Abstract
Cancer cachexia afflicts many advanced cancer patients with many progressing to death. While there have been many advancements in understanding the molecular mechanisms that contribute to the development of cancer cachexia, substantial gaps still exist. Chemotherapy drugs often target ribosome biogenesis to slow or blunt tumor cell growth and proliferation. Some of the most frequent side-effects of chemotherapy are loss of skeletal muscle mass, muscular strength and an increase in fatigue. Given that ribosome biogenesis has emerged as a main mechanism regulating muscle hypertrophy, and more recently, also implicated in muscle atrophy, we propose that some chemotherapy drugs can cause further muscle wasting via its effect on skeletal muscle cells. Many chemotherapy drugs, including the most prescribed drugs such as doxorubicin and cisplatin, affect ribosomal DNA transcription, or other pathways related to ribosome biogenesis. Furthermore, middle-aged and older individuals are the most affected population with cancer, and advanced cancer patients often show reduced levels of physical inactivity. Thus, aging and inactivity can themselves affect muscle ribosome biogenesis, which can further worsen the effect of chemotherapy on skeletal muscle ribosome biogenesis and, ultimately, muscle mass and function. We propose that chemotherapy can accelerate the onset or worsen cancer cachexia via its inhibitory effects on skeletal muscle ribosome biogenesis. We end our review by providing recommendations that could be used to ameliorate the negative effects of chemotherapy on skeletal muscle ribosome biogenesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer is the second most fatal disease in the United States [1] and a leading cause of death worldwide [2]. Although cancer can affect almost all tissues, a primary tumor or metastasis in skeletal muscle is a very rare condition [3]. Despite the fact that skeletal muscle is rarely a tumorigenic site, cancer in other tissues can often affect muscle profoundly. Patients with advanced stage cancer may suffer from cachexia, a wasting syndrome in which there is a marked loss of skeletal muscle mass with or without loss of body fat as well as increased fatigue, weakness and potentially developing anemia [4].
Early diagnosis of cancer cachexia is an important factor towards positive outcomes, as late-stage cachexia is generally considered untreatable [5]. Hence, the concepts of pre-cachexia (initial stage) and refractory cachexia (later stage) have been developed in the medical literature in an attempt to modify the course of the syndrome while still reversible [5, 6]. However, the criteria used to determine the stage of cachexia remains arbitrary and may lack validation under different clinical conditions [7, 8]. Nonetheless, there is a general consensus that a diagnosis of cancer cachexia at an early stage enhances the ability to effectively treat the condition [6]. Hence, understanding the molecular mechanism of skeletal muscle wasting associated with cachexia is of the upmost importance towards developing strategies to mitigate or prevent cancer cachexia. Also, a greater understanding of the other factors that synergistically operate to worsen cancer cachexia will lead to the development of more effective therapeutic approaches to treat cachexia.
Chemotherapy is often used to treat cancer. However, many chemotherapy drugs can also affect skeletal muscle, contributing to the development of cancer cachexia [9,10,11,12]. Chemotherapy drugs are able to target cancer cells because their high rate of proliferation which is heavily dependent on ribosome biogenesis to provide the necessary translational capacity to support growth [13, 14]. As such, many chemotherapy drugs aim to damage DNA to induce apoptosis [15, 16], while many others directly target ribosome biogenesis to slow or blunt cell growth and proliferation. A growing body of evidence has revealed that ribosome biogenesis has a central role in the regulation of skeletal muscle mass [17] in addition to other cellular and molecular mechanisms [18]. By targeting ribosome biogenesis via chemotherapy, we propose that not only tumor cells are gravely affected but also skeletal muscle tissue, which further exacerbates cancer cachexia progression. The purpose of this review is to bring attention to the notion that targeting ribosome biogenesis to inhibit cancer growth is inadvertently contributing to cachexia by enhancing the loss of skeletal muscle while likely blunting therapeutic efforts to restore or prevent further decreases in skeletal muscle mass and function.
Cancer cachexia
Cancer cachexia is a highly prevalent condition among advanced cancer patients [19]. Cancer cachexia is viewed as a wasting syndrome in which patients lose a disproportionate amount of muscle tissue which is tightly linked to a poor prognosis [4]. Accordingly, a high proportion of cancer patients die as the result of cachexia or with cachexia [19]. The significant loss of skeletal muscle mass observed in advanced cancer patients leads to a corresponding loss of muscle strength, an increase in disabilities, decrease in the quality of life and is a predictor of poor prognosis and mortality [4, 20,21,22,23,24]. The degree of muscle loss in cancer patients affects the survival curve compared to patients with stable or minor muscle loss [12, 25]. Therefore, the progressive loss of skeletal muscle mass has been considered the most clinically relevant aspect of cachexia [5].
It is well established that muscle strength is a predictor of all-cause mortality in the general population [26,27,28]. Similar relationship has also been observed among cancer patients. Muscular strength has been negatively associated with cancer mortality [22], regardless of the level of cardiorespiratory fitness or adiposity [20, 21]. Additionally, a significant decrease in skeletal muscle mass has been found to be an independent predictor of poor prognosis in cachectic patients [24] and associated with increased risk of mortality in cancer survivors [23]. Similarly, patients with lower muscle mass are more likely to experience toxicity from chemotherapy [29, 30], possibly by lowering drug uptake in skeletal muscle, making it more available for other tissues. Thus, muscle loss in cachectic patients predicts increased toxicity to chemotherapy and mortality [4].
Although a clear association between the reduction in skeletal muscle mass and increased mortality in cancer patients has been observed, it has been viewed as a merely indirect feature of poor health or an end-of-life condition [4], i.e., diseases lead to wasting in many tissues including skeletal muscle. This concept was elegantly tested by Zhou et al. [31]. These researchers treated tumor-bearing mice with soluble ActRIIB (Activin receptor IIB) to promote muscle growth by blocking the pro-muscle wasting myostatin and activin A signaling. As expected, the vehicle-control group developed cancer cachexia as the result of progressive muscle wasting and was associated with higher mortality rates. In contrast, administration of soluble ActRIIB prevented the loss of muscle mass and significantly increased life span, despite there being no effect on tumor size. These findings provide strong pre-clinical evidence that muscle mass has a direct and independent effect on life span in cachexia, as shown in population-based studies [23, 24, 32].
There are several candidates that have been thought to play a role in the development of cancer cachexia, such as inflammatory cytokines (e.g., Tumor Necrosis Factor (TNF)-α and Interleukin-6), and myokines (e.g. myostatin), which appears to be more or less relevant depending on the type of cancer [33]. Regardless of the primary molecule (or molecules) triggering muscle loss, muscle wasting associated with cachexia has been primarily explained by increased protein degradation rates [34,35,36,37] and/or decreased rates of muscle protein synthesis [35,36,37,38,39] that results in a negative muscle protein balance. A lower level of ribosome biogenesis and subsequent reduced translational capacity (total quantities of ribosomes) could be a key factor underlying the decrease in skeletal muscle protein synthesis. In a mouse model of cancer cachexia using colon-26 tumor cell line, a loss of ribosomal RNA was observed in skeletal muscle [40]. Similarly, in a mouse model of ovarian cancer, which causes rapid muscle wasting, ribosomal content is markedly reduced, which was explained by lower muscle ribosome biogenesis [41]. These recent findings provide clear evidence that ribosome biogenesis and translational capacity are negatively affected during cancer cachexia. It is likely that the lower protein synthesis rates in skeletal muscle with cachexia is, at least partially, explained by lower ribosome biogenesis.
