Abstract
Chemotherapy has been associated with increased mitochondrial reactive oxygen species production, mitochondrial dysfunction and skeletal muscle atrophy leading to severe patient clinical complications including skeletal muscle fatigue, insulin resistance and wasting. The exact mechanisms behind this skeletal muscle toxicity are largely unknown, and as such co-therapies to attenuate chemotherapy-induced side effects are lacking. Here, we review the current literature describing the clinical manifestations and molecular origins of chemotherapy-induced myopathy with a focus on the mitochondria as the target organelle via which chemotherapeutic agents establish toxicity. We explore the likely mechanisms through which myopathy is induced, using the anthracycline doxorubicin, and the platinum-based alkylating agent oxaliplatin, as examples. Finally, we recommend directions for future research and outline the potential significance of these proposed directions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer is a leading cause of morbidity and mortality worldwide, with approximately 14 million new cases diagnosed and 8.2 million cancer related deaths occurring in 2012 alone [1]. In most cases, the first-line anti-neoplastic treatment option is systemic chemotherapy administration, in which cells undergoing rapid, abnormal division (such as cancer cells) are successfully targeted for the chemical induction of autologous cell death pathways secondary to DNA damage [2], thereby reducing the size of, or completely abolishing, tumours [3]. However, due to their non-specific mode of action, chemotherapeutic drugs elicit significant side effects by also targeting healthy cells that maintain high proliferative potential throughout the lifespan. These include cells of the integumentary, immune, nervous, and gastrointestinal systems, which induce the notorious side effects associated with chemotherapy treatment including nausea, vomiting, hair and weight loss, and fatigue [4–6].
Much less characterised, however, is the effect of chemotherapeutic agents on the muscular system—in particular the skeletal musculature, which relies on mitotic activity to maintain “skeletal muscle turnover” and mass throughout the lifespan. Skeletal muscle also has a theoretically high propensity for DNA-mediated toxicity due to its dense nucleation compared to other cells [7]. While skeletal muscle wasting and dysfunction due to cancerous cells and the inflammatory cytokines they release (commonly termed cancer cachexia) is well documented [4], little is known about the effect chemotherapeutic agents have on the skeletal musculature. Emerging research indicates that long-term effects persist in skeletal muscle for many years after chemotherapy treatment and that they are independent of those induced by cancer cachexia [8–10]. These adverse effects include skeletal muscle atrophy, dysfunction, insulin resistance, weakness, and fatigue, which, in addition to a multitude of side effects in other organ systems, leads to poor tolerance and treatment discontinuation and limits therapeutic efficacy [5]. Skeletal muscle-specific co-morbidities correlate with a range of negative clinical outcomes in cancer patients—these include reduced participation in activities of daily living, quality of life, and a higher risk of morbidity and mortality [11].
Mitochondria are increasingly emerging as key players in the pathogenesis of a variety of diseases. Due to the highly metabolic nature of the skeletal muscle, mitochondrial density is also high [12], and mitochondrial dysfunction and toxicity can therefore manifest as skeletal muscle-specific symptomatology which include fatigue, muscle wasting, impaired regenerative capacity, pain, exercise intolerance, and sometimes, mild-to-severe neurological symptoms. Indeed, these symptoms have been well documented in chemotherapy-treated cancer patients [5, 13–15] suggesting that anti-cancer chemotherapy may be non-specifically targeting the skeletal musculature, and perhaps even more specifically, the mitochondria to induce a variety of persistent adverse side effects.
Here, we review the current literature describing the clinical manifestations and molecular origins of chemotherapy-induced myopathy with a focus on the mitochondria as the target organelle via which chemotherapeutic agents establish toxicity. We explore the likely mechanisms through which myopathy is induced, using the anthracycline doxorubicin, and the platinum-based alkylating agent oxaliplatin as examples. Finally, we recommend directions for future research and outline the potential significance of these proposed directions with respect to the development of suitable musculoskeletal protective treatment regimes.
Potential mechanisms of chemotherapy-induced mitochondrial myopathy
Chemotherapy drugs target rapidly dividing mitotic cells to arrest specific phases of the cell cycle, with the exact mode of action varying between drugs and the chemical classes from which they derive (as summarised in Table 1)—for this reason, specific agents are used to target specific neoplasms. The anthracycline family, for example, has been used for over 50 years to treat a number of different cancers including leukaemia, prostate, ovarian, lung and breast [16, 17], with doxorubicin hydrochloride (Adriamycin®)—an anthracycline with limited therapeutic tolerability and efficacy due to its highly toxic effects on the heart—used primarily to treat solid tumours [18, 19]. A number of mechanisms of action have been proposed to explain the neoplastic cytotoxic and cytostatic nature of doxorubicin, which include: (1) DNA intercalation thus inhibiting protein biosynthesis and affecting transcription processes [20, 21]; (2) free radical formation resulting in cellular damage and apoptosis signalling and/or necrosis [22, 23]; (3) inhibition of topoisomerase II [24], an important nuclear DNA transcription enzyme; and (4) intrinsic mitochondrial apoptotic signalling [25]. As doxorubicin targets DNA as its primary cytotoxic action, it has also been hypothesised that the circular and covalently closed nature of mtDNA allows easier intercalation of chemotherapies, and thus an increased rate of transcriptional error occurs leading to mitochondrial dysfunction [25].
