Cisplatin was administered up to 6 cycles. In later cycles, only DTX and S-1 were administered. In patients who received taxanes in the third-line, irinotecan for the second-line chemotherapy was administered at 150 mg/m2 intravenously every two weeks. As to the rechallenge of taxane monotherapy, patients received either weekly PTX (PTX 80 or 100 mg/m2 intravenous infusion on days 1, 8 and 15 in a 28-day cycle); triweekly Nab-PTX (Nab-PTX 260 mg/m2 intravenous infusion on day 1 in a 21-day cycle); weekly Nab-PTX (Nab-PTX 100 mg/m2 intravenous infusion on days 1, 8, 15 in a 21-day cycle). The treatment was continued until appearance of objective or clinical disease progression, unacceptable toxicity, or patient refusal of further treatment.
Evaluation
CT examination was repeated every two months or when indicated. Tumor response was assessed by each attending physician in accordance with the Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1. OS was defined as the time from the first day of initiating taxane rechallenge in the second- or third-line treatment setting to death or censored at the last date confirming survival. PFS was defined as the time from the first day of initiating taxane rechallenge in the second- or third-line setting to clinically judged or objective disease progression or death. Adverse events (AEs) were graded according to the Common Terminology Criteria for Adverse Events (CTCAE), version 4.0.
Statistical analysis
PFS and OS were estimated by the Kaplan–Meier method, and the median survival time and its 95% interval were estimated by the Brookmeyer–Crowley method with the log-transformation. Analyses were performed by EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan) in R software (The R foundation for Statistical Computing, Vienna, Austria) [13].
Results
Patients
Patient selection is shown in Fig. 1. By April 2015, first-line DCS therapy was discontinued in 92 patients. Of these, 43 patients received either taxane (n = 15) or irinotecan (n = 28) as second-line chemotherapy, and then 15 received taxane as third-line chemotherapy. In total, 30 patients who received taxane rechallenge and met the selection criteria were included in the analysis. This cohort of patients was divided into either second- or third-line groups by the treatment line of rechallenge with taxane monotherapy. Informed consent was not required for this study because of its retrospective nature.
Patient characteristics are shown in Table 1. Median age for the second- and third-line groups was 64.0 (range 27–75) and 62.0 (range 42–75), respectively. ECOG PS was 0 or 1 in 14 (93.3%) and 13 (86.7%) patients from each group. The response rate and median PFS during DCS therapy were 77.8% and 9.4 months in the second-line group, and 64.3% and 5.4 months in the third-line group.
Treatment delivery and compliance
Table 2 summarizes the initial dose of taxane rechallenge; there were no patients whose dose of taxane rechallenge was reduced initially. The median relative dose intensity was 96.4% in the second-line group and 98.5% in the third-line group. The dose was reduced in 4 patients due to neutropenia in the second-line group, and 1 patient due to febrile neutropenia and 2 patients due to their request in the third-line group. Causes of discontinuation of taxane rechallenge in the second-line and the third-line groups were disease progression in 13 (86.7%) and 14 (93.3%) patients, and AEs in 2 (13.3%) and 1 (6.7%) patients, respectively.
Subsequent chemotherapy was given to 9 patients (60.0%) in the second-line group and 6 patients (42.9%) in the third-line group.
Efficacy
Responses of the taxane rechallenge are shown in Table 3. There were no complete or partial responses in either second-line or third-line groups. The disease control rates of 8 and 14 patients with target lesions in the second-line and the third-line groups were 37.5%, and 35.7%, respectively. The disease control rate across all 22 patients with target lesions was 36.4%.
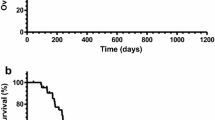
The median PFS was 2.2 months [95% confidence interval (95% CI) 1.3–3.5 months] for all patients, and 3.4 (95% CI 0.8–5.6 months) and 2.0 months (95% CI 0.9–2.8 months) in the second-line and the third-line groups, respectively (Fig. 2).
Progression-free survival a by chemotherapy line or b across all patients, and overall survival, c by chemotherapy line, or d across all patients. In a, c solid line refers to the second-line group, and dotted line represents the third-line group. mPFS median progression-free survival, CI confidence interval, mOS median overall survival
The median OS was 4.8 months (95% CI 3.4–8.8 months) for all patients, and 5.8 (95% CI 2.2–10.6 months) and 4.5 months (95% CI 2.1–8.3 months) in the second-line and third-line groups, respectively (Fig. 2).