Cancer is often associated with dysregulation of ribosome biogenesis
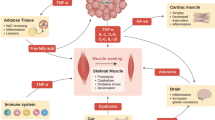
A common denominator among different types of cancer is the dysregulation of cell proliferation as the result of defective cell-cycle check points [42,43,44,45]. This includes proteins involved in cell-cycle progression, such as pRB, c-Myc, cyclin-dependent kinases (Cdks) and their cyclin partners, which are frequently dysregulated [43, 44, 46]. Additionally, proteins involved in biosynthetic pathways to support cell proliferation, such as PI3K, Akt, mTOR and TSC are also commonly dysregulated in cancer cells [47,48,49,50]. Each of these proteins and pathways converges upon a central point involved in the regulation of cancer cell proliferation: ribosome biogenesis. Ribosome biogenesis is the de novo synthesis of ribosomes, in which the ribosomal DNA is transcribed by RNA Polymerase I (Pol I) to produce the precursor 47S pre-rRNA. The pre-rRNA is, in turn, processed into the mature ribosomal (r)RNAs (18S, 28S and 5.8S) which along the 5S rRNA (transcribed by the RNA Polymerase III) and ~ 80 ribosomal proteins forms the mature ribosome (for reviews detailing this process, there are excellent reviews available [51,52,53]). The synthesis of new ribosomes is essential for cancer cells and is the basic mechanism supporting cell proliferation and tumorigenesis [46, 54,55,56,57,58]. Thus, not surprisingly, drugs that target the nucleolus (the primarily nuclear site of ribosomal DNA transcription) and ribosome biogenesis have been shown to be a powerful tool against a wide range of cancer types [46, 52, 55, 58,59,60,61,62,63,64]. Simply put, inhibiting ribosome biogenesis will prevent or dramatically slow cell proliferation and thus severely limit subsequent tumor growth. Many drugs routinely prescribed in chemotherapy, such as cisplatin, doxorubicin and methotrexate, act at different steps of ribosome biogenesis (either transcription of ribosomal DNA and/or processing of ribosomal RNAs) [58], in addition to ribosome maturation and assembly (Fig. 1).
Chemotherapy drugs affect ribosome biogenesis at multiple levels. Ribosome biogenesis supports cancer cell growth and proliferation, hence, it is a common target of many chemotherapy drugs. Chemotherapy drugs may affect ribosomal DNA transcription, Pol I activy, pre-rRNA processing and maturation into mature ribosomes. Some drugs may affect translation of ribosomal and growth-related proteins, also affecting ribosome biogenesis
Furthermore, drugs that affect upstream pathways leading to rDNA transcription or other steps, such as synthesis of ribosomal proteins, can also reduce ribosome biogenesis. Akt regulates ribosome biogenesis through mTOR-dependent and -independent mechanisms [65], and mTOR regulates the synthesis of the ribosomal proteins and pre-rRNA synthesis [66]. c-Myc is involved in ribosome biogenesis at multiple steps, such as synthesis and processing of pre-rRNA as well as the transcription of ribosomal proteins mRNAs [67, 68]. Thus, drugs that inhibit Akt (such MK-2206 [69, 70], and mTOR (rapamycin and other rapalogs) [71, 72] have also been used to treat cancer (Fig. 1).
Another promising cancer target is RNA Polymerase I itself. Pol I is the dedicated enzymatic complex that transcribes rDNA, as stated above. Several proteins and transcription factors, such as the Upstream Binding Factor (UBF), the TIF-IA/RRN3, Selectivity Factor 1 (SL-1), act together to recruit the Pol I forming the Pre-Initiation Complex (PIC) to the rDNA promoter region. In the last few years, several molecules have been identified to target Pol I. The compounds CX-3543 [73], CX-5461 [74, 75], BMH-21 [76], and PMR-116 have been shown to have anti-cancer effects likely due to its interaction and inhibitory effect on the PIC formation and inhibition of rDNA transcription [77]. These drugs have the potential to directly target ribosome biogenesis and slow cancer progression. Indeed, CX-5461 has already been tested in a human clinical trial [78] with others scheduled to follow [77]. However, the effects of drugs that inhibit Pol I, on healthy tissues such as skeletal muscle, remain largely unknown.
Ribosome biogenesis in skeletal muscle
Given that the pathways involved in regulating cell growth are highly conserved, it comes as no surprise that many of cancer growth pathways have been shown to be involved in the regulation of skeletal muscle mass, hypertrophy in particular. For instance, the classical oncogenes c-Myc [79, 80] and Akt [81,82,83] have been shown to either be involved or sufficient to induce muscle growth. The mTOR complex I (mTORC1), as well, has been shown to be master regulator of cancer and myofiber hypertrophy [84]. The mTORC1 pathway is often dysregulated in cancer, thus the use of rapamycin to block mTOR activity has been extensively evaluated for treating certain types of cancer [47]. In skeletal muscle, rapamycin has been also widely employed to block muscle growth induced by resistance exercise or mechanical loading, demonstrating a requirement for mTOR signaling [82, 85,86,87].
Furthermore, in the last few years, ribosome biogenesis has emerged as a central process in skeletal muscle growth [17, 88, 89]. The synthesis of new ribosomes enhances the translational capacity of myofibers [17]. Both rodent models of hypertrophy and resistance training in humans have been shown to increase ribosomal DNA transcription with the subsequent accumulation of ribosomes [79, 90,91,92,93,94]. Moreover, similar pathways involved in the regulation of ribosome biogenesis discovered in cancer cells, are also upregulated following mechanical overload/resistance exercise which include canonical hubs such as mTORC1, MAPK, c-Myc and cell-cycle regulators, such as Cyclin D1 [17].
Lower ribosome biogenesis and reduced translational capacity have also been reported to occur during muscle wasting [95, 96] and growth impairment [97, 98]. Furthermore, the increase in ribosomal mass is also a critical determinant for the recovery from malnutrition during postnatal muscle development [99]. A growing body of evidence demonstrate that ribosome biogenesis is not only important for muscle hypertrophy but may also be an underlying mechanism in the maintenance of muscle mass [96, 100]. The shared canonical growth pathways between cancer cells and muscle tissue, provides a plausible mechanism for how chemotherapy negatively impacts skeletal muscle. Attempts to mitigate the loss of skeletal muscle mass in cancer cachexia when a patient is receiving chemotherapy known to target ribosome biogenesis will likely become more difficult precisely because the pathways and cellular process that dictates muscle growth will be targeted by such compounds.
Chemotherapy that targets ribosome biogenesis causes muscle atrophy
The cytotoxic use of chemotherapy seeks to specifically target cancer cells based on their high rate of cell proliferation via the cell cycle to prevent cell growth, mitosis and/or induce apoptosis. However, often chemotherapy leads to a variety of side-effects due to the uptake and accumulation of the drug by healthy tissues [101]. Indeed, in some cases, the uptake of a chemotherapy drug is higher in normal healthy tissue than in tumor tissue. This can be especially true for solid tumors with a significant distance from blood vessels [102]. Accumulation of chemotherapy drugs and its metabolites can occur in many tissues and organs, such as liver, kidney, heart and skeletal muscle [103, 104]. While other tissues, in particular, liver and kidney, have been shown to display higher concentration of chemotherapy drugs, skeletal muscle also shows significant accumulation of these compounds [103, 104]. As the most abundant tissue in the body with a large surface area and highly vascularized, the uptake of chemotherapy drugs by muscle cells cannot be disregarded. In addition to the natural progression of cancer cachexia, chemotherapy may further exacerbate cachexia [9, 10]. Indeed, chemotherapy often leads to substantial muscle loss [105, 106], and sequeales related to muscle function and fatigue persist for months after cessation of chemotherapy [107].
Doxorubicin—a potent and highly prescribed anti-tumorigenic drug—is one of the most studied chemotherapy drugs on skeletal muscle physiology. Doxorubicin and its metabolites can accumulate for long periods in skeletal and cardiac muscle [108,109,110] leading to loss of muscle strength [108] and increase in fatigue. In rats, doxorubicin causes a decrease in muscle fiber size and muscle mass [111]. The main mechanism of action of doxorubicin is through inhibition of DNA Topoisomerase II [112], which directly interacts with RNA Polymerase I [113]. Indeed, doxorubicin inhibits ribosome biogenesis in cancer cells [58]; therefore, it is possible that the detrimental effects of doxorubicin may be driven by blunting ribosome biogenesis in muscle tissue, thereby further promoting cachexia. There is evidence from a cell culture study that doxorubicin treatment decreases rates of protein synthesis in skeletal muscle cells [114], which could be explained by its inhibitory effects on ribosome biogenesis.