Mitochondrial function, and perhaps even more so, mitochondrial dysfunction, is physiologically complex and is modulated by a variety of regulators including the mitochondrial (mtDNA) and nuclear (nDNA) DNA, reactive oxygen species (ROS), nuclear and cellular signalling molecules and ATP production amongst others [26, 27]. In the first instance, chemotherapy-induced mitochondrial dysfunction has been associated with elevated levels of mitochondrial ROS (mtROS). It is well established that doxorubicin treatment causes increased ROS production as a by-product of its metabolism via a redox cycling process unique to the anthracycline class of chemotherapeutics [28–32]. Doxorubicin, which has a high affinity for the inner mitochondrial membrane (IMM) [33], accumulates on the matrix side and undergoes a single-electron reduction process at complex I (NADH oxidase) of the electron transport chain (ETC) removing electrons vital to ATP production. This process forms the free radical semiquinone species that reduces molecular oxygen to produce the highly reactive superoxide (\({\text{O}}_{2}^{ - }\)) molecule and subsequently the less reactive hydrogen peroxide (H2O2) molecule [17, 28, 32, 34, 35]. \({\text{O}}_{2}^{ - }\) and H2O2 collectively constitute the mtROS, which directly increase the state of cellular oxidative stress if not buffered effectively by endogenous antioxidants [33, 34, 36, 37]. Thus, doxorubicin acts via a two-hit mode of action on the mitochondria acting as a powerful reducer when stable, depleting ATP production and available ATP stores, and as an efficient oxidiser in its semiquinone state, producing excess mtROS.
In addition to decreasing electron flow through the ETC and thus decreasing ATP production, the single-strand DNA breaks induced by doxorubicin (and indeed other chemotherapies that directly damage DNA) induces the activation of enzymes that repair such damage albeit to the detriment of ATP stores. Poly-ADP-ribose polymerases (PARPs) are highly conserved proteins that respond to DNA damage by stimulating repair through the use of energy co-factors, in particular, the use of NAD+ (a key mitochondrial substrate) by PARP-1 [38]. Within minutes of PARP-1 activation, the NAD+ pool depletes by up to 20 % with the cell required to replenish this loss through ATP consumption, further exacerbating inner mitochondrial membrane potential (ΔΨ) depletion and energy homeostasis perturbation [39, 40]. Additionally, the depleted NAD+ pool impacts upon other metabolic pathways including glycolysis and the tricarboxylic acid cycle, of which various steps are dependent upon NAD+ availability [41, 42] which culminates in decreased substrate delivery to, and ATP synthesis at, the ETC (refer to Fig. 1). Considering that skeletal muscle is a highly metabolic organ, such reductions in the capacity to generate ATP would be undesirable and ultimately lead to functional perturbations. While skeletal muscle could tolerate acute PARP activity, the chronic activation of PARP following repeated systemic exposure would be detrimental and energetically expensive to skeletal muscle [41–43].
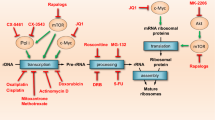
Effects of chemotherapeutic agents on mitochondria and the promotion of skeletal muscle wasting. (A) The metabolism of anthracycline chemotherapies occurs at Complex I of the electron transport chain (ETC) within the mitochondria. The anthracycline molecule is reduced by Complex I, removing vital electrons from the ETC and transforming the anthracycline into its oxidative semiquinone, which reduces molecular oxygen to superoxide. This final reduction process returns the anthracycline to its non-semiquinone state. (B) Chemotherapy-induced nuclear DNA (nDNA) damage stimulates PARP-1 activity, which consumes NAD+ (a vital mitochondrial substrate) to repair the nDNA damage. In doing so, the NAD+ pool is rapidly depleted. This loss of NAD+ negatively effects ATP production as well as negatively impacting various metabolic pathways including glycolysis and TCA cycle. (C) While the precise mechanism of oxaliplatin toxicity is unknown, likely mechanisms are intercalation of the platinum derivative into the mtDNA and the ETC complexes, and depletion of the mitochondrial Cu2 + pool. (D) mtDNA encodes for multiple components of the ETC and as such damage to the mtDNA through chemotherapy treatment and increased ROS levels perpetuates a positive-feedback loop of damage and dysfunction to the mtDNA and cellular components of the mitochondria. (E) mtROS can oxidise the mitochondrial membranes and damage the proteins of the ETC resulting in electron leak and an increase in mtROS production. As ROS levels increase within the cell, they upregulate atrophic pathways leading to muscle cell degradation, necrosis due to oxidative damage, autophagy and macroautophagy. (F) As a result of ROS-induced (and possibly chemotherapy-induced) mitochondrial dysfunction and mtDNA damage, mitophagic pathways are stimulated in order to curb the number of dysfunctional and mutated mitochondria within the total mitochondrial pool. As dysfunctional mitochondria are destroyed, the capacity of the mitochondrial pool to produce ATP is reduced resulting in depletion of the cellular ATP pool and thus induction of various autophagic, necrotic and apoptotic pathways
Although PARP is classified as a nuclear protein, the discovery of a truncated mitochondrial PARP-1 suggests that PARP-1 has a direct effect on mitochondrial respiration potentially through the PARylation of mitochondrial proteins [43, 44]. In addition, PARP activation leads to reduced SIRT1 activity, mitochondrial biogenesis, and glucose clearance, and shifts skeletal muscle from the oxidative fibre type [38, 45]. Together, these metabolic changes would promote further metabolic dysfunction in chemotherapy-treated skeletal muscle. As deletion of PARP-1/2 in mice demonstrably reverses metabolic suppression by promoting mitochondrial biogenesis, improving the oxidation of fats and enhancing appetite [38, 45, 46], the pharmacological inhibition of PARPs may assist in maintaining the NAD+ pool and preventing metabolic compromise during chemotherapy.
As previously mentioned, an increase in chemotherapy-induced mtROS production is strongly linked to mitochondrial dysfunction and damage. However, mtROS are also thought to function as signalling molecules that activate several proteolytic pathways within skeletal muscle, including caspase-3 and calpain [33, 47–49]. These pathways in-turn catalyse the release of myofilament proteins, allowing activation of the ubiquitin–proteasome system and resulting in skeletal muscle degradation [49–51]. Activation of the ATP-dependent ubiquitin–proteasome system is responsible for the muscular degradation seen in homeostatic regulation of skeletal muscle mass and is amplified in many chronic diseases including cancer cachexia and diabetes [52–54]. Thus, doxorubicin-induced skeletal muscle atrophy is strongly associated with mitochondrial dysfunction. This dysfunction is a direct result of increased ROS production via drug metabolism as well as that due to non-specific electron leak from the mitochondrial respiratory chain which is likely induced by mtDNA and respiratory chain protein damage. These negative effects have been the basis of several investigations into chemotherapy-induced myopathies. Adachi et al. [55] have demonstrated strong evidence that the prevalence of mtDNA deletions increases with doxorubicin dosage, and exponentially more so with long-term exposure. In cardiomyocytes, mtDNA deletions could be prevented with co-therapy of the antioxidant and electron carrier coenzyme Q10 [55], suggesting that the aetiology of mtDNA mutation is via doxorubicin-induced mtROS rather than the doxorubicin semiquinone itself.
