Abstract
Background
Little progress has been made, and there is an unmet medical need for treatment of metastatic gastric cancer (MGC). Docetaxel + cisplatin + 5-fluororacil (DCF) combination is an effective regimen with high rate of toxicity and is not well tolerated. We aimed to evaluate the efficacy and toxicity of a modified DCF (mDCF) combination regimen and capecitabine maintenance in MGC.
Method
Data of MGC patients were treated with first-line mDCF regimen (two weekly docetaxel 60 mg/m2 day 1 iv, cisplatin 50 mg/m2 day 1 iv, 5-fluouracil 400 mg/m2 day 1 iv push, 2400 mg/m2; day 1–day 2 iv infusion, leucovorin 400 mg/m2 day 1 iv push) were recorded. Capecitabine maintenance was given as 2500 mg/m2/ day 1–day 14 po, every 3 weeks, to patients who do not have progressive disease and grade 3 treatment-related toxicity. A retrospective analysis was made.
Results
Forty patients were included. Mean age was 53 ± 11. Thirty-two patients had de novo metastasis. All patients’ performance status was ECOG 1 or 2 (32/8). Median number of mDCF cycles given was 9 (min–max: 1–23). Overall response rate was 47.5%. Ten patients (25%) received capecitabine maintenance. Grade 3/4 toxicity was seen in 20 patients (50%). Hematologic grade 3/4 toxicity occurred in 13 patients (32.5%), and grade 3/4 neutropenia occurred in 11 patients (27.5%) and in 15 cycles. Nonhematologic grade 3/4 toxicity was seen in 7 patients (17.5%). Median follow-up time was 17.2 months. Median time to progression (TTP) was 10.8 ± 1.9 months (95% CI: 6.89–14.64). Median overall survival was 14.7 ± 1.73 months (95% CI: 11.30–18.10).
Conclusions
mDCF protocol was a tolerable chemotherapy regimen for the first-line treatment of MGC with higher ORR and longer TTP compared to standard DCF protocol. Capecitabine maintenance might increase TTP.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer is the 2nd most common cause of death from cancer [1]. It is with poor prognosis in advanced stage [1]. Chemotherapy provides a significant overall survival benefit compared to the best supportive care which translates into an improvement in median survival from 4.3 to 11 months [2]. Little progress has been made with few positive randomized studies, and there is an unmet medical need (Table 1). Several cytotoxic agents including fluoropyrimidines, epirubicin, platinum agents, taxanes, and irinotecan are active against metastatic gastric cancer (MGC). Trastuzumab, a human epidermal growth factor receptor 2 (HER2) monoclonal antibody, is active in HER2-overexpressing tumors in combination with cytotoxic chemotherapy [7]. There is no standard first-line chemotherapy regimen in the first-line treatment of HER-2 negative MGC. Platin + fluoropyrimidines ± docetaxel/epirubicin are systemic combination chemotherapy choices. Combination chemotherapy regimens provide better response rates and longer progression-free survival durations but contributes a few weeks or months to overall survival in the cost of excess toxicity. Docetaxel + cisplatin + 5-fluororacil (DCF) combination is an effective regimen with high rate of toxicity and is not well tolerated especially in patients with poor condition [4]. Grade 3/4 neutropenia and infections were the main toxicities reported with this protocol. Different modified regimens of DCF are tested in several phase 2 studies of MGC [10]. Modification of the parental DCF regimen was made within chemotherapy doses or time intervals of treatment in those studies. Divided doses of drugs in a 2-week protocol might decrease the toxicity and adherence of patients to treatment. Increased doses of drugs might be given in a 2-week protocol. Two-week protocol might give help to closely follow up patients regarding toxicity and dosing manipulations, and treatments for toxicity might also be given immediately. We generated a biweekly modified DCF protocol with increasing the dose intensity and also addition of leucovorin. Maintenance capecitabine in available patients was the second part of the protocol which is not a standard approach. Primary granulocyte colony-stimulating factor (GCSF) prophylaxis was given routinely with this protocol due to high rates of grade 3/4 neutropenia and infection in the V325 study. We aimed to evaluate the efficacy and toxicity of a modified DCF (mDCF) combination regimen and capecitabine maintenance in MGC.