Importantly, the detrimental effects of chemotherapy on skeletal muscle mass not only impact muscle function, strength, quality of life but also may affect mortality rates. Blauwhoff-Buskermolen et al. [25] analyzed the changes in muscle mass in addition to the survival rates in advanced cancer patients undergoing chemotherapy. After 3 months, there was a significant decrease in muscle mass (1.7 kg in men and 1.1 kg in women). Moreover, patients with the highest muscle loss (≥ 9% muscle loss) had significantly different survival curve compared to patients that showed stable or minor muscle loss. It is noteworthy that the vast majority of those patients were receiving a pro-drug that is converted to 5-fluorouracil and oxaliplatin, which both are known to effect cancer cells by inhibiting ribosome biogenesis [58, 115]. Other combinations of different chemotherapy drugs that target ribosome biogenesis, such as cisplatin, fluorouracil, paclitaxel and etoposide also result in substantial muscle loss within a few weeks [116]. As a comparison, drugs that do not act on ribosome biogenesis, appears to have minor, if any, effect on skeletal muscle mass. Gemcitabine, a chemotherapy drug does not appear to affect ribosomal DNA transcription alone [117, 118] has little or no effect after eight weeks of treatment on lean mass [119]. Gemcitabine’s main mechanism of action is through inhibition of DNA synthesis [120], having greater effect on inhibiting DNA synthesis than RNA synthesis [121]. Interestingly, Selumetinib—an MAPK inhibitor—is one of the few chemotherapy drugs that may actually have beneficial effects on skeletal muscle mass [122, 123]. While its mechanism of action in muscle is currently unknown, it is a promising drug to treat sensitive tumors while possibly having a beneficial effect on cancer cachexia by preserving skeletal muscle mass [122, 123].
It is important to highlight that chemotherapy drugs could affect muscle ribosome biogenesis indirectly, in addition to the direct mechanism described here. Chemotherapy and cancer itself can reduce appetite and protein intake [124]. Given that sufficient protein and caloric intake is important for muscle mass maintenance [125, 126], the anorexigenic effects of chemotherapy can further impact muscle anabolism. Protein and amino acids, especially leucine, is a known modulator of protein synthesis, via the mTOR pathway. Leucine and protein intake has been shown to modulate the muscle ribosome biogenesis response to exercise [127, 128], and mTOR can directly regulate muscle ribosome biogenesis [129]. Therefore, in addition to directly targeting rDNA transcription, it is possible that reduced protein/amino acid intake due to loss of appetite also impacts muscle ribosome biogenesis and further exacerbates muscle loss during chemotherapy.
Recently, a new drug that targets RNA Polymerase I (Pol I) transcription activity—CX-5461—has been tested [60, 130, 131]. CX-5461, and other similar drugs targeting Pol I, such as CX-3543 and BMH-21, have been shown to reduce tumor growth in vitro and in vivo [73, 75, 130,131,132,133]. However, these drugs will also target RNA Pol I in muscle cells. CX-5461 has been recently shown to block rDNA transcription leading to impaired muscle growth in tissue culture [93, 129]. Hence, although the results on cancer growth appear promising, we are particularly concerned that chemotherapy drugs that targets Pol I activity in cancer cells, will also affect ribosome biogenesis in healthy tissue, specifically in skeletal muscle. The long-term effects of chemotherapy drugs on skeletal muscle are not well understood, and clearly warrants further research.
Physical inactivity further inhibit muscle ribosome biogenesis
A meta-analysis showed that recreational physical activity overall reduces cancer mortality in the general population in a dose-dependent way [134], and in cancer survivors [134, 135]. Whereas increased physical activity is protective, inactivity has also been linked to increased mortality risk among cancer patients [136]. We recently proposed that a minimal amount of physical activity is required to maintain ribosome biogenesis and translational capacity [100], which may help explain the relationship between physical activity, muscle maintenance and survival among cancer patients. Advanced cancer patients often show significantly lower levels of daily physical activities for various reasons such as bed rest from surgery and hospitalization, or increased fatigue and perception of effort from simple daily tasks [137, 138]. It is well established that repeated muscle disuse events can result in rapid muscle loss [139]. We recently demonstrated that muscle disuse rapidly reduces skeletal muscle ribosome biogenesis, preceding muscle loss [100]. Within several hours to a single day, it is already possible to detect lower levels of rDNA transcription in the mouse soleus muscle that is associated with decreased protein synthesis [100, 140]. In addition to ribosome biogenesis, ribosome degradation (possible via ribophagy) can further negatively affect translational capacity in skeletal muscle. Indeed, bouts of disuse robustly increased ribosome degradation rates in skeletal muscle [100].
Combined, chemotherapy and muscle disuse may exacerbate or accelerate the onset of muscle wasting, which we hypothesized is due to an even greater negative effect on muscle ribosome biogenesis than the effects of cancer cachexia alone. Furthermore, muscle disuse can also affect ribosome degradation which further reduce translational capacity. The detrimental effect on ribosome biogenesis and degradation impairs translational capacity in muscle cells, which, we argue may be part of the persistent anabolic resistance, in which muscle lost is not easily regained. We hypothesize that the refractory state [141], where muscle lost is difficult to regain is partially due to a loss of ribosomal content as a result of impaired ribosome biogenesis during chemotherapy. Our data also demonstrate that during return to ambulation, rats restore ribosome biogenesis and muscle ribosomal mass. We further speculate that cancer patients may have an impaired ability to respond to an increase in activity due to a lower capacity to restore ribosome biogenesis precisely due to chemotherapy treatment.
Older population is more likely to suffer from cancer and muscle loss
Although cancer can develop at any age, the majority of cancer patients are found among middle-aged and elderly population (above 55 years old) [142, 143]. The incidence of invasive cancer is predominantly among persons of 55–85 years of age [143]. The elderly are already a cohort of the population vulnerable to muscle loss [144]. Sarcopenia, the loss of skeletal muscle mass with aging, is highly prevalent among men and women over 60 years old [145]. This adds another layer of complexity into the relationship between cancer, chemotherapy and skeletal muscle. Older individuals, with—or in the process of developing—sarcopenia, presenting lower levels of physical activity with treatment with chemotherapy drugs targeting ribosome biogenesis may pose a significant additional burden on the ability of skeletal muscle to maintain size.
Initially, different studies have suggested that aging decreases rates of basal muscle protein synthesis [146, 147], while others did not find such effect [148,149,150]. More recently, the underlying mechanism leading to the progression of sarcopenia has been suggested to be the result of ‘anabolic resistance’, which is the impaired response of muscle (particular muscle protein synthesis) to an anabolic stimulus (exercise and nutrition) [151]. Indeed, it appears that older individuals may require twice the recommended amount to protein just to maintain muscle mass [125, 152]. Also, it has also been proposed a “catabolic crisis”, in which acute events of muscle disuse in the elderly (hospitalization, bed rest, etc.) reduces muscle protein synthesis accelerating muscle atrophy [153]. Furthermore, ribosome biogenesis, in particular the response to an anabolic stimulus, has also been implicated in anabolic resistance observed with aging [154,155,156]. Hence, the initiation and progression of sarcopenia are multifactorial and likely have more than one underlying molecular mechanism which may involve ribosome biogenesis and muscle protein synthesis, whether at rest and/or in response to exercise or nutrition.
Most of the mechanisms thought to explain the progression of sarcopenia, combined or individually, will potentially be affected by chemotherapy [124]. In particular, chemotherapy, as stated here, can affect muscle ribosome biogenesis, which may further accelerate sarcopenia. Thus, entering the middle-age with “reserve” muscle mass may be an important ally to counterattack the detrimental effects of chemotherapy and cancer cachexia on physical disabilities. We believe that promoting exercise programs that are focused in muscle mass gains during middle-age, have the potential to improve life-span and health-span in the elderly.
Promoting muscle ribosome biogenesis to countermeasure cachexia
As proposed herein, targeting ribosome biogenesis in cancer patients via chemotherapy is counterproductive for efforts directed at minimizing additional loses in skeletal muscle during cachexia. Naturally, the primary goal of chemotherapy is to eradicate cancer cells or mitigate tumor growth. However, since skeletal muscle is one of the primary tissues involved in the development and progression of cancer cachexia, with direct effects on patient toxicity and survival rates, we posit that skeletal muscle health must be taken into consideration in the treatment of cancer.