Long-term doxorubicin treatment induces significant reductions in skeletal muscle mass, strength, and endurance in cancer survivors (for detailed review, refer to [10]). Scheede-Bergdahl et al. [10] postulated that the molecular basis of these effects was due to the progressive amplification and proliferation of mtDNA mutations. Gouspillou et al. [56] have also demonstrated a reduction in muscle mass and function—thought to be due to an increase in mtROS and a reduction in mitochondrial respiration—in female C57BL/6 mice when treated with four cycles of doxorubicin (with one cycle equivalent to two 10 mg/kg doses on days one and five with 3 weeks recovery). However, no evidence of mtDNA damage post-doxorubicin therapy (as detected by long range PCR) was found [10].
Another effective class of chemotherapeutics are the platinum-derived alkylating agents, of which oxaliplatin is used predominantly for the treatment of colorectal cancer. Oxaliplatin exerts its antibiotic effects by forming platinum–DNA adducts which efficiently block DNA replication forcing cell cycle arrest and ultimately apoptosis in mitotic cells [57–59]. Both doxorubicin and oxaliplatin—although differing in their precise modes of action—have been shown to negatively affect mitochondrial function [22] and to induce deleterious effects on skeletal muscle that clinically manifest as muscle weakness [5, 17, 60] (refer to Fig. 2). Gourdier et al. [61] demonstrated that oxaliplatin treatment induces mitochondrial and energy homeostasis dysregulation in colorectal cancer cells, potentially through the direct mutation of mtDNA or via mutation of the nuclear-encoded mitochondrial proteins (refer to Fig. 1). While this effect is of obvious benefit to the induction of cell death pathways in neoplastic cells, suppression of mitochondrial function and the disruption of energy homeostasis would have detrimental consequences to somatic cells, especially in highly metabolic tissues such as the skeletal muscle. Our group has recently demonstrated that oxaliplatin treatment increases mtROS production, and reduces mitochondrial and cell viability in an in vitro C2C12 myotube culture model [22]. Oxaliplatin seems to exert immediate but reversible inhibition of key respiratory enzymes, as shown by induction of a metabolic shift towards an anaerobic glycolytic phenotype following acute administration [22]. We speculate that this phenotype shift occurs to buffer the acute suppression of respiratory function that occurs during the transport of oxaliplatin into skeletal muscle, and more specifically, the mitochondria. We speculate that oxaliplatin, specifically the platinum component, is competitively substituted for copper (Cu2 +) at receptor sites on the copper transporter 1 (CT1), limiting the availability of the transporter to Cu2 + and thereby reducing the mitochondrial Cu2 + pool, which is essential for normal complex IV function and oxidative phosphorylation. A study by Lutsenko et al. [62] suggests that the mitochondrial Cu2 + transporter, COX17, transports Cu2+ into the mitochondria and, with the assistance of Sco proteins, incorporates the Cu2 + molecule into complex IV. Thus in addition to, or instead of, reducing the mitochondrial Cu2 + pool, it is possible that the entire oxaliplatin molecule is incorporated into complex IV with the potential to induce malfunction of electron flow and acceptance by molecular oxygen. An acute effect of oxaliplatin administration thus seems to be inhibition of the mitochondrial respiratory chain.
Hypothetical model of chemotherapy-induced myopathy in skeletal muscle. a Chemotherapy is delivered to skeletal muscle which detrimentally effects nuclear DNA and potentially mtDNA. b Chemotherapy induces mitochondrial dysfunction resulting in increased mtROS production leading to damage of the skeletal muscle. c Damage sustained to the nuclear DNA is exemplified during mitosis causing a failure of satellite cell replication, and therefore, of regeneration mechanisms. d Long-term chemotherapy treatment results in progressive skeletal muscle damage and dysfunction due to blunted repair mechanisms
The chronic effects of oxaliplatin treatment, however, seem intrinsically related to mtDNA damage and mutation resulting in gene polymorphisms as per the single-stranded breaks induced in nuclear DNA, rather than due to compounding effects of acute respiratory chain inhibition. As mtDNA encodes for the matrix-residing components of the respiratory chain complexes which are responsible for proton pumping and initial electron transfer, a natural consequence of such damage would be reduced mitochondrial function and increased mtROS production leading to skeletal muscle atrophy, damage and wasting (refer to Fig. 1). A recent study by Wisnovsky et al. [63] highlighted the capacity for oxaliplatin to induce single-stranded breaks in the mtDNA. The group isolated the nuclear DNA damaging component of oxaliplatin and conjugated it with the N terminus of a mitochondrial-penetrating peptide (mPP). When delivered to ovarian cancer lines, the oxaliplatin-mPP molecule localised solely within the mitochondria and induced mtDNA mutation followed by mitochondrial death and the induction of cellular apoptosis. Although Wisnovsky et al.’s data [63] show that oxaliplatin is capable of causing mtDNA damage, the group failed to establish that oxaliplatin was able to independently penetrate the mitochondria in its natural form. Studies currently being undertaken by our laboratory have shown interesting results in this area. Our preliminary data shows that oxaliplatin-induced platinum–DNA adducts are present within neuronal mitochondrial fractions in addition to the nuclear fraction, both in a cell culture model and following systemic oxaliplatin treatment of BalbC mice [64]. We are currently investigating whether the same is true for skeletal muscle.