Method
Data of MGC patients who were treated with first-line mDCF regimen (docetaxel 60 mg/m2 on day 1 intravenous (iv), cisplatin 50 mg/m2 on day 1 iv, 5-fluouracil 400 mg/m2 on day 1 iv push and 2400 mg/m2; 46-h iv infusion, leucovorin 400 mg/m2 on day 1 iv push every 2 weeks) and capecitabine maintenance given to patients who did not have progressive disease (capecitabine 2500 mg/m2/ day 1–day 14 po; every 3 weeks) in Izmir Tepecik Research and Training Hospital, Department of Medical Oncology, between the years 2011 and 2015 were recorded. Primary prophylaxis with filgrastim was given at 30 or 48 MU according to body weight (≤ 60 kg or >) on days 4 to 8, sc, every 2 weeks. Doses of the chemotherapy drugs and dose intensity of mDCF regimen compared to original DCF protocol are summarized in Table 2. Inclusion criteria for the mDCF treatment were as follows: age ≥ 18, Eastern Cooperative Oncology Group (ECOG) performance status 0 to 2, no prior treatment for MGC, no HER-2( +) disease, no secondary malignancy, adequate organ functions (cardiac, renal, hepatic, and hematological). Treatment was continued until progression, intolerable toxicity, or patient’s refusal. Intolerable toxicity was defined as recurrent grade 3 or 4 toxicity despite dose reduction/delay or preventive measures.
Response evaluations were made according to RECIST 1.1 every 12 weeks or earlier if there was a suspicion of clinical progression [11]. Responses were recorded after every disease evaluation. Toxicities were evaluated and recorded according to Common terminology Criteria for Adverse Events (CTCAE) version 4.0 on the 1st day of each treatment cycle or on the unscheduled admission dates due to toxicity [12]. Dose reductions were made if there was grade 4 toxicity or recurrent grade 3 toxicity.
Stopping mDCF treatment and continuing with capecitabine maintenance (capecitabine 2500 mg/m2; day 1–day 14 per oral, every 21 days) treatment was discussed with the nonprogressing patients after the sixth cycle of mDCF and every response evaluation. Maintenance treatment with capecitabine after the first-line treatment of MGC was planned for patients who did not have progressive disease and willing to have that kind of treatment.
Time to progression was defined as the time interval from the date of diagnosis for metastatic or recurrent disease to the date of disease progression. Overall survival was defined as the time from the date of diagnosis for metastatic or recurrent disease to the date of death. A retrospective analysis was made. Descriptive statistical analyses and survival analysis were made with SPSS program version 18.0 for Windows.
Results
Patient characteristics are summarized in Table 3. Median follow-up time was 17.2 months (min–max: 1.7–76.3). Total number of mDCF cycles given was 358 to 40 patients. Median number of mDCF cycles given was 9 (min–max: 1–23). Dose reduction was made in 11 of 40 patients (27.5%). Median number of cycles of dose reduction made was 6 (min–max: 3–8). Total number of cycle dose reduction made was 65 (18.16%).
Efficacy
There was one complete response (2.5%) and 18 partial responses (45%). Twelve patients (30%) had stable disease as the best response with mDCF treatment (Table 4). Response evaluation could not be made in four due to early deaths. Three of those four patients had clinical disease progression without any response during treatment. Each of those 3 patients had metastases at 3 sites. One of them had liver, bone, and peritoneal carcinomatosis; the 2nd one had lung, bone, and peritoneal carcinomatosis; and the 3rd one had liver, lung, and peritoenal carcinomatosis.