Safety
Table 4 shows AEs observed within this patient cohort. The incidences of grade 3 or worse AEs in the second-line and third-line groups were 60.0 and 33.3%, respectively. The AEs leading to dose reduction were neutropenia in four patients (26.7%) from the second-line group and febrile neutropenia in one patient (6.7%) from the third-line group. There was no treatment-related death.
Discussion
A survival benefit of second-line chemotherapy, using taxanes or irinotecan, for AGC has been confirmed by several phase III trials [14,15,16]. At present, first-line chemotherapy with fluoropyrimidine and platinum followed by second-line chemotherapy with taxanes or irinotecan is a widely accepted treatment strategy against AGC worldwide. Recently, Nab-PTX showed an equivalent efficacy to weekly PTX as the second-line chemotherapy [17]. Furthermore, gastric cancer treatment guidelines provided by the Japanese Gastric Cancer Association recommend third-line chemotherapy with either taxanes or irinotecan which is not used in the prior chemotherapy [18]. Weekly PTX showed a very similar efficacy in either second- or third-line treatment [7]. Although the efficacy of Nab-PTX in the third-line setting is not fully investigated, it is anticipated that its efficacy may be similar in both the second and third line, considering that Nab-PTX contains PTX in its albumin particles and there is generally no cross-resistance between taxanes and irinotecan. It is, therefore, considered to be appropriate that both PTX and Nab-PTX were examined in this study investigating the efficacy of taxane rechallenge.
Basic science and clinical studies in other cancer types suggested incomplete cross-resistance between taxanes [8, 19,20,21,22]. However, three studies that assessed the efficacy of a second taxane in AGC have showed inconsistent results with wide ranges of outcomes, as follows: response rates of 5.0–14.2%, disease control rates of 5.0–47.6%, PFS of 1.6–2.6 months, and OS of 3.9–6.7 months [10,11,12]. There are some reasons for this inconsistency. All the studies were retrospective and assessed small numbers of patients in various treatment lines after prior taxane failure due to a variety of reasons. In this study, the number of patients is larger than these reports. Furthermore, unlike other reports of taxane rechallenge [10,11,12], the chemotherapy regimen including prior taxane was limited to DCS as the first-line treatment and disease progression during DCS was confirmed in all the subjects.
In this study, there were no apparent differences in efficacy of taxane rechallenge in terms of response rate, disease control rate, PFS, and OS between the second-line and third-line groups. These results seem to be consistent with the reports that weekly PTX showed a very similar efficacy either in the second- or third-line treatment for taxane-naïve gastric cancer patients [7]. Moreover, the efficacy of taxane rechallenge seemed worse than previous reports of weekly PTX for second- and third-line treatment in patients without prior taxane (response rates 16–23.2%; disease control rates 64–65.9%; PFS 2.9–3.6 months; OS 6.6–9.5 months) [4, 6, 7]. Moreover, the median OS was close to those of patients who received supportive care only in second-line and third-line trials (3.6–4.3 months) [5, 14, 23]. From these results, it is suggested that taxane rechallenge may have little activity against AGC after taxane failure. The background condition of the patients included in this study, with a median age of 64.0/62.0 and PS 0/1 in 93.3/86.7% for the second/third line, does not seem poor compared to that of patients that took part in previous studies. Also, none of the patients had initial dose reduction and the relative dose intensity was maintained. These results suggest that the poor efficacy was not due to the baseline condition of patients or inadequate dosing, but rather due to true lack of efficacy of taxane readministration.
There are some limitations in this study. Firstly, there may be some bias related to its retrospective nature. For example, the frequencies and timings of tumor and AEs assessments were not uniform among the patients studied. Secondly, the type of second taxane (PTX or Nab-PTX) and its dosing (80 or 100 mg/m2 weekly or 260 mg/m2 triweekly) were not uniform. Thirdly, it remains unknown whether PTX + ramucirumab, which is the present standard second-line chemotherapy [4], is ineffective after failure of DCS therapy. Finally, the relationship between patient background, including the efficacy of DCS in the first-line treatment and interval between the last DTX and taxane rechallenge, and efficacy of taxane rechallenge could not be explored by multivariate analysis due to the limiting number of patients in the study.
In conclusion, our findings suggest that PTX/Nab-PTX is tolerable but ineffective either for second-line or third-line therapy after DCS failure. While it is still awaited whether DCS therapy will be a future standard first-line treatment or not, rechallenge of the other taxane cannot be recommended for patients with AGC refractory to the first taxane.