While we are yet to determine the precise mechanisms of oxaliplatin toxicity in skeletal muscle, our data suggests that in addition to the anthracyclines, chemotherapeutic agents from other drug classes that do not necessarily induce mtROS formation as a consequence of drug metabolism (i.e. the alkylating platinum-based chemotherapies) are also detrimental to mitochondrial function and myofiber survivability. The molecular mechanisms underlying doxorubicin toxicity in skeletal muscle and the consequential repercussions on physiological function are being increasingly documented [23, 28, 33, 65] and have established that mitochondrial dysfunction and heightened ROS production are key players. However, skeletal myopathy is a common side effect of chemotherapy exposure across all drug classes, and thus, if it has a mitochondrial origin, the initial defect seems not to be intrinsically associated with a particular mode of drug action, i.e. DNA damage versus inhibition of DNA replication. Indeed, our preliminary data in a myotube culture model indicate that both increased mitochondrial ROS production and reduced mitochondrial pool viability are consequences of treatment with chemotherapies from various drug classes including the anti-metabolite (5-fluorouracil) and topoisomerase inhibitor (irinotecan) families [22]; however, we did not observe functional deficits in myotubular mitochondrial function following exposure to these drugs as per doxorubicin and oxaliplatin. This highlights that there are both similarities and differences in the precise effects different chemotherapy agents have at the mitochondrial level and warrants further investigation. Indeed, since doxorubicin is the only chemotherapy agent that has been even moderately characterised in the literature with respect to the skeletal muscular system, there is an immediate need for the investigation of all chemotherapeutic agents in current clinical use and whether their toxic effects induce similar levels of myopathy, such that appropriate therapies can be devised to address them.
Consequences of mitochondrial dysfunction and ROS production on skeletal muscle mass
A number of recent studies investigating the molecular origin of skeletal muscle atrophy in various diseases/conditions have concluded that atrophy is almost always preceded in the first instance by increased levels of mtROS [66–70]. In a 2015 review, Sena et al. [71] outlined that mtROS production is a tightly regulated cell signalling pathway that, when excessive, induces mitochondrial and cellular protein damage thus leading to autologous mitochondrial destruction. Termed as mitophagy, this type of targeted autophagy is promoted in an attempt to attenuate elevated mtROS production by stressed mitochondria, which would otherwise inevitably induce oxidative damage, cellular energy depletion, and apoptotic/necrotic cell death. Attaix and Taillandier [72] have demonstrated that skeletal muscle mitophagy, regardless of cause, is a potent inducer of skeletal muscle wasting. Thus our hypothesis that chemotherapeutic agents (irrespective of the chemical class from which they derive) promote skeletal muscle atrophy and wasting via a mtROS/mutation-dependent mechanism seems pertinent—especially since we have demonstrated elevated mtROS levels following exposure to chemotherapy agents from a variety of drug classes [22, 73]. Indeed, Gilliam et al. [33] have shown that chemotherapy (doxorubicin) treatment causes an immediate increase (16 h post-treatment) in the established upstream muscular atrophy regulators, E3 ubiquitin ligase and Atrogin-1/MAFbx, in cardiomyocytes via a mtROS-dependent pathway. Although there is a lack of recent data on the effect mitochondrial dysfunction has on skeletal muscle atrophy, two studies have implicated ROS molecules in the induction of the FoxO family of transcription factors, which have been shown to upregulate Atrogin-1/MAFbx atrophic signals [74, 75] (for detailed review see Bonaldo and Sandri [76]).
In addition to direct modulation of atrophic signalling pathways, mtROS have the capability to oxidatively modify protein structures [71, 77]. As chemotherapeutic agents demonstrably increase mtROS production [22], it is rational to link the subsequent increase in mtROS concentration with the modification of mitochondrial as well as other cellular proteins. A study by Kurihara et al. [78] has linked excessive ROS production with dysfunctional mitochondrial respiratory proteins which perpetuated a positive-feedback cycle of increased mtROS production, respiratory chain defects, mtDNA deletion, and ultimately mitophagy. As mitochondrial dysfunction increases, cellular energy depletion occurs which, as demonstrated by Neel et al. [79], leads to macroautophagy within the skeletal muscle in an attempt to increase substrate availability to oxidative phosphorylation and restore energy homeostasis—albeit a futile effort in the event of respiratory chain inhibition and/or defects/dysfunction. As conclusively established by Argilés et al. [13], negative alterations in energy balance act as a potent stimulus of muscle atrophy. Furthermore, Maccarrone et al. [80] have implicated increased ROS levels with the propagation of lipoxygenases which induce structural defects within the cell leading to necrosis-induced cell death, with others [81] associating ROS-induced necrosis with organelle or plasma membrane modification. With these findings reinforcing a number of previous studies [69, 82, 83], targeting mitochondrial dysfunction to reduce mtROS production is a logical intervention point through which to attenuate the initiation of muscular atrophy, macroautophagy, necrosis, and apoptosis signalling pathways, all of which have been strongly associated with chemotherapy treatment [4, 37, 84, 85].
Clinical manifestations of chemotherapy-induced myopathy
Chemotherapy-induced skeletal myopathy is thought to manifest clinically as a variety of symptoms which include varying indices of muscle pain and weakness [86], exercise intolerance [87] and intense and persistent fatigue. Incidentally, these are common symptoms associated with various metabolic diseases with mitochondrial aetiology. Emerging data also describe various metabolic syndrome-like co-morbidities in testicular cancer patients following cisplatin-based chemotherapy treatment, in which acute insulin resistance, hyperlipidemia, and abdominal visceral and subcutaneous adiposity are observed [88]. Collectively, these symptomologies highlight a probable mitochondrial aetiology, with the systemic effects resulting from an insufficiency to effectively utilise energy substrates such as glucose (leading to insulin resistance) and fats (leading to hyperlipidemia and adiposity), and the skeletal muscle-specific effects resulting from a failure of intracellular energy synthesis and homeostasis regulation.