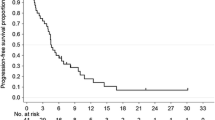
Median time to progression (TTP) was 10.8 ± 1.9 months (95% confidence interval: 6.89–14.64) (Fig. 1). Twenty-nine patients died at the time of last evaluation date. Median overall survival was 14.7 ± 1.73 months (95% confidence interval: 11.30–18.10) (Fig. 2).
Subsequent treatments and toxicity
Twenty of 40 patients received treatment after the first-line mDCF (50%). Ten patients (25%) who did not have progressive disease after the first-line mDCF received capecitabine as maintenance treatment and median duration of that treatment with capecitabine was 9.97 ± 7.56 months (min–max: 1–15.9 months). Ten patients (25%) received FOLFIRI (5-fluorouracil, leucovorin, and irinotecan) for the second-line treatment due to disease progression. One patient who did not have grade 3/4 chemotherapy-related toxicity was treated with rechallenge mDCF regimen after 1-year progression-free interval after the first-line mDCF treatment.
TTP was longer in the patients who received capecitabine maintenance treatment compared to patients who did not take (15.87 ± 4.34 months: %95 CI: 7.35–24.38 vs 7.26 ± 1.25 months: %95 CI: 4.82–9.72; p: 0.02). Overall survival was also longer in the patients who received capecitabine maintenance treatment compared to patients who did not take (24.94 ± 2.87 months: %95 CI: 19.31–30.57 vs 10.85 ± 1.48 months: %95 CI: 7.96–13.74; p: 0.001).
Grade 3/4 toxicity was seen in 20 patients (50%) and 35 of the 358 cycles of mDCF given (9.8%). Hematologic grade 3/4 toxicity occurred in 13 patients (32.5%) and 25 of the 358 cycles given (7%). Five patients (12.5%) had grade 3/4 anemia in a total of 5 cycles and 3 patients had grade 3/4 thrombocytopenia in a total of 3 cycles. Grade 3/4 leucopenia occurred in 7 patients (17.5%) and in 11 cycles. Grade 3/4 neutropenia occurred in 11 patients (27.5%) and in 15 cycles. Four patients (10%) lived neutropenic fever in total 4 cycles.
Nonhematologic grade 3/4 toxicity was seen in 7 patients (17.5%) and 10 of the 358 of mDCF cycles given (2.8%). Three patients had grade 3/4 neuropathy in a total of 4 cycles. One patient lived with grade 3/4 nephropathy. Grade 3/4 mucositis occurred in 9 patients (22.5%) in 13 cycles of treatment. Grade 3/4 nausea occurred in 3 patients and in 5 cycles, and vomiting occurred in 5 patients (12.5%) and in 7 cycles. All grade 3/4 toxicities are summarized in Table 5.
There were no hematologic or nonhematologic grade 3/4 toxicity in patients treated with capecitabine maintenance.
There were four early deaths (in first 3 months) in this study. Clinical disease progression was present in 3 of those 4 patients. The remaining one was lost due to toxicity (neutropenic infection and mucositis). The patient was 70 years old.
Subgroup analysis of patients with long overall survival
A subgroup analysis was made for patients who lived more than a year. There were 14 patients (35%) who lived longer than 12 months. Six (42.9%) of 14 patients had a single site of metastasis. Peritoneum (n: 5; 35.6%), liver (n: 4; 28.6%), and lung (n: 4; 28.6%) were the most frequent sites of metastasis. Primary tumor localization of those 14 patients were most frequently cardia (n: 4/14; 28.6%) and antrum (n: 6/14; 42.9%). Four patients (28.6%) had de novo metastatic disease. Four patients (28.6%) received adjuvant or neoadjuvant chemotherapy and radiotherapy for nonmetastatic disease. Thirteen of 14 patients’ performance status was ECOG 1 (92.9%). All of those 14 patients received more than 6 cycles of mDCF treatment and 9 (64.2%) patients received 12 or more cycles of mDCF treatment. Grade 3/4 toxicities were seen in 7 of 13 patients (53.8%). Dose reduction was made in 4 patients (28.6%) due to toxicity. Twelve of those 14 patients received treatment after the first-line mDCF. Four patients (8.5%) received FOLFIRI, one patient received rechallenge treatment with mDCF after progression, and 8 patients (57.1%) received capecitabine maintenance after the first-line mDCF treatment.