References
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136(5):E359–E386. doi:10.1002/ijc.29210
Koizumi W, Narahara H, Hara T, Takagane A, Akiya T, Takagi M, Miyashita K, Nishizaki T, Kobayashi O, Takiyama W, Toh Y, Nagaie T, Takagi S, Yamamura Y, Yanaoka K, Orita H, Takeuchi M (2008) S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol 9(3):215–221. doi:10.1016/s1470-2045(08)70035-4
Van Cutsem E, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, Boni C, Rodrigues A, Fodor M, Chao Y, Voznyi E, Risse ML, Ajani JA (2006) Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol 24(31):4991–4997. doi:10.1200/jco.2006.06.8429
Wilke H, Muro K, Van Cutsem E, Oh SC, Bodoky G, Shimada Y, Hironaka S, Sugimoto N, Lipatov O, Kim TY, Cunningham D, Rougier P, Komatsu Y, Ajani J, Emig M, Carlesi R, Ferry D, Chandrawansa K, Schwartz JD, Ohtsu A (2014) Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol 15(11):1224–1235. doi:10.1016/s1470-2045(14)70420-6
Fuchs CS, Tomasek J, Yong CJ, Dumitru F, Passalacqua R, Goswami C, Safran H, dos Santos LV, Aprile G, Ferry DR, Melichar B, Tehfe M, Topuzov E, Zalcberg JR, Chau I, Campbell W, Sivanandan C, Pikiel J, Koshiji M, Hsu Y, Liepa AM, Gao L, Schwartz JD, Tabernero J (2014) Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 383(9911):31–39. doi:10.1016/s0140-6736(13)61719-5
Hironaka S, Ueda S, Yasui H, Nishina T, Tsuda M, Tsumura T, Sugimoto N, Shimodaira H, Tokunaga S, Moriwaki T, Esaki T, Nagase M, Fujitani K, Yamaguchi K, Ura T, Hamamoto Y, Morita S, Okamoto I, Boku N, Hyodo I (2013) Randomized, open-label, phase III study comparing irinotecan with paclitaxel in patients with advanced gastric cancer without severe peritoneal metastasis after failure of prior combination chemotherapy using fluoropyrimidine plus platinum: WJOG 4007 trial. J Clin Oncol 31(35):4438–4444. doi:10.1200/jco.2012.48.5805
Shimoyama R, Yasui H, Boku N, Onozawa Y, Hironaka S, Fukutomi A, Yamazaki K, Taku K, Kojima T, Machida N, Todaka A, Tomita H, Sakamoto T, Tsushima T (2009) Weekly paclitaxel for heavily treated advanced or recurrent gastric cancer refractory to fluorouracil, irinotecan, and cisplatin. Gastric Cancer 12(4):206–211. doi:10.1007/s10120-009-0524-9
Yonemori K, Katsumata N, Uno H, Matsumoto K, Kouno T, Tokunaga S, Yamanaka Y, Shimizu C, Ando M, Takeuchi M, Fujiwara Y (2005) Efficacy of weekly paclitaxel in patients with docetaxel-resistant metastatic breast cancer. Breast Cancer Res Treat 89(3):237–241. doi:10.1007/s10549-004-2184-0
Taguchi T, Aihara T, Takatsuka Y, Shin E, Motomura K, Inaji H, Noguchi S (2004) Phase II study of weekly paclitaxel for docetaxel-resistant metastatic breast cancer in Japan. Breast J 10(6):509–513. doi:10.1111/j.1075-122X.2004.21555.x
Ando T, Hosokawa A, Kajiura S, Itaya Y, Ueda A, Fujinami H, Nishikawa J, Kobayashi T, Horikawa N, Tsukioka Y, Yabushita K, Note M, Ogawa K, Sugiyama T (2012) Efficacy of weekly paclitaxel in patients with advanced gastric cancer refractory to docetaxel-based chemotherapy. Gastric Cancer 15(4):427–432. doi:10.1007/s10120-011-0135-0
Kondoh C, Takahari D, Shitara K, Mizota A, Nomura M, Yokota T, Ura T, Ito S, Kawai H, Sawaki A, Muro K (2012) Efficacy of docetaxel in patients with paclitaxel-resistant advanced gastric cancer. Gan To Kagaku Ryoho 39(10):1511–1515
Shimura T, Kitagawa M, Yamada T, Yoshida M, Ebi M, Hirata Y, Mizushima T, Mizoshita T, Tanida S, Kataoka H, Kamiya T, Joh T (2012) The impact of cross-resistance between paclitaxel and docetaxel for metastatic gastric cancer. Onkologie 35(4):176–183. doi:10.1159/000337400
Kanda Y (2013) Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transpl 48(3):452–458. doi:10.1038/bmt.2012.244