At the myocellular level, skeletal muscle fatigue—which has been traditionally researched following intense and/or prolonged exercise [89, 90]—is associated with significant alterations in the intracellular and extracellular ionic environment, concentration and functional ratios of intracellular metabolites, calcium sensitivity of the contractile apparatus, and the production of ROS [91–93]. Indeed, these perturbations result in pain, weakness, and exercise intolerance. Fatigue is a complex phenomenon that is influenced by a plethora of factors, both physical and psychological. True skeletal muscle fatigue has been defined as any decline in performance associated with muscle activity and is strongly influenced by perturbations in neural and myocellular function [5, 94]. In the first instance, skeletal muscle mass and function is strongly regulated by both the central and peripheral nervous system, and as such, chemotherapy-induced neuropathy would strongly promote skeletal myopathy [84]. Interestingly, chemotherapy-induced peripheral neuropathy is also associated with mitochondrial dysfunction and induces escalating myopathy and weakness as dosages increase [95]. In the second instance, skeletal muscle mass and function is strongly regulated by loading, thus central and/or psychological fatigue alongside extensive hospitalisation for anti-cancer treatment would promote disuse deconditioning and atrophy [96, 97]. In the third instance, muscle mass is positively correlated with nutritional status, and thus chemotherapy-induced dysregulation of gastrointestinal function and appetite alongside promotion of nutrient malabsorption (i.e. via vomiting and/or diarrhoea) [98] would reduce the nutritional status and promote skeletal muscle wasting. This highlights the multifactorial nature of chemotherapy-induced skeletal myopathy. While there is limited research to date that has examined the mechanisms through which non-anthracycline chemotherapeutic agents might induce skeletal muscle fatigue, there is mounting literature demonstrating that symptoms persist long after chemotherapy exposure, and as such, the fatiguing effects of chemotherapeutic agents are unlike that of the reversible phenomenon observed during exercise.
A study conducted by Ness et al. [15] examined skeletal muscle function in chemotherapy-treated childhood cancer survivors, confirming that patients experience significant limitations to physical performance and are restricted from participation in daily activities several years following treatment. Post-chemotherapy-treated children also have a significantly reduced maximal exercise capacity [8, 9, 15, 60], reduced fat-free [8] and skeletal muscle mass [15], and significant skeletal muscle weakness [8, 9, 15, 99]. Importantly Järvelä et al. [8] have established that the skeletal muscle dysfunction observed in childhood cancer survivors is not secondary to impaired cardiac muscle function, as the same results are observed in the absence of detectable cardiac dysfunction and in comparison with age-, gender-, and physical activity-matched controls [60]. Thus, chemotherapy-induced exercise intolerance, weakness, and fatigue seem intrinsically rooted in physiological maladaptations at the skeletal muscle level. A number of other groups have shown strong associations between skeletal muscle impairment and atrophy induction, which is thought to be preceded by mitochondrial dysfunction and mtROS production [10, 65–70].
Without a doubt, the chronic, long-term side effects of chemotherapy treatment on the skeletal muscular system seem most profound when chemotherapy is administered during childhood [8–10, 15, 60, 99]. Hyperplastic skeletal muscle growth and therefore, mitotic activity, is prolific during foetal and neonatal growth and ceases during early childhood in which the total fibre number is set [100, 101]. During this time, there would be a much higher propensity for chemotherapy-induced DNA damage and mutations to be quickly incorporated into the somatic skeletal muscle genome and induce repercussions on skeletal muscle structure and function that persist for life. For those chemotherapies that act to stop mitosis altogether, the result would be a systemic reduction in skeletal muscle fibre number that is unlikely to ever be entirely recovered. However, chemotherapy-induced myopathy and metabolic syndrome-like co-morbidities are also well reported in the adult population [87, 102, 103], highlighting that the damaging and atrophic side effects elicited by chemotherapy treatment are not merely segregated to cell cycle manipulation, but also directly affect mitochondrial function and energy production, skeletal muscle physiology and ultimately, the regulation of muscle mass. Thus chemotherapy-induced myopathy is likely a two-tiered phenomenon in which skeletal muscle damage and necrotic/apoptotic cell death is propagated, and the capacity for repair of that damage—especially during energy homeostasis dysregulation—is severely impaired (as summarised in Fig. 2).
Future direction and significance
Chemotherapy-induced mtROS production and DNA damage has been implicated in mitochondrial dysfunction, energy homeostasis dysregulation, mitophagy, and subsequently skeletal muscle atrophy and wasting. As a result, cancer survivors are prone to low muscle mass, poor function and heightened fatigue. Thus therapeutic interventions to ameliorate these unwanted side effects are greatly needed. We propose that the precise mechanisms through which chemotherapeutic agents induce mitochondrial and skeletal muscle toxicity and wasting be carefully characterised—particularly for those that are in current widespread clinical use—in the first instance. Further, while no single treatment has been identified to clearly ameliorate chemotherapy-induced mitochondrial dysfunction, a number of treatments have been used to treat other myopathies with similar symptomatology and which are specifically underscored by mitophagy [104, 105]. Thus targeting the mitochondria with either established or novel mitochondrial targeted therapeutics (MTT) could provide a therapeutic avenue through which to provide the skeletal musculature with protection against chemotherapy-induced toxicity. This is indeed a promising pharmacotherapeutic direction for future research.
Conclusions
The clinical repercussions of chemotherapy-induced skeletal muscle toxicity range from reduced participation in activities of daily living, chronic fatigue, exercise intolerance, depression and treatment discontinuation, to an increased risk of morbidity and mortality from myopathy-related disease [5, 94]. We have presented compelling evidence to suggest that the mitochondria are an etiological pharmacotoxic target of chemotherapy treatment which induces various co-morbidities that are overwhelmingly manifested in the skeletal muscular system. Given the persistent and severe nature of these co-morbidities, we stress the importance for a concerted research effort to develop appropriate (co-)/therapeutics to address the deleterious effects of chemotherapy-based anti-cancer therapy on the mitochondria to mitigate impacts on the skeletal muscular system.