Discussion
Triplet chemotherapy combinations mainly DCF and FLOT (5-fluorouracil, leucovorin, oxaliplatin, and docetaxel) are still the most effective treatment options in the first-line treatment of HER-2 nonexpressing MGC despite all efforts and studies on this field [4, 13]. The toxicity of those regimens is the main shortcoming. We showed that biweekly, mDCF regimen with a higher dose intensity provides increased response rates, TTP, and OS compared to standard DCF regimen. The ORR with mDCF regimen was 47.5% and median TTP and OS were 10.7 and 14.7 months. Maintenance treatment with capecitabine increased TTP and OS in nonprogressing patients after mDCF. Twenty-five percent of the patients who did not have progressive disease after the 1st-line mDCF treatment received capecitabine maintenance treatment. Grade ¾ toxicity rates are lower in mDCF-treated patients compared to parental DCF regimen [4]. However primary prophylaxis of GCSF was routinely given after each cycle of mDCF treatment in this protocol.
mDCF treatment was given biweekly in this protocol, and chemotherapy dose intensity was different from the parental protocol (Table 2). When compared, weekly doses of the chemotherapy drugs with parental DCF regimen leucovorin were added to the protocol with 200 mg/m2 on day 1 and docetaxel dose was 5 mg/m2 higher than the standard DCF regimen [4]. Weekly dose intensity of 5-fluorouracil was higher than the DCF regimen as 150 mg/m2. Cisplatin dose was the same as the parental DCF regimen.
In the phase 3 V325 study, mean TTP and OS increased to 5.6 and 9.2 months with the addition of docetaxel to cisplatin plus 5-fluorouracil in the expense of 69% grade 3–4 toxicities and 10% treatment-related deaths [4]. Overall response rate was higher in our study compared to standard treatment regimen (47.5 vs 37%). Median TTP (10.7 vs 5.6 months) and OS (14.7 vs 9.2 months) were longer in mDCF-treated patients of our study compared to standard DCF study [4].
All grade 3–4 toxicity and neutropenia rates were higher in standard DCF protocol compared to mDCF regimen in our study (70% vs 50% and 81 vs 27.5%, respectively) [4]. However, primary GCSF prophylaxis was given to all patients in our study. Neutropenic fever rate was also lower in our study compared to V325 study (29 vs 10%). There are a number of mDCF studies which used different regimens in the literature. Twenty-four mDCF studies were analyzed in a systematic review by Petrelli et al. [10]. In the study by Petrelli et al., toxic deaths in mDCF studies were reported 1.2 to 5.7%. Toxic death was seen in one patient (2.5%) in our study. Primary prophylaxis with GCSF was reported in only 3 of 24 studies and GCSF was used in 16 of 24 studies. Median grade 3/4 neutropenia, diarrhea, and neurotoxicity were reported as 29.9%, 8.9%, and 9.9% in the studies of mDCF. Grade 3/4 neurotoxicity was seen as 7.5%, and grade 3/4 diarrhea was not seen in our study. Dose reductions were made in 19 of 24 studies and ranged from 38 to 80%. Dose reductions were made 27.5% in our study.
The duration of first-line treatment in responding patients with MGC is not clear. In general, treatment is given until disease progression or untolerable toxicity. Median treatment cycles were 6 in the V325 DCF study and median treatment cycles were 9 in our study [4]. Median number of treatment cycles was 6 in the first-line mDCF studies in MGC [10].