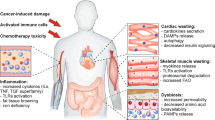
The trio—chemotherapy, physical inactivity and aging—may have combined effects on skeletal muscle ribosome biogenesis and ribophagy, leading to muscle loss and persistent difficulty in restoring muscle mass in cancer patients, worsening cancer cachexia (Fig. 2). So far, the best-known tool to promote of muscle ribosome biogenesis is exercise, particularly resistance training. However, increasing physical activity alone or avoiding sedentary lifestyle is already beneficial to muscle and overall health. Physical activity promotes positive changes in quality of life, such as improved fatigue resistance among cancer patients [157,158,159,160]. Furthermore, physical activity has also been associated with improved survival rates among cancer patients [161, 162].
Blocking ribosome biogenesis can blunt cell growth in tumor and muscle tissue. Chemotherapy drugs, such as RNA Pol I inhibitors, can blunt rDNA transcription in both cancer and muscle cells. Other risk factors, such as aging/sarcopenia and physical inactivity will further impact negatively on muscle ribosome biogenesis. These factors can decrease translation capacity on muscle via decreased ribosome biogenesis combined with the effect of physical inactivity on ribophagy. Resistance training and physical activity can be alternative strategy to drive ribosome biogenesis specifically in muscle tissue which can help mitigate the detrimental effects of chemotherapy and cancer on skeletal muscle. This figure was created using graphic elements from Servier Medical Art (https://smart.servier.com/)
However, whenever possible, cancer patients should be directed to resistance training programs aiming to gain muscle mass to counterattack the detrimental effects of the aforementioned trio on muscle translational capacity. Because resistance training has been shown to promote muscle ribosome biogenesis in healthy humans [92, 93, 156, 163, 164], resistance exercise can be an effective strategy to counteract the inhibitory effects of chemotherapy on muscle specifically regarding muscle ribosome biogenesis. Resistance training increases muscle strength in cancer patients [157, 165, 166], cancer survivors [167, 168], even in those undergoing chemotherapy [169, 170]. Additionally, resistance training also helps maintain or increase lean mass/muscle size in cancer patients [106, 171,172,173]. Hence, resistance training can be a treatment to restore or prevent the further loss of muscle mass in cancer patients while undergoing chemotherapy.
Indeed, both physical activity and resistance training has been recommended for cancer patients [21, 158, 174]. High-intensity resistance exercise has been shown to be well tolerated in advanced stage cancer patients undergoing chemotherapy [170]; however, this may require further assessment regarding disease stage. Even though exercise is generally well tolerated and guidelines have been formulated to assist oncologist in the prevention or treatment of disabilities in cancer patients [174], its prescription has been neglected among oncologists for some types of cancer [175]. Although that is not without reasoning, since it appears that the risk-to-benefit ratio has not been delineated enough to provide oncologists with a high-level of evidence to endorse safe prescription of resistance exercise [175]. That is especially true for unsupervised exercise [175]. However, this could be overcome by proper pre-exercise screening, individualized prescription [176], supervised training [177], and development of exercise facilities where cancer patients have proper supervision by trained staff when patients require closer care during exercise [174, 175].
In addition to avoiding physical inactivity behavior and prescribing resistance training as tools to promote muscle mass in cancer patients undergoing chemotherapy, we hope that this review bring the perspective of muscle ribosome biogenesis into cancer treatment and, perhaps in the future, be part of the drug selection criteria. While a chemotherapy drug that affects ribosome biogenesis may be well tolerated in non-cachectic patients, cachectic or pre-cachetic patients may benefit more from chemotherapy that does not affect directly ribosome biogenesis, which the benefits on muscle mass may outweigh the potential benefits on the tumor. Drugs that have no direct effect on ribosome biogenesis, such as Gemcitabine or Selumetinib, may be an option. Chemotherapy prescription is complex and depends on a variety of factors [178]; however, the mechanism of action of a particular chemotherapy drug may be important to take in consideration depending on the patient health conditions, i.e., drugs targeting cancer cells via ribosome biogenesis may impact muscle mass and patient ability to recover muscle mass. Novel treatments are clearly required to treat muscle wasting and we think targeting skeletal muscle ribosome biogenesis should be a key factor for these endeavors.
Conclusion and future directions
Treating cancer via chemotherapy drugs can lead to robust detrimental effects in advanced cancer patients that may lead to severe sequelae. Treating cancer cachexia is a major challenge that must be addressed to prevent skeletal muscle mass loss and physical disabilities, and improve of quality of life [4]. In fact, treating muscle mass loss may increase survival and life span in cancer patients. Future studies should address whether targeting ribosome biogenesis via chemotherapy may actually turn out to be counterproductive to survival rates in cachectic patients. Chemotherapy in those patients should be carefully evaluated to avoid further loss of muscle mass, for instance, a chemotherapy that targets Pol I and ribosome biogenesis will also affect skeletal muscle. The combination of chemotherapy, aging and physical inactivity may further affect muscle ribosome biogenesis and the capacity of muscle cells to maintain adequate protein synthesis, leading to muscle wasting. Whenever possible, patients should be counseled to perform exercise to mitigate loss of muscle mass and its maintenance, as resistance exercise training is the only strategy currently known to promote skeletal muscle ribosome biogenesis specifically and solely in skeletal muscle. Pre-cachectic patients should be directed towards program that aim for muscle hypertrophy. It would be beneficial that cancer patients start a resistance exercise training program before chemotherapy treatment, maintained during chemotherapy, and following chemotherapy to recover loss of muscle mass. A future therapeutic challenge will be to design more efficient modes of delivery to minimize the uptake of chemotherapy drugs by skeletal muscle and other healthy tissues in combination with novel drugs that selectively promote ribosome biogenesis in skeletal muscle.
Availability of data and material
Not applicable.
Code availability
Not applicable.