References
Steward B, Wild C (2014) World Cancer Report 2014. International Agency for Research on Cancer, Lyon
de Gramont AD et al (2000) Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol 18(16):2938–2947
Ogston KN et al (2003) A new histological grading system to assess response of breast cancers to primary chemotherapy: prognostic significance and survival. The Breast 12(5):320–327
Zitvogel L et al (2008) Immunological aspects of cancer chemotherapy. Nat Rev Immunol 8(1):59–73
Gilliam LAA, St. Clair DK (2011) Chemotherapy-induced weakness and fatigue in skeletal muscle: the role of oxidative stress. Antioxid Redox Signal 15(9):2543–2563
Greene D et al (1993) A comparison of patient-reported side effects among three chemotherapy regimens for breast cancer. Cancer Pract 2(1):57–62
Yin H, Price F, Rudnicki MA (2013) Satellite cells and the muscle stem cell niche. Physiol Rev 93(1):23–67
Järvelä LS et al (2012) Effects of a home-based exercise program on metabolic risk factors and fitness in long-term survivors of childhood acute lymphoblastic leukemia. Pediatr Blood Cancer 59(1):155–160
van Brussel M et al (2006) Physical function and fitness in long-term survivors of childhood leukaemia. Pediatr Rehabil 9(3):267–274
Scheede-Bergdahl C, Jagoe RT (2013) After the chemotherapy: potential mechanisms for chemotherapy-induced delayed skeletal muscle dysfunction in survivors of acute lymphoblastic leukaemia in childhood. Front Pharmacol 4:49. doi:10.3389/fphar.2013.00049
Miyamoto Y et al (2015) Negative impact of skeletal muscle loss after systemic chemotherapy in patients with unresectable colorectal cancer. PLoS ONE 10(6):e0129742
Larsen S et al (2012) Biomarkers of mitochondrial content in skeletal muscle of healthy young human subjects. J Physiol 590(14):3349–3360
Argilés JM, López-Soriano FJ, Busquets S (2015) Muscle wasting in cancer: the role of mitochondria. Curr Opin Clin Nutr Metab Care 18(3):221–225
Dobs AS et al (2013) Effects of enobosarm on muscle wasting and physical function in patients with cancer: a double-blind, randomised controlled phase 2 trial. Lancet Oncol 14(4):335–345
Ness KK et al (2007) Body composition, muscle strength deficits and mobility limitations in adult survivors of childhood acute lymphoblastic leukemia. Pediatr Blood Cancer 49(7):975–981
Gewirtz D (1999) A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochem Pharmacol 57(7):727–741
Gilliam LA et al (2011) Doxorubicin causes diaphragm weakness in murine models of cancer chemotherapy. Muscle Nerve 43(1):94–102
Schelman WR et al (2009) A phase I study of Triapine® in combination with doxorubicin in patients with advanced solid tumors. Cancer Chemother Pharmacol 63(6):1147–1156
Lu P (2005) Monitoring cardiac function in patients receiving doxorubicin. Semin Nucl Med 35(3):197–201
Agudelo D et al (2014) Intercalation of antitumor drug doxorubicin and its analogue by DNA duplex: structural features and biological implications. Int J Biol Macromol 66:144–150
Quach B, Birk A, Szeto H (2014) Mechanism of preventing doxorubicin-induced mitochondrial toxicity with cardiolipin-targeted peptide, SS-31 (966.1). FASEB J 28(1 Supplement):966.1
Cheregi B et al (2015) Chemotherapy-induced mitochondrial respiratory dysfunction, oxidant production and death in healthy skeletal muscle C2C12 myoblast and myotube models. Neuromuscul Disord 25(Supplement 2):S202
Deavall DG et al (2012) Drug-induced oxidative stress and toxicity. J Toxicol 2012:645460
Sawyer DB et al (2010) Mechanisms of anthracycline cardiac injury: Can we identify strategies for cardioprotection? Prog Cardiovasc Dis 53(2):105–113
Sarosiek KA, Chonghaile TN, Letai A (2013) Mitochondria: gatekeepers of response to chemotherapy. Trends Cell Biol 23(12):612–619
Wallace DC (2012) Mitochondria and cancer. Nat Rev Cancer 12(10):685–698
Zorov DB, Juhaszova M, Sollott SJ (2014) Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol Rev 94(3):909–950
Davies K, Doroshow J (1986) Redox cycling of anthracyclines by cardiac mitochondria. I. Anthracycline radical formation by NADH dehydrogenase. J Biol Chem 261(7):3060–3067
Doroshow J, Davies K (1986) Redox cycling of anthracyclines by cardiac mitochondria. II. Formation of superoxide anion, hydrogen peroxide, and hydroxyl radical. J Biol Chem 261(7):3068–3074
Chen Q et al (2003) Production of reactive oxygen species by mitochondria central role of complex III. J Biol Chem 278(38):36027–36031
Chen Y et al (2007) Collateral damage in cancer chemotherapy: oxidative stress in nontargeted tissues. Mol Interv 7(3):147
Dirks-Naylor AJ et al (2013) The effects of acute doxorubicin treatment on proteome lysine acetylation status and apical caspases in skeletal muscle of fasted animals. J Cachexia Sarcopenia Muscle 4(3):239–243
Gilliam LAA et al (2012) Doxorubicin acts via mitochondrial ROS to stimulate catabolism in C2C12 myotubes. Am J Physiol Cell Physiol 302:C195–C202
Xu X, Persson HL, Richardson DR (2005) Molecular pharmacology of the interaction of anthracyclines with iron. Mol Pharmacol 68(2):261–271
Finn NA, Findley HW, Kemp ML (2011) A switching mechanism in doxorubicin bioactivation can be exploited to control doxorubicin toxicity. PLoS Comput Biol 7(9):e1002151
Ismail HM et al (2013) Inhibition of iPLA2β and of stretch-activated channels by doxorubicin alters dystrophic muscle function. Br J Pharmacol 169(7):1537–1550
Conklin KA (2004) Chemotherapy-associated oxidative stress: impact on chemotherapeutic effectiveness. Integr Cancer Ther 3(4):294–300