Ford HE, Marshall A, Bridgewater JA, Janowitz T, Coxon FY, Wadsley J, Mansoor W, Fyfe D, Madhusudan S, Middleton GW, Swinson D, Falk S, Chau I, Cunningham D, Kareclas P, Cook N, Blazeby JM, Dunn JA (2014) Docetaxel versus active symptom control for refractory oesophagogastric adenocarcinoma (COUGAR-02): an open-label, phase 3 randomised controlled trial. Lancet Oncol 15(1):78–86. doi:10.1016/s1470-2045(13)70549-7
Thuss-Patience PC, Kretzschmar A, Bichev D, Deist T, Hinke A, Breithaupt K, Dogan Y, Gebauer B, Schumacher G, Reichardt P (2011) Survival advantage for irinotecan versus best supportive care as second-line chemotherapy in gastric cancer–a randomised phase III study of the Arbeitsgemeinschaft Internistische Onkologie (AIO). Eur J Cancer 47(15):2306–2314. doi:10.1016/j.ejca.2011.06.002
Kang JH, Lee SI, Limdo H, Park KW, Oh SY, Kwon HC, Hwang IG, Lee SC, Nam E, Shin DB, Lee J, Park JO, Park YS, Lim HY, Kang WK, Park SH (2012) Salvage chemotherapy for pretreated gastric cancer: a randomized phase III trial comparing chemotherapy plus best supportive care with best supportive care alone. J Clin Oncol 30(13):1513–1518. doi:10.1200/JCO.2011.39.4585
Shitara K, Takashima A, Fujitani K, Koeda K, Hara H, Nakayama N, Hironaka S, Nishikawa K, Makari Y, Amagai K, Ueda S, Yoshida K, Shimodaira H, Nishina T, Tsuda M, Kurokawa Y, Tamura T, Sasaki Y, Morita S, Koizumi W (2017) Nab-paclitaxel versus solvent-based paclitaxel in patients with previously treated advanced gastric cancer (ABSOLUTE): an open-label, randomised, non-inferiority, phase 3 trial. Lancet 2(4):277–287
Japanese Gastric Cancer Association (2017) Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer 20(1):1–19. doi:10.1007/s10120-016-0622-4
Maeda S, Saikawa Y, Kubota T, Aoki M, Otani Y, Furukawa T, Watanabe M, Kumai K, Kitajima M (2003) No cross-resistance of taxotere and taxol to conventional chemotherapeutic agents against gastric cancers as detected by MTT assay. Anticancer Res 23(4):3147–3150
Verweij J, Clavel M, Chevalier B (1994) Paclitaxel (Taxol) and docetaxel (Taxotere): not simply two of a kind. Ann Oncol 5(6):495–505
Imai H, Komine K, Takahashi S, Saijo K, Okada Y, Kobayashi A, Okita A, Chikamatsu S, Kasahara Y, Takahashi M, Oishi T, Shirota H, Takahashi M, Shimodaira H, Ishioka C (2016) Efficacy and safety assessment of paclitaxel in patients with docetaxel-resistant esophageal squamous cell carcinoma. Chemotherapy 61(5):262–268. doi:10.1159/000444122
Sonpavde G, Pond GR, Mullane S, Qu AQ, Di Lorenzo G, Federico P, Necchi A, Rosenberg JE, Bellmunt J, Choueiri TK (2015) Incomplete cross-resistance between taxanes for advanced urothelial carcinoma: implications for clinical practice and trial design. Clin Genitourin Cancer 13(3):250–256. doi:10.1016/j.clgc.2014.10.005
Ohtsu A, Ajani JA, Bai YX, Bang YJ, Chung HC, Pan HM, Sahmoud T, Shen L, Yeh KH, Chin K, Muro K, Kim YH, Ferry D, Tebbutt NC, Al-Batran SE, Smith H, Costantini C, Rizvi S, Lebwohl D, Van Cutsem E (2013) Everolimus for previously treated advanced gastric cancer: results of the randomized, double-blind, phase III GRANITE-1 study. J Clin Oncol 31(31):3935–3943. doi:10.1200/jco.2012.48.3552
Acknowledgements
We would like to express our sincere thanks to all patients and investigators.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared no conflicts of interest.
Funding
None.
Ethical approval
This study was approved by the ethics committee of each participating hospital and was performed in accordance with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was not required for this study.
Rights and permissions
About this article
Cite this article
Iizumi, S., Takashima, A., Narita, Y. et al. Efficacy and safety of taxane monotherapy in advanced gastric cancer refractory to triplet chemotherapy with docetaxel, cisplatin, and S-1: a multicenter retrospective study. Cancer Chemother Pharmacol 80, 575–582 (2017). https://doi.org/10.1007/s00280-017-3397-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-017-3397-3