References
Siegel RL, Miller KD, Jemal A (2016) Cancer statistics, 2016. CA Cancer J Clin 66(1):7–30
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A (2015) Global cancer statistics, 2012. CA Cancer J Clin 65(2):87–108
Plaza JA, Perez-Montiel D, Mayerson J, Morrison C, Suster S (2008) Metastases to soft tissue: a review of 118 cases over a 30-year period. Cancer 112(1):193–203
Dodson S, Baracos VE, Jatoi A, Evans WJ, Cella D, Dalton JT et al (2011) Muscle wasting in cancer cachexia: clinical implications, diagnosis, and emerging treatment strategies. Annu Rev Med 62:265–279
Muscaritoli M, Anker SD, Argiles J, Aversa Z, Bauer JM, Biolo G et al (2010) Consensus definition of sarcopenia, cachexia and pre-cachexia: joint document elaborated by Special Interest Groups (SIG) “cachexia-anorexia in chronic wasting diseases” and “nutrition in geriatrics.” Clin Nutr 29(2):154–159
Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL et al (2011) Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 12(5):489–495
Blauwhoff-Buskermolen S, de van der Schueren MA, Verheul HM, Langius JA (2014) “Pre-cachexia”: a non-existing phenomenon in cancer? Ann Oncol 25(8):1668–1669
Blum D, Stene GB, Solheim TS, Fayers P, Hjermstad MJ, Baracos VE et al (2014) Validation of the consensus-definition for cancer cachexia and evaluation of a classification model—a study based on data from an international multicentre project (EPCRC-CSA). Ann Oncol 25(8):1635–1642
Pin F, Barreto R, Couch ME, Bonetto A, O’Connell TM (2019) Cachexia induced by cancer and chemotherapy yield distinct perturbations to energy metabolism. J Cachexia Sarcopenia Muscle 10(1):140–154
Damrauer JS, Stadler ME, Acharyya S, Baldwin AS, Couch ME, Guttridge DC (2018) Chemotherapy-induced muscle wasting: association with NF-κB and cancer cachexia. Eur J Transl Myol 28(2):7590
Naito T, Okayama T, Aoyama T, Ohashi T, Masuda Y, Kimura M et al (2017) Skeletal muscle depletion during chemotherapy has a large impact on physical function in elderly Japanese patients with advanced non-small-cell lung cancer. BMC Cancer 17(1):571
Daly LE, Ni Bhuachalla EB, Power DG, Cushen SJ, James K, Ryan AM (2018) Loss of skeletal muscle during systemic chemotherapy is prognostic of poor survival in patients with foregut cancer. J Cachexia Sarcopenia Muscle 9(2):315–325
Thomas G (2000) An encore for ribosome biogenesis in the control of cell proliferation. Nat Cell Biol 2(5):E71–E72
Derenzini M, Montanaro L, Trere D (2017) Ribosome biogenesis and cancer. Acta Histochem 119(3):190–197
Goldstein M, Kastan MB (2015) The DNA damage response: implications for tumor responses to radiation and chemotherapy. Annu Rev Med 66:129–143
Bouwman P, Jonkers J (2012) The effects of deregulated DNA damage signalling on cancer chemotherapy response and resistance. Nat Rev Cancer 12(9):587–598
Figueiredo VC, McCarthy JJ (2019) Regulation of ribosome biogenesis in skeletal muscle hypertrophy. Physiology (Bethesda) 34(1):30–42
Schiaffino S, Dyar KA, Ciciliot S, Blaauw B, Sandri M (2013) Mechanisms regulating skeletal muscle growth and atrophy. FEBS J 280(17):4294–4314
von Haehling S, Anker SD (2010) Cachexia as a major underestimated and unmet medical need: facts and numbers. J Cachexia Sarcopenia Muscle 1(1):1–5
Ruiz JR, Sui X, Lobelo F, Morrow JR, Jackson AW, Sjöström M et al (2008) Association between muscular strength and mortality in men: prospective cohort study. BMJ (Clin. Res Ed) 337:439
Ruiz JR, Sui X, Lobelo F, Lee D-C, Morrow JR, Jackson AW et al (2009) Muscular strength and adiposity as predictors of adulthood cancer mortality in men. Cancer Epidemiol Biomarkers Prev 18(5):1468–1476
Gale CR, Martyn CN, Cooper C, Sayer AA (2007) Grip strength, body composition, and mortality. Int J Epidemiol 36(1):228–235
Villaseñor A, Ballard-Barbash R, Baumgartner K, Baumgartner R, Bernstein L, McTiernan A et al (2012) Prevalence and prognostic effect of sarcopenia in breast cancer survivors: the HEAL Study. J Cancer Survivorship Res Pract 6(4):398–406
Martin L, Birdsell L, Macdonald N, Reiman T, Clandinin MT, McCargar LJ et al (2013) Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol 31(12):1539–1547
Blauwhoff-Buskermolen S, Versteeg KS, de van der Schueren MAE, den Braver NR, Berkhof J, Langius JAE et al (2016) Loss of muscle mass during chemotherapy is predictive for poor survival of patients with metastatic colorectal cancer. J Clin Oncol 34(12):1339–1344
Metter EJ, Talbot LA, Schrager M, Conwit R (2002) Skeletal muscle strength as a predictor of all-cause mortality in healthy men. J Gerontol A Biol Sci Med Sci 57(10):B359–B365
Newman AB, Kupelian V, Visser M, Simonsick EM, Goodpaster BH, Kritchevsky SB et al (2006) Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J Gerontol A Biol Sci Med Sci 61(1):72–77
Rantanen T, Harris T, Leveille SG, Visser M, Foley D, Masaki K et al (2000) Muscle strength and body mass index as long-term predictors of mortality in initially healthy men. J Gerontol A Biol Sci Med Sci 55(3):M168–M173
Prado CMM, Baracos VE, McCargar LJ, Reiman T, Mourtzakis M, Tonkin K et al (2009) Sarcopenia as a determinant of chemotherapy toxicity and time to tumor progression in metastatic breast cancer patients receiving capecitabine treatment. Clin Cancer Res 15(8):2920–2926
da Rocha IMG, Marcadenti A, de Medeiros GOC, Bezerra RA, Rego JFM, Gonzalez MC et al (2019) Is cachexia associated with chemotherapy toxicities in gastrointestinal cancer patients? A prospective study. J Cachexia Sarcopenia Muscle 10(2):445–454
Zhou X, Wang JL, Lu J, Song Y, Kwak KS, Jiao Q et al (2010) Reversal of cancer cachexia and muscle wasting by ActRIIB antagonism leads to prolonged survival. Cell 142(4):531–543
Wannamethee SG, Shaper AG, Lennon L, Whincup PH (2007) Decreased muscle mass and increased central adiposity are independently related to mortality in older men. Am J Clin Nutr 86(5):1339–1346
Mueller TC, Bachmann J, Prokopchuk O, Friess H, Martignoni ME (2016) Molecular pathways leading to loss of skeletal muscle mass in cancer cachexia—can findings from animal models be translated to humans? BMC Cancer 8(16):75
Tisdale MJ (2002) Cachexia in cancer patients. Nat Rev Cancer 2(11):862–871
Smith KL, Tisdale MJ (1993) Increased protein degradation and decreased protein synthesis in skeletal muscle during cancer cachexia. Br J Cancer 67(4):680–685
Samuels SE, Knowles AL, Tilignac T, Debiton E, Madelmont JC, Attaix D (2001) Higher skeletal muscle protein synthesis and lower breakdown after chemotherapy in cachectic mice. Am J Physiol Regul Integr Comp Physiol 281(1):R133–R139
Lundholm K, Bylund AC, Holm J, Scherstén T (1976) Skeletal muscle metabolism in patients with malignant tumor. Eur J Cancer 12(6):465–473
White JP, Baynes JW, Welle SL, Kostek MC, Matesic LE, Sato S et al (2011) The regulation of skeletal muscle protein turnover during the progression of cancer cachexia in the Apc(Min/+) mouse. PLoS ONE 6(9):e24650
Emery PW, Edwards RH, Rennie MJ, Souhami RL, Halliday D (1984) Protein synthesis in muscle measured in vivo in cachectic patients with cancer. Br Med J (Clin Res Ed) 289(6445):584–586
Lautaoja JH, Lalowski M, Nissinen TA, Hentila J, Shi Y, Ritvos O et al (2019) Muscle and serum metabolomes are dysregulated in colon-26 tumor-bearing mice despite amelioration of cachexia with activin receptor type 2B ligand blockade. Am J Physiol Endocrinol Metab 316(5):E852–E865