Bai P et al (2011) PARP-1 inhibition increases mitochondrial metabolism through SIRT1 activation. Cell Metab 13(4):461–468
Goodwin P et al (1978) The effect of gamma radiation and neocarzinostatin of NAD and ATP levels in mouse leukaemia cells. Biochimica et Biophysica Acta (BBA) Gen Subj 543(4):576–582
Skidmore CJ et al (1979) The Involvement of poly (ADP-ribose) polymerase in the degradation of NAD caused by γ-radiation and N-methyl-N-nitrosourea. Eur J Biochem 101(1):135–142
Ying W, Garnier P, Swanson RA (2003) NAD+ repletion prevents PARP-1-induced glycolytic blockade and cell death in cultured mouse astrocytes. Biochem Biophys Res Commun 308(4):809–813
Zong WX et al (2004) Alkylating DNA damage stimulates a regulated form of necrotic cell death. Genes Dev 18(11):1272–1282
Niere M et al (2008) Functional localization of two poly (ADP-ribose)-degrading enzymes to the mitochondrial matrix. Mol Cell Biol 28(2):814–824
Lai Y et al (2008) Identification of poly-ADP-ribosylated mitochondrial proteins after traumatic brain injury. J Neurochem 104(6):1700–1711
Bai P et al (2011) PARP-2 regulates SIRT1 expression and whole-body energy expenditure. Cell Metab 13(4):450–460
Devalaraja-Narashimha K, Padanilam BJ (2010) PARP1 deficiency exacerbates diet-induced obesity in mice. J Endocrinol 205(3):243–252
Kourie JI (1998) Interaction of reactive oxygen species with ion transport mechanisms. Am J Physiol Cell Physiol 275:C1–C24
Leeuwenburgh C (2003) Role of apoptosis in sarcopenia. J Gerontol Ser A Biol Sci Med Sci 58(11):999–1001
Powers SK, Kavazis AN, DeRuisseau KC (2005) Mechanisms of disuse muscle atrophy: role of oxidative stress. Am J Physiol Regul Integr Comp Physiol 288:R337–R344
Du J et al (2004) Activation of caspase-3 is an initial step triggering accelerated muscle proteolysis in catabolic conditions. J Clin Investig 113(1):115–123
Smuder AJ et al (2011) Exercise protects against doxorubicin-induced oxidative stress and proteolysis in skeletal muscle. J Appl Physiol 110:935–942
Lecker SH et al (1999) Muscle protein breakdown and the critical role of the ubiquitin–proteasome pathway in normal and disease states. J Nutr 129(1):227S–237S
Lecker SH, Goldberg AL, Mitch WE (2006) Protein degradation by the ubiquitin–proteasome pathway in normal and disease states. J Am Soc Nephrol 17(7):1807–1819
Bonnard C et al (2008) Mitochondrial dysfunction results from oxidative stress in the skeletal muscle of diet-induced insulin-resistant mice. J Clin Investig 118(2):789–800
Adachi K et al (1993) A deletion of mitochondrial DNA in murine doxorubicin-induced cardiotoxicity. Biochem Biophys Res Commun 195(2):945–951
Gouspillou G et al (2015) Anthracycline-containing chemotherapy causes long-term impairment of mitochondrial respiration and increased reactive oxygen species release in skeletal muscle. Sci Rep 5:8717. doi:10.1038/srep08717
Raymond E et al (1998) Oxaliplatin: mechanism of action and antineoplastic activity. Semin Oncol 25(2 Suppl 5):4–12
André T et al (2004) Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med 350(23):2343–2351
Raymond E et al (1998) Oxaliplatin: a review of preclinical and clinical studies. Ann Oncol 9(10):1053–1071
Talvensaari KK et al (1995) Decreased isokinetic trunk muscle strength and performance in long-term survivors of childhood malignancies: correlation with hormonal defects. Arch Phys Med Rehabil 76(11):983–988
Gourdier I et al (2004) Oxaliplatin-induced mitochondrial apoptotic response of colon carcinoma cells does not require nuclear DNA. Oncogene 23(45):7449–7457
Lutsenko S et al (2007) Function and regulation of human copper-transporting ATPases. Physiol RevVol. 87:1011–1046
Wisnovsky SP et al (2013) Targeting mitochondrial DNA with a platinum-based anticancer agent. Chem Biol 20(11):1323–1328
Stojanovska V, McQuade RM, Stewart M, Timpani CA, Sorensen J, Orbell J, Rybalka E, Nurgali K (2015) Platinum accumulation and changes in mitochondrial function of the longitudinal muscle & myenteric plexus following oxaliplatin administration. In: Proceedings of the Australian Physiology Society
Gilliam LAA et al (2013) The anticancer agent doxorubicin disrupts mitochondrial energy metabolism and redox balance in skeletal muscle. Free Radic Biol Med 65:988–996
Powers SK et al (2012) Mitochondrial signaling contributes to disuse muscle atrophy. Am J Physiol Endocrinol Metab 303:E31–E39
Min K et al (2011) Mitochondrial-targeted antioxidants protect skeletal muscle against immobilization-induced muscle atrophy. J Appl Physiol 111:1459–1466
Singh K, Hood DA (2011) Effect of denervation-induced muscle disuse on mitochondrial protein import. American Physiol Cell Physiol 300:C138–C145
Min K et al (2015) Increased mitochondrial emission of reactive oxygen species and calpain activation are required for doxorubicin-induced cardiac and skeletal muscle myopathy. J Physiol 593(8):2017–2036
Powers SK, Talbert EE, Adhihetty PJ (2011) Reactive oxygen and nitrogen species as intracellular signals in skeletal muscle. J Physiol 589(9):2129–2138
Sena LA, Chandel NS (2012) Physiological roles of mitochondrial reactive oxygen species. Mol Cell 48(2):158–167
Lokireddy S et al (2012) The ubiquitin ligase Mul1 induces mitophagy in skeletal muscle in response to muscle-wasting stimuli. Cell Metab 16(5):613–624
Sorensen J, Timpani CA, Campelj D, Petersen AC, Hayes A, Rybalka E (2015) Idebenone protects against chemotherapy-induced skeletal muscle wasting and mitochondrial dysfunction in mice. Proc Aust Physiol Soc 46:142