Thirteen of 24 studies were with reduced doses of 3 weekly schedule and 11 studies were splitted with weekly or two weekly schedules in the systematic review of mDCF regimens by Petrelli et al. [10]. Median ORR was 49% (%95 CI: 43.4–54.4), median TTP (or PFS) was 7.2 months (%95 CI: 5.9–8.8), and median OS was 12.3 months (%95 CI: 10.6–14.3). Median TTP and OS were longer in weekly and biweekly regimens compared to three weekly regimens (7.8 vs 6.7 and 13 vs 11.8 months). However, the ORRs were similar with three weekly and weekly or biweekly regimens (50.2 vs 47.5%, respectively). Hematological and nonhematological toxicity rates could not be compared between the three weekly and biweekly mDCF regimens due to heterogeneity of the dose reduction and prophylactic treatment approaches. Neutropenia and infection rates might be decreased with primary prophylaxis of GCSF in those mDCF regimens. However, primary prophylaxis was given in only 3 studies of mDCF in the literature [14,15,16]. Grade 3/4 neutropenia rates were as low as 20, 22.5, and 37.5% in those studies.
Median TTP was longer in our study than the median of other mDCF regimens analyzed in systematic review by Petrelli et al. (10.7 vs 7.2 months) [10]. One of the reasons for that longer TTP was maintenance capecitabine treatment given in nonprogressing and good-performance status patients (25%). The TTP and OS were longer in the patients treated with capecitabine maintenance compared to nontreated patients in our study (15.87 vs 7.26 and 24.94 vs 10.85 months, respectively). Median duration of treatment with capecitabine was 9.97 ± 7.56 months (min–max: 1–15.9 months).
Maintenance treatment is not a standard approach in MGC. However, in our study, it was recommended to the patients who did not have progressive disease under mDCF treatment and have no grade 3/4 toxicity and willing to have maintenance treatment with the aim of prolonging TTP. In a phase 2 study, maintenance with capecitabine after the first-line DCF treatment by Oyan et al. showed median TTP and OS of 10.4 and 20.3 months [17]. The median number of maintenance capecitabine cycles was 5, and 2-year survival rate was reported as 26%. Median duration of capecitabine treatment in our study was 10 months. In the phase 2 study by Qiu et al., they reported longer PFS with capecitabine maintenance compared to observation (11.4 vs 7.1 months) after the first-line capecitabine plus oxaliplatin in MGC patients who suffered from neurotoxicity (grade 2 or higher) and were without progressive disease [18]. Results of the phase 2 randomized MATEO study are awaited, which explore the efficacy and toxicity of S-1 maintenance after 12 weeks of platinum plus fluoropyrimidine treatment in advanced esophageal and gastric cancer [19].
Wang et al. tested their own mDCF regimen in a phase 3 study in comparison with CF (cisplatin at 75 mg/m2 (day 1) followed by fluorouracil at 600 mg/m2/day (days 1–5, CF) every 3 weeks) in Chinese first-line MGC patients. mDCF regimen was defined as docetaxel and cisplatin at 60 mg/m2 (day 1) followed by fluorouracil at 600 mg/m2/day (days 1–5) every 3 weeks [9]. The primary endpoint of the study was PFS. There were 243 patients in that one to one randomized study and PFS was longer in mDCF compared to CF arm (7.2 and 4.9 months, respectively; HR: 0.58, p = 0.0008). Median duration of mDCF treatment was 17 weeks (range 3–46 weeks). The median OS was 10.2 and 8.5 months, in mDCF and CF arms, respectively (HR = 0.71, P = 0.0319). The ORR was improved to 48.7% with the mDCF regimen versus 33.9% with CF (P = 0.0244). But grade 3 and 4 treatment-related adverse events increased from 46.1% in CF-treated patients to 77.3% in mDCF-treated patients (p < 0.001). The most frequent grade 3–4 toxicity was neutropenia (60.5%) in mDCF arm. Febrile neutropenia and diarrhea were the other frequent grade 3–4 toxicities which occurred in 12.6 of the patients treated with mDCF. Our regimen was different from the one by Wang et al. [9]. Chemotherapy doses were higher and treatment was given biweekly in our regimen. Primary prophylaxis with GCSF was also given in our study. The ORR was similar (47.5 vs 48.7) with those different mDCF regimens in our study and the study by Wang et al. But TTP (10.8 months) and OS (14.7 months) were longer in our study and grade 3–4 overall (50%) and hematologic (32.5%) toxicity rates were also less frequent compared to the study results by Wang et al. [9].