Kim HG, Huot JR, Pin F, Guo B, Bonetto A, Nader GA (2021) Reduced rDNA transcription diminishes skeletal muscle ribosomal capacity and protein synthesis in cancer cachexia. FASEB J 35(2):e21335
Preston-Martin S, Pike MC, Ross RK, Jones PA, Henderson BE (1990) Increased cell division as a cause of human cancer. Can Res 50(23):7415–7421
Giancotti FG (2014) Deregulation of cell signaling in cancer. FEBS Lett 588(16):2558–2570
Evan GI, Vousden KH (2001) Proliferation, cell cycle and apoptosis in cancer. Nature 411(6835):342–348
DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB (2008) The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab 7(1):11–20
van Riggelen J, Yetil A, Felsher DW (2010) MYC as a regulator of ribosome biogenesis and protein synthesis. Nat Rev Cancer 10(4):301–309
Guertin DA, Sabatini DM (2007) Defining the role of mTOR in cancer. Cancer Cell 12(1):9–22
Bader AG, Vogt PK (2004) An essential role for protein synthesis in oncogenic cellular transformation. Oncogene 23(18):3145–3150
Altomare DA, Testa JR (2005) Perturbations of the AKT signaling pathway in human cancer. Oncogene 24(50):7455–7464
Janku F, Yap TA, Meric-Bernstam F (2018) Targeting the PI3K pathway in cancer: are we making headway? Nat Rev Clin Oncol 15(5):273–291
Henras AK, Plisson-Chastang C, O’Donohue MF, Chakraborty A, Gleizes PE (2015) An overview of pre-ribosomal RNA processing in eukaryotes. Wiley Interdiscipl Rev RNA 6(2):225–242
Drygin D, Rice WG, Grummt I (2010) The RNA polymerase I transcription machinery: an emerging target for the treatment of cancer. Annu Rev Pharmacol Toxicol 50(1):131–156
Mayer C, Grummt I (2006) Ribosome biogenesis and cell growth: mTOR coordinates transcription by all three classes of nuclear RNA polymerases. Oncogene 25(48):6384–6391
van Sluis M, McStay B (2014) Ribosome biogenesis: achilles heel of cancer? Genes Cancer 5(5–6):152–153
Ruggero D, Pandolfi PP (2003) Does the ribosome translate cancer? Nat Rev Cancer 3(3):179–192
Rosenwald IB (2004) The role of translation in neoplastic transformation from a pathologist’s point of view. Oncogene 23(18):3230–3247
Montanaro L, Treré D, Derenzini M (2008) Nucleolus, ribosomes, and cancer. Am J Pathol 173(2):301–310
Burger K, Mühl B, Harasim T, Rohrmoser M, Malamoussi A, Orban M et al (2010) Chemotherapeutic drugs inhibit ribosome biogenesis at various levels. J Biol Chem 285(16):12416–12425
Freed EF, Bleichert F, Dutca LM, Baserga SJ (2010) When ribosomes go bad: diseases of ribosome biogenesis. Mol BioSyst 6(3):481–493
Hannan RD, Drygin D, Pearson RB (2013) Targeting RNA polymerase I transcription and the nucleolus for cancer therapy. Expert Opin Ther Targets 17(8):873–878
Hein N, Hannan KM, George AJ, Sanij E, Hannan RD (2013) The nucleolus: an emerging target for cancer therapy. Trends Mol Med 19(11):643–654
Poortinga G, Quinn LM, Hannan RD (2015) Targeting RNA polymerase I to treat MYC-driven cancer. Oncogene 34(4):403–412
Quin JE, Devlin JR, Cameron D, Hannan KM, Pearson RB, Hannan RD (2014) Targeting the nucleolus for cancer intervention. Biochem Biophys Acta 1842(6):802–816
Tsai RYL, Pederson T (2014) Connecting the nucleolus to the cell cycle and human disease. FASEB J 28(8):3290–3296
Chan JC, Hannan KM, Riddell K, Ng PY, Peck A, Lee RS et al (2011) AKT promotes rRNA synthesis and cooperates with c-MYC to stimulate ribosome biogenesis in cancer. Sci signal 4(188):ra56-ra
Iadevaia V, Liu R, Proud CG (2014) mTORC1 signaling controls multiple steps in ribosome biogenesis. Semin Cell Dev Biol 36:113–120
Guo QM, Malek RL, Kim S, Chiao C, He M, Ruffy M et al (2000) Identification of c-myc responsive genes using rat cDNA microarray. Can Res 60(21):5922–5928
Schlosser I, Hölzel M, Mürnseer M, Burtscher H, Weidle UH, Eick D (2003) A role for c-Myc in the regulation of ribosomal RNA processing. Nucleic Acids Res 31(21):6148–6156
Hirai H, Sootome H, Nakatsuru Y, Miyama K, Taguchi S, Tsujioka K et al (2010) MK-2206, an allosteric Akt inhibitor, enhances antitumor efficacy by standard chemotherapeutic agents or molecular targeted drugs in vitro and in vivo. Mol Cancer Ther 9(7):1956–1967
Ahn DH, Li J, Wei L, Doyle A, Marshall JL, Schaaf LJ et al (2015) Results of an abbreviated phase-II study with the Akt Inhibitor MK-2206 in patients with advanced biliary cancer. Sci Rep 5:12122
Meng LH, Zheng XF (2015) Toward rapamycin analog (rapalog)-based precision cancer therapy. Acta Pharmacol Sin 36(10):1163–1169
Xie J, Wang X, Proud CG. mTOR inhibitors in cancer therapy. F1000Res. 2016;5
Drygin D, Siddiqui-Jain A, O’Brien S, Schwaebe M, Lin A, Bliesath J et al (2009) Anticancer activity of CX-3543: a direct inhibitor of rRNA biogenesis. Can Res 69(19):7653–7661
O’Brien S, Drygin D, Harrison SJ, Khot A, Cullinane C, Geoff M et al (2013) Inhibition of RNA polymerase I transcription by CX-5461 as a therapeutic strategy for the cancer-specific activation of p53 in highly refractory haematological malignancies. Blood 122(21):3941
Drygin D, Lin A, Bliesath J, Ho CB, O’Brien SE, Proffitt C et al (2011) Targeting RNA polymerase I with an oral small molecule CX-5461 inhibits ribosomal RNA synthesis and solid tumor growth. Cancer Res 71(4):1418–1430
Peltonen K, Colis L, Liu H, Trivedi R, Moubarek MS, Moore HM et al (2014) A targeting modality for destruction of RNA polymerase I that possesses anticancer activity. Cancer Cell 25(1):77–90
Ferreira R, Schneekloth JS Jr, Panov KI, Hannan KM, Hannan RD (2020) Targeting the RNA polymerase I transcription for cancer therapy comes of age. Cells 9(2):266
Khot A, Brajanovski N, Cameron DP, Hein N, Maclachlan KH, Sanij E et al (2019) First-in-human RNA polymerase I transcription inhibitor CX-5461 in patients with advanced hematologic cancers: results of a phase I dose-escalation study. Cancer Discov 9(8):1036–1049
von Walden F, Casagrande V, Ostlund Farrants K, Nader G (2012) Mechanical loading induces the expression of a Pol I regulon at the onset of skeletal muscle hypertrophy. AJP Cell Physiol 302(10):C1523–C1530
Whitelaw PF, Hesketh JE (1992) Expression of c-myc and c-fos in rat skeletal muscle. Evidence for increased levels of c-myc mRNA during hypertrophy. Biochem J 281(Pt 1):143–147
Lai K-MV, Gonzalez M, Poueymirou WT, Kline WO, Na E, Zlotchenko E et al (2004) Conditional activation of akt in adult skeletal muscle induces rapid hypertrophy. Mol Cell Biol 24(21):9295–9304
Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R et al (2001) Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol 3(11):1014–1019
Blaauw B, Canato M, Agatea L, Toniolo L, Mammucari C, Masiero E et al (2009) Inducible activation of Akt increases skeletal muscle mass and force without satellite cell activation. FASEB J 23(11):3896–3905
Saxton RA, Sabatini DM (2017) mTOR signaling in growth, metabolism, and disease. Cell 169(2):361–371
Goodman CA, Miu MH, Frey JW, Mabrey DM, Lincoln HC, Ge Y et al (2010) A phosphatidylinositol 3-kinase/protein kinase B-independent activation of mammalian target of rapamycin signaling is sufficient to induce skeletal muscle hypertrophy. Mol Biol Cell 21(18):3258–3268
Drummond MJ, Fry CS, Glynn EL, Dreyer HC, Dhanani S, Timmerman KL et al (2009) Rapamycin administration in humans blocks the contraction-induced increase in skeletal muscle protein synthesis. J Physiol 587(Pt 7):1535–1546