Jackman RW, Kandarian SC (2004) The molecular basis of skeletal muscle atrophy. Am J Physiol Cell Physiol 287:C834–C843
Jang YC et al (2010) Increased superoxide in vivo accelerates age-associated muscle atrophy through mitochondrial dysfunction and neuromuscular junction degeneration. FASEB J 24(5):1376–1390
Bonaldo P, Sandri M (2013) Cellular and molecular mechanisms of muscle atrophy. Dis Models Mech 6(1):25–39
Lenaz G (2012) Mitochondria and reactive oxygen species. Which role in physiology and pathology? In: Scatena R, Bottoni P, Giardina B (eds) Advances in mitochondrial medicine. Springer, Dordrecht, pp 93–136
Kurihara Y et al (2012) Mitophagy plays an essential role in reducing mitochondrial production of reactive oxygen species and mutation of mitochondrial DNA by maintaining mitochondrial quantity and quality in yeast. J Biol Chem 287(5):3265–3272
Neel BA, Lin Y, Pessin JE (2013) Skeletal muscle autophagy: a new metabolic regulator. Trends Endocrinol Metab TEM. doi:10.1016/j.tem.2013.09.004
Maccarrone M, Melino G, Finazzi-Agro A (2001) Lipoxygenases and their involvement in programmed cell death. Cell Death Differ 8(8):776–784
England K, Cotter T (2005) Direct oxidative modifications of signalling proteins in mammalian cells and their effects on apoptosis. Redox Rep 10(5):237–245
Moylan JS, Reid MB (2007) Oxidative stress, chronic disease, and muscle wasting. Muscle Nerve 35(4):411–429
Ranek M, Wang X (2009) Activation of the ubiquitin–proteasome system in doxorubicin cardiomyopathy. Curr Hypertens Rep 11(6):389–395
Corradetti RM (2014) Chemotherapy-induced peripheral neuropathy. Chemotherapy 249:279
Lind MJ (2008) Principles of cytotoxic chemotherapy. Medicine 36(1):19–23
Coates A et al (1983) On the receiving end—patient perception of the side-effects of cancer chemotherapy. Eur J Cancer Clin Oncol 19(2):203–208
Courneya KS et al (2004) Exercise issues in older cancer survivors. CritRev Oncol Hematol 51(3):249–261
Willemse P-PM et al (2014) Abdominal visceral and subcutaneous fat increase, insulin resistance and hyperlipidemia in testicular cancer patients treated with cisplatin-based chemotherapy. Acta Oncol 53(3):351–360
Kent-Braun JA, Fitts RH, Christie A (2012) Skeletal muscle fatigue. Compr Physiol 2:997–1044
MacIntosh BR, Holash RJ, Renaud J-M (2012) Skeletal muscle fatigue–regulation of excitation–contraction coupling to avoid metabolic catastrophe. J Cell Sci 125(9):2105–2114
Jacobs RA et al (2013) Improvements in exercise performance with high-intensity interval training coincide with an increase in skeletal muscle mitochondrial content and function. J Appl Physiol 115(6):785–793
Gejl K et al. (2015) Repeated high-intensity exercise modulates Ca2+ sensitivity of human skeletal muscle fibers. Scand J Med Sci Sports 26(5):488–97
Kano Y et al (2012) Mechanisms of exercise-induced muscle damage and fatigue: intracellular calcium accumulation. J Phys Fit Sports Med 1(3):505–512
Hydock D et al (2015) Protective effects of endurance or resistance training exercise on chemotherapy-induced skeletal muscle weakness and fatigue. FASEB J 29(1 Supplement):LB660
Grisold W, Cavaletti G, Windebank AJ (2012) Peripheral neuropathies from chemotherapeutics and targeted agents: diagnosis, treatment, and prevention. Neuro-oncology 14(suppl 4):iv45–iv54
Wall BT, Dirks ML, van Loon LJC (2013) Skeletal muscle atrophy during short-term disuse: implications for age-related sarcopenia. Ageing Res Rev 12(4):898–906
Franchi M et al (2014) Architectural, functional and molecular responses to concentric and eccentric loading in human skeletal muscle. Acta Physiol 210(3):642–654
McQuade RM, Bornstein JC, Nurgali K (2014) Anti-colorectal cancer chemotherapy-induced diarrhoea: current treatments and side-effects. Int J Clin Med 5(7):393
Hovi L et al (1993) Impaired muscle strength in female adolescents and young adults surviving leukemia in childhood. Cancer Phila 72:276
Malina RM, Bouchard C, Bar-Or O (2004) Growth, maturation, and physical activity. Human Kinetics, Champaign
Mouly V et al (2005) The mitotic clock in skeletal muscle regeneration, disease and cell mediated gene therapy. Acta Physiol Scand 184(1):3–15
Scully RE, Lipshultz SE (2007) Anthracycline cardiotoxicity in long-term survivors of childhood cancer. Cardiovasc Toxicol 7(2):122–128
Syrjala KL et al (2005) Late effects of hematopoietic cell transplantation among 10-year adult survivors compared with case-matched controls. J Clin Oncol 23(27):6596–6606
Lynch DR, Perlman SL, Meier T (2010) A phase 3, double-blind, placebo-controlled trial of idebenone in Friedreich ataxia. Arch Neurol 67(8):941–947
Literati-Nagy B et al (2009) Improvement of insulin sensitivity by a novel drug, BGP-15, in insulin-resistant patients: a proof of concept randomized double-blind clinical trial. Hormone Metab Res Hormon-und Stoffwechselforschung Hormones et metabolisme 41(5):374–380
Acknowledgments
This work was supported by grants from the Centre for Chronic Disease and the Institute of Sport, Exercise and Active Living (ISEAL) Clinical Exercise Program funding schemes (both Victoria University). The authors all contributed to the writing of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Rights and permissions
About this article
Cite this article
Sorensen, J.C., Cheregi, B.D., Timpani, C.A. et al. Mitochondria: Inadvertent targets in chemotherapy-induced skeletal muscle toxicity and wasting?. Cancer Chemother Pharmacol 78, 673–683 (2016). https://doi.org/10.1007/s00280-016-3045-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-016-3045-3