Shah et al. studied a mDCF regimen in a phase 2 randomized fashion in comparison with standard parental DCF regimen [20]. They have defined mDCF regimen as follows: fluorouracil 2000 mg/m2 iv over 48-h infusion, docetaxel 40 mg/m2 iv on day 1, cisplatin 40 mg/m2 iv on day 3, every 2 weeks. They enrolled 85 patients into the study, and 54 patients were treated with mDCF regimen. Grade 3–4 toxicity rates were reported as 76% in mDCF regimen. Six-month PFS rate was higher in mDCF compared to standard DCF arm (63%; 95% CI, 48 to 75% vs 53%; 95% CI, 34 to 69%, respectively). Median OS was also improved with mDCF treatment (18.8 vs 12.6 months; p = 0.007). Dose density of mDCF regimen in the study by Shah et al. is lower compared to our mDCF regimen [20]. However, toxicity rates were higher in this study despite lower doses. Lower toxicity and especially hematological toxicity rates in our study might be related with routine primary GCSF prophylaxis in the protocol.
In a modified protocol, three weekly DCF with reduced doses of chemotherapy agents was compared with standard DCF regimen in a phase 2 study of the first-line MGC treatment by Inal et al. [21]. The ORR was similar 46.7% vs 45.8%; PFS and OS were longer in standard DCF arm compared to mDCF arm (7.4 vs 6.5 and 9.9 vs 8.6, respectively). Grade 3/4 neutropenia was higher in standard DCF arm compared to mDCF arm (44.7% vs 13.6%). Kos et al. reported their mDCF treatment results (N: 40) compared to cisplatin plus 5-fluorouracil plus leucovorin in a retrospective phase 2 study in the first-line treatment of MGC patients [22]. Median follow-up time was 10.3 months in this study. The ORR reported as 30%; mean PFS was 6.2 and OS was 8.7 months in mDCF-treated MGC patients. Grade 3/4 neutropenia was seen in 20% of the patients. ORR rate was lower and PFS and OS were shorter compared to our study results mainly attributable to increased dose density and intensity in our protocol. However, grade 3/4 neutropenia rate was lower compared to our study (20% vs 27.5%) might be related with the same factor.
The same mDCF regimen in our study was tested by Koca et al. and Unek et al. in phase 2 studies in MGC patients [15, 16]. In the study by Koca et al., median follow-up time was short as 8.6 months, and median number of treatment cycles given was six [15]. The ORR rate was 43.7%; PFS and OS were 7 and 11 months. In the study by Unek et al., median follow-up time was not given but median number of chemotherapy cycles given was 10 [16]. The ORR was 41.4%; PFS and OS were 9 and 10.8 months [16].
Grade 3/4 neutropenia was seen in 20% of the patients in the study by Koca et al. and in 37.1% of the patients in the study by Unek et al. [15, 16]. Primary prophylaxis with GCSF was given in both studies. Primary prophylaxis with GCSF reduces grade 3/4 neutropenia rates as in our study (27.5%) and must be taken into consideration when giving three drug combinations in the treatment of MGC.
Median follow-up time, PFS and OS in the study by Koca et al., and PFS and OS durations in the study by Unek et al. were shorter compared to our study [15, 16]. However, ORR rates were similar in those 2 studies with our study. Maintenance capecitabine treatment was differently given in our study and might be related with higher TTP and OS durations compared to the results by Koca and Unek [15, 16].
The second-line treatment was given to 30% of the patients and mostly was irinotecan based in the study by Unek et al. Twenty-five percent of the patients received second-line treatment with FOLFIRI in our study.