Anthony JC, Yoshizawa F, Anthony TG, Vary TC, Jefferson LS, Kimball SR (2000) Leucine stimulates translation initiation in skeletal muscle of postabsorptive rats via a rapamycin-sensitive pathway. J Nutr 130(10):2413–2419
Wen Y, Alimov AP, McCarthy JJ (2016) Ribosome biogenesis is necessary for skeletal muscle hypertrophy. Exerc Sport Sci Rev 44(3):110–115
Figueiredo VC (2019) Revisiting the roles of protein synthesis during skeletal muscle hypertrophy induced by exercise. Am J Physiol Regul Integr Comp Physiol 317(5):R709–R718
Nakada S, Ogasawara R, Kawada S, Maekawa T, Ishii N (2016) Correlation between ribosome biogenesis and the magnitude of hypertrophy in overloaded skeletal muscle. PLoS ONE 11(1):e0147284
Figueiredo VC, Englund DA, Vechetti IJ Jr, Alimov A, Peterson CA, McCarthy JJ (2019) Phosphorylation of eukaryotic initiation factor 4E is dispensable for skeletal muscle hypertrophy. Am J Physiol Cell Physiol 317(6):C1247–C1255
Figueiredo VC, Caldow MK, Massie V, Markworth JF, Cameron-Smith D, Blazevich AJ (2015) Ribosome biogenesis adaptation in resistance training-induced human skeletal muscle hypertrophy. Am J Physiol Endocrinol Metab 309(1):E72-83
Stec MJ, Kelly NA, Many GM, Windham ST, Tuggle SC, Bamman MM (2016) Ribosome biogenesis may augment resistance training-induced myofiber hypertrophy and is required for myotube growth in vitro. Am J Physiol Endocrinol Metab 310:E652–E661
Hammarstrom D, Ofsteng S, Koll L, Hanestadhaugen M, Hollan I, Apro W et al (2020) Benefits of higher resistance-training volume are related to ribosome biogenesis. J Physiol 598(3):543–565
Machida M, Takeda K, Yokono H, Ikemune S, Taniguchi Y, Kiyosawa H et al (2012) Reduction of ribosome biogenesis with activation of the mTOR pathway in denervated atrophic muscle. J Cell Physiol 227(4):1569–1576
Connolly M, Paul R, Farre-Garros R, Natanek SA, Bloch S, Lee J et al (2018) miR-424-5p reduces ribosomal RNA and protein synthesis in muscle wasting. J Cachexia Sarcopenia Muscle 9(2):400–416
Figueiredo VC, Markworth JF, Durainayagam BR, Pileggi CA, Roy NC, Barnett MP et al (2016) Impaired ribosome biogenesis and skeletal muscle growth in a murine model of inflammatory bowel disease. Inflamm Bowel Dis 22(2):268–278
Von Walden F, Gantelius S, Liu C, Borgstrom H, Bjork L, Gremark O et al (2018) Muscle contractures in patients with cerebral palsy and acquired brain injury are associated with extracellular matrix expansion, pro-inflammatory gene expression, and reduced rRNA synthesis. Muscle Nerve 58(2):277–285
Fiorotto ML, Davis T, Sosa H, Villegas-Montoya C, Estrada I, Fleischmann R (2014) Ribosome abundance regulates the recovery of skeletal muscle protein mass upon recuperation from postnatal undernutrition in mice. J Physiol 592:5269–5286
Figueiredo VC, D’Souza RF, Van Pelt DW, Lawrence MM, Zeng N, Markworth JF et al (2021) Ribosome biogenesis and degradation regulate translational capacity during muscle disuse and reloading. J Cachexia Sarcopenia Muscle 12(1):130–143
Blagosklonny MV, Pardee AB (2001) Exploiting cancer cell cycling for selective protection of normal cells. Cancer Res 61(11):4301–4305
Minchinton AI, Tannock IF (2006) Drug penetration in solid tumours. Nat Rev Cancer 6(8):583–592
van Asperen J, van Tellingen O, Tijssen F, Schinkel AH, Beijnen JH (1999) Increased accumulation of doxorubicin and doxorubicinol in cardiac tissue of mice lacking mdr1a P-glycoprotein. Br J Cancer 79(1):108–113
Anderson LL, Collins GJ, Ojima Y, Sullivan RD (1970) A study of the distribution of methotrexate in human tissues and tumors. Cancer Res 30(5):1344–1348
Jang MK, Park C, Hong S, Li H, Rhee E, Doorenbos AZ (2020) Skeletal muscle mass change during chemotherapy: a systematic review and meta-analysis. Anticancer Res 40(5):2409–2418
Mijwel S, Cardinale DA, Norrbom J, Chapman M, Ivarsson N, Wengstrom Y et al (2018) Exercise training during chemotherapy preserves skeletal muscle fiber area, capillarization, and mitochondrial content in patients with breast cancer. FASEB J 32(10):5495–5505
Jacobsen PB, Donovan KA, Small BJ, Jim HS, Munster PN, Andrykowski MA (2007) Fatigue after treatment for early stage breast cancer: a controlled comparison. Cancer 110(8):1851–1859
Hydock DS, Lien C-Y, Jensen BT, Schneider CM, Hayward R (2011) Characterization of the effect of in vivo doxorubicin treatment on skeletal muscle function in the rat. Anticancer Res 31(6):2023–2028
Hayward R, Hydock D, Gibson N, Greufe S, Bredahl E, Parry T (2013) Tissue retention of doxorubicin and its effects on cardiac, smooth, and skeletal muscle function. J Physiol Biochem 69(2):177–187
Fabris S, MacLean DA (2015) Skeletal muscle an active compartment in the sequestering and metabolism of doxorubicin chemotherapy. PLoS ONE 10(9):e0139070
de Lima Junior EA, Yamashita AS, Pimentel GD, De Sousa LG, Santos RV, Goncalves CL et al (2016) Doxorubicin caused severe hyperglycaemia and insulin resistance, mediated by inhibition in AMPk signalling in skeletal muscle. J Cachexia Sarcopenia Muscle 7(5):615–625
Nitiss JL (2009) Targeting DNA topoisomerase II in cancer chemotherapy. Nat Rev Cancer 9(5):338–350
Ray S, Panova T, Miller G, Volkov A, Porter AC, Russell J et al (2013) Topoisomerase IIalpha promotes activation of RNA polymerase I transcription by facilitating pre-initiation complex formation. Nat Commun 4:1598
Guigni BA, Fix DK, Bivona JJ 3rd, Palmer BM, Carson JA, Toth MJ (2019) Electrical stimulation prevents doxorubicin-induced atrophy and mitochondrial loss in cultured myotubes. Am J Physiol Cell Physiol 317(6):C1213–C1228
Bruno PM, Liu Y, Park GY, Murai J, Koch CE, Eisen TJ et al (2017) A subset of platinum-containing chemotherapeutic agents kills cells by inducing ribosome biogenesis stress. Nat Med 23(4):461–471
Guinan EM, Doyle SL, Bennett AE, O’Neill L, Gannon J, Elliott JA et al (2018) Sarcopenia during neoadjuvant therapy for oesophageal cancer: characterising the impact on muscle strength and physical performance. Support Care Cancer 26(5):1569–1576
Donati G, Bertoni S, Brighenti E, Vici M, Treré D, Volarevic S et al (2011) The balance between rRNA and ribosomal protein synthesis up- and downregulates the tumour suppressor p53 in mammalian cells. Oncogene 30(29):3274–3288
Valdez BC, Wang G, Murray D, Nieto Y, Li Y, Shah J et al (2013) Mechanistic studies on the synergistic cytotoxicity of the nucleoside analogs gemcitabine and clofarabine in multiple myeloma: relevance of p53 and its clinical implications. Exp Hematol 41(8):719–730
Wiedenmann B, Malfertheiner P, Friess H, Ritch P, Arseneau J, Mantovani G et al (2008) A multicenter, phase II study of infliximab plus gemcitabine in pancreatic cancer cachexia. J Support Oncol 6(1):18–25
Jiang HY, Hickey RJ, Abdel-Aziz W, Malkas LH (2000) Effects of gemcitabine and araC on in vitro DNA synthesis mediated by the human breast cell DNA synthesome. Cancer Chemother Pharmacol 45(4):320–328
RuizvanHaperen VW, Veerman G, Vermorken JB, Peters GJ (1993) 2’,2’-Difluoro-deoxycytidine (gemcitabine) incorporation into RNA and DNA of tumour cell lines. Biochem Pharmacol 46(4):762–766
Prado CM, Bekaii-Saab T, Doyle LA, Shrestha S, Ghosh S, Baracos VE et al (2012) Skeletal muscle anabolism is a side effect of therapy with the MEK inhibitor: selumetinib in patients with cholangiocarcinoma. Br J Cancer 106(10):1583–1586
Quan-Jun Y, Yan H, Yong-Long H, Li-Li W, Jie L, Jin-Lu H et al (2017) Selumetinib attenuates skeletal muscle wasting in murine cachexia model through ERK inhibition and AKT activation. Mol Cancer Ther 16(2):334–343