FLOT is another biweekly three drug combination alternative regimen for the first-line treatment of MGC [13]. Fifty-nine MGC patients were treated with FLOT in this phase 2 study. The ORR was 57.7%, median PFS was 5.7, and median OS was 11.1 months. FLOT also became a standard regimen in last few years in the perioperative setting of gastric cancer [23]. Eight cycles of treatment is standard in perioperative setting with grade 3/4 neutropenia, infection, and neuropathy of 51%, 18%, and 7%. However, in metastatic setting, 8 cycles of treatment might be inadequate for control of metastatic disease and grade 3/4 toxicities might be a bigger problem with additional cycles of FLOT in this setting. Cumulative neurotoxicity related with docetaxel and oxaliplatin might be the rate-limiting toxicity and might not let additional cycles of FLOT in metastatic setting. Oxaliplatin has a higher neurotoxicity potential than cisplatin, and mDCF regimen might be more tolerable compared to FLOT regarding cumulative neurotoxicity rate in the treatment of metastatic disease. In our study, grade 3/4 neurotoxicity rate was 7.5% despite median of 9 cycles (max: 23 cycles) of mDCF treatment. However, ORR rate was lower (47.5% vs 57.7%) and TTP (10.7 vs 5.7 months) and OS (14.7 vs 11.1 months) were longer in our study compared to FLOT regimen in metastatic setting [13]. However, capecitabine maintenance was given 25% of the patients in our study.
In conclusion, little progress has been made in the first-line treatment of MGC in patients without HER-2 amplification, since DCF combination regimen was established in 2006. Toxicity is a major concern with standard DCF and with another triplet regimen FLOT; however, efficacy results are quite acceptable. Thus, modified regimens of DCF are still among the best alternative choices regarding lower toxicity and higher efficacy rates. Primary GCSF prophylaxis must be routinely considered in these triplet regimens however they are modified. Primary GCSF prophylaxis might decrease grade 3/4 neutropenia and infection rates. Maintenance capecitabine might prolong time to progression in advanced gastric cancer patients who did not have progressive disease and willing to take such treatment. Our regimen of mDCF is quite effective and with low toxicity rates compared to modified protocols of DCF in the literature, standard DCF, and FLOT regimens. Cumulative neurotoxicity potential might be lower with mDCF regimen compared to FLOT in metastatic setting of gastric cancer.
Data availability
The authors declare that all data and material are available.
Code availability
Not applicable.
References
Ferlay J, Soerjomataram I, Dikshit R et al (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136:E359–E386
Wagner AD, Syn NL, Moehler M, et al. Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev. 8: CD004064, 2017.
Kang YK, Kang WK, Shin DB et al (2009) Capecitabine/cisplatin versus 5-fluorouracil/cisplatin as first-line therapy in patients with advanced gastric cancer: a randomised phase III noninferiority trial. Ann Oncol 20:666–673
Van Cutsem E, Moiseyenko VM, Tjulandin S et al (2006) Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol 24:4991–4997
Ross P, Nicolson M, Cunningham D et al (2002) Prospective randomized trial comparing mitomycin, cisplatin, and protracted venous-infusion fluorouracil (PVI 5-FU) With epirubicin, cisplatin, and PVI 5-FU in advanced esophagogastric cancer. J Clin Oncol 20:1996–2004
Cunningham D, Starling N, Rao S et al (2008) Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med 358:36–46
Bang YJ, Van Cutsem E, Feyereislova A et al (2010) Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 376:687–697
Ohtsu A, Shah MA, Van Cutsem E et al (2011) Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. J Clin Oncol 29:3968–3976
Wang J, Xu R, Li J et al (2016) Randomized multicenter phase III study of a modified docetaxel and cisplatin plus fluorouracil regimen compared with cisplatin and fluorouracil as first-line therapy for advanced or locally recurrent gastric cancer. Gastric Cancer 19:234–244
Petrelli F, Tomasello G, Ghidini M et al (2017) Modified schedules of DCF chemotherapy for advanced gastric cancer: a systematic review of efficacy and toxicity. Anticancer Drugs 28:133–141
Eisenhauer EA, Therasse P, Bogaerts J, et al New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–47, 2009.
Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. Published on May 2009. https://www.eortc.be/services/doc/ctc/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf (Access date 16.11.2019)
Al-Batran SE, Hartmann JT, Hofheinz R et al (2008) Biweekly fluorouracil, leucovorin, oxaliplatin, and docetaxel (FLOT) for patients with metastatic adenocarcinoma of the stomach or esophagogastric junction: a phase II trial of the Arbeitsgemeinschaft Internistische Onkologie. Ann Oncol 19:1882–1887
Tomasello G, Liguigli W, Poli R et al (2014) Efficacy and tolerability of chemotherapy with modified dose-dense TCF regimen (TCF-dd) in locally advanced or metastatic gastric cancer: final results of a phase II trial. Gastric Cancer 17:711–717
Koca D, Dogan E, Yardim H et al (2013) A modified DCF regimen as primary treatment for patients with metastatic gastric cancer. J BUON 18:377–384
Unek IT, Akman T, Oztop I et al (2013) Bimonthly regimen of high-dose leucovorin, infusional 5-fluorouracil, docetaxel, and cisplatin (modified DCF) in advanced gastric adenocarcinoma. Gastric Cancer 16:428–434
Oyan B, Eren OO, Sonmez O. Capecitabine maintenance after first-line induction chemotherapy with docetaxel, cisplatin, and fluorouracil in patients with advanced gastric cancer. J Clin Oncol 32, 2014 (suppl; abstr e15003)
Qiu MZ, Wei XL, Zhang DS et al (2014) Efficacy and safety of capecitabine as maintenance treatment after first-line chemotherapy using oxaliplatin and capecitabine in advanced gastric adenocarcinoma patients: a prospective observation. Tumour Biol 35:4369–4375
Haag GM, Stocker G, Quidde J et al (2017) Randomized controlled trial of S-1 maintenance therapy in metastatic esophagogastric cancer - the multinational MATEO study. BMC Cancer 17:509
Shah MA, Janjigian YY, Stoller R et al (2015) Randomized multicenter phase II study of modified docetaxel, cisplatin, and fluorouracil (DCF) versus DCF plus growth factor support in patients with metastatic gastric adenocarcinoma: a study of the US Gastric Cancer Consortium. J Clin Oncol 33:3874–3879
Inal A, Kaplan MA, Kucukoner M, Isikdogan A (2012) Docetaxel and cisplatin plus fluorouracil compared with modified docetaxel, cisplatin, and 5-fluorouracil as first-line therapy for advanced gastric cancer: a retrospective analysis of single institution. Neoplasma 59:233–236
Kos FT, Uncu D, Ozdemir N et al (2011) Comparison of cisplatin-5-fluorouracil-folinic acid versus modified docetaxel-cisplatin-5-fluorouracil regimens in the first-line treatment of metastatic gastric cancer. Chemotherapy 57:230–235
Al-Batran SE, Pauligk C, Homann N, et al. LBA-008 Docetaxel, oxaliplatin, and fluorouracil/leucovorin (FLOT) versus epirubicin, cisplatin, and fluorouracil or capecitabine (ECF/ECX) as perioperative treatment of resectable gastric or gastro-esophageal junction adenocarcinoma: the multicenter, randomized phase 3 FLOT4 trial (German Gastric Group at AIO). Ann Oncol. 28 (suppl_3), 2017. https://doi.org/10.1093/annonc/mdx302.007
Author information
Authors and Affiliations
Contributions
C.A. and F.D.A. wrote and edited the manuscript.
Corresponding author
Ethics declarations
Ethics approval
Ethical approval date: 13 February 2019, ethical approval no: 2019/2–3.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Arslan, C., Atilla, F.D. Modified docetaxel, cisplatin, and 5-fluorouracil combination regimen and capecitabine maintenance in metastatic gastric cancer: toxicity and efficacy results. Support Care Cancer 30, 4447–4455 (2022). https://doi.org/10.1007/s00520-022-06859-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-022-06859-0