Prado CM, Purcell SA, Laviano A (2020) Nutrition interventions to treat low muscle mass in cancer. J Cachexia Sarcopenia Muscle 11(2):366–380
Mitchell CJ, Milan AM, Mitchell SM, Zeng N, Ramzan F, Sharma P et al (2017) The effects of dietary protein intake on appendicular lean mass and muscle function in elderly men: a 10-wk randomized controlled trial. Am J Clin Nutr 106(6):1375–1383
McKendry J, Thomas ACQ, Phillips SM (2020) Muscle mass loss in the older critically ill population: potential therapeutic strategies. Nutr Clin Pract 35(4):607–616
Figueiredo VC, Zeng N, D’Souza RF, Markworth JF, Della Gatta PA, Petersen A et al (2018) High dose of whey protein after resistance exercise promotes 45 S preribosomal RNA synthesis in older men. Nutrition 50:105–107
Mobley CB, Fox CD, Thompson RM, Healy JC, Santucci V, Kephart WC et al (2015) Comparative effects of whey protein versus L-leucine on skeletal muscle protein synthesis and markers of ribosome biogenesis following resistance exercise. Amino Acids 48(3):733–750
von Walden F, Liu C, Aurigemma N, Nader GA (2016) mTOR signaling regulates myotube hypertrophy by modulating protein synthesis, rDNA transcription and chromatin remodeling. Am J Physiol Cell Physiol 311(4):C663–C672
Haddach M, Schwaebe MK, Michaux J, Nagasawa J, O’Brien SE, Whitten JP et al (2012) Discovery of CX-5461, the first direct and selective inhibitor of RNA polymerase I, for cancer therapeutics. ACS Med Chem Lett 3(7):602–606
Bywater MJ, Poortinga G, Sanij E, Hein N, Peck A, Cullinane C et al (2012) Inhibition of RNA polymerase I as a therapeutic strategy to promote cancer-specific activation of p53. Cancer Cell 22(1):51–65
Negi SS, Brown P (2015) rRNA synthesis inhibitor, CX-5461, activates ATM/ATR pathway in acute lymphoblastic leukemia, arrests cells in G2 phase and induces apoptosis. Oncotarget 6(20):18094–18104
Negi SS, Brown P (2015) Transient rRNA synthesis inhibition with CX-5461 is sufficient to elicit growth arrest and cell death in acute lymphoblastic leukemia cells. Oncotarget 6(33):34846–34858
Li T, Wei S, Shi Y, Pang S, Qin Q, Yin J et al (2016) The dose-response effect of physical activity on cancer mortality: findings from 71 prospective cohort studies. Br J Sports Med 50(6):339–345
Lahart IM, Metsios GS, Nevill AM, Carmichael AR (2015) Physical activity, risk of death and recurrence in breast cancer survivors: a systematic review and meta-analysis of epidemiological studies. Acta Oncol (Stockholm, Sweden) 54(5):635–654
Campbell PT, Patel AV, Newton CC, Jacobs EJ, Gapstur SM (2013) Associations of recreational physical activity and leisure time spent sitting with colorectal cancer survival. J Clin Oncol 31(7):876–885
Morikawa A, Naito T, Sugiyama M, Okayama T, Aoyama T, Tanuma A et al (2018) Impact of cancer cachexia on hospitalization-associated physical inactivity in elderly patients with advanced non-small-cell lung cancer. Asia Pac J Oncol Nurs 5(4):377–382
Huneidi SA, Wright NC, Atkinson A, Bhatia S, Singh P (2018) Factors associated with physical inactivity in adult breast cancer survivors—a population-based study. Cancer Med 7(12):6331–6339
Kilroe SP, Fulford J, Holwerda AM, Jackman SR, Lee BP, Gijsen AP et al (2020) Short-term muscle disuse induces a rapid and sustained decline in daily myofibrillar protein synthesis rates. Am J Physiol Endocrinol Metab 318(2):E117–E130
Tyganov SA, Mochalova EP, Belova SP, Sharlo KA, Rozhkov SV, Vilchinskaya NA et al (2019) Effects of plantar mechanical stimulation on anabolic and catabolic signaling in rat postural muscle under short-term simulated gravitational unloading. Front Physiol 10:1252
Bruggeman AR, Kamal AH, LeBlanc TW, Ma JD, Baracos VE, Roeland EJ (2016) Cancer cachexia: beyond weight loss. J Oncol Pract 12(11):1163–1171
Smith BD, Smith GL, Hurria A, Hortobagyi GN, Buchholz TA (2009) Future of cancer incidence in the United States: burdens upon an aging, changing nation. J Clin Oncol 27(17):2758–2765
White MC, Holman DM, Boehm JE, Peipins LA, Grossman M, Henley SJ (2014) Age and cancer risk: a potentially modifiable relationship. Am J Prev Med 46(3 Suppl 1):S7–S15
Evans WJ (2010) Skeletal muscle loss: cachexia, sarcopenia, and inactivity. Am J Clin Nutr 91(4):1123S-S1127
Shafiee G, Keshtkar A, Soltani A, Ahadi Z, Larijani B, Heshmat R (2017) Prevalence of sarcopenia in the world: a systematic review and meta-analysis of general population studies. J Diab Metab Disord 16:21
Yarasheski KE, Zachwieja JJ, Bier DM (1993) Acute effects of resistance exercise on muscle protein synthesis rate in young and elderly men and women. Am J Physiol 265(2 Pt 1):E210–E214
Welle S, Thornton C, Statt M (1995) Myofibrillar protein synthesis in young and old human subjects after three months of resistance training. Am J Physiol 268(3 Pt 1):E422–E427
Volpi E, Mittendorfer B, Wolf SE, Wolfe RR (1999) Oral amino acids stimulate muscle protein anabolism in the elderly despite higher first-pass splanchnic extraction. Am J Physiol 277(3):E513–E520
Paddon-Jones D, Sheffield-Moore M, Zhang XJ, Volpi E, Wolf SE, Aarsland A et al (2004) Amino acid ingestion improves muscle protein synthesis in the young and elderly. Am J Physiol Endocrinol Metab 286(3):E321–E328
Volpi E, Sheffield-Moore M, Rasmussen BB, Wolfe RR (2001) Basal muscle amino acid kinetics and protein synthesis in healthy young and older men. JAMA 286(10):1206–1212
Shad BJ, Thompson JL, Breen L (2016) Does the muscle protein synthetic response to exercise and amino acid-based nutrition diminish with advancing age? A systematic review. Am J Physiol Endocrinol Metab 311(5):E803–E817
Campbell WW, Trappe TA, Wolfe RR, Evans WJ (2001) The recommended dietary allowance for protein may not be adequate for older people to maintain skeletal muscle. J Gerontol A Biol Sci Med Sci 56(6):M373–M380
English KL, Paddon-Jones D (2010) Protecting muscle mass and function in older adults during bed rest. Curr Opin Clin Nutr Metab Care 13(1):34–39
Brook MS, Wilkinson DJ, Mitchell WK, Lund JN, Phillips BE, Szewczyk NJ et al (2016) Synchronous deficits in cumulative muscle protein synthesis and ribosomal biogenesis underlie age-related anabolic resistance to exercise in humans. J Physiol 594(24):7399–7417
Kirby TJ, Lee JD, England JH, Chaillou T, Esser KA, McCarthy JJ (2015) Blunted hypertrophic response in aged skeletal muscle is associated with decreased ribosome biogenesis. J Appl Physiol 1985 119(4):321–327
Stec MJ, Mayhew DL, Bamman MM (2015) The effects of age and resistance loading on skeletal muscle ribosome biogenesis. J Appl Physiol 1985 119(8):851–857
Adamsen L, Quist M, Midtgaard J, Andersen C, Møller T, Knutsen L et al (2006) The effect of a multidimensional exercise intervention on physical capacity, well-being and quality of life in cancer patients undergoing chemotherapy. Support Care Cancer 14(2):116–127
Keogh JWL, MacLeod RD (2012) Body composition, physical fitness, functional performance, quality of life, and fatigue benefits of exercise for prostate cancer patients: a systematic review. J Pain Symptom Manage 43(1):96–110
Mustian KM, Peppone L, Darling TV, Palesh O, Heckler CE, Morrow GR (2009) A 4-week home-based aerobic and resistance exercise program during radiation therapy: a pilot randomized clinical trial. J Support Oncol 7(5):158–167
Segal RJ, Reid RD, Courneya KS, Malone SC, Parliament MB, Scott CG et al (2003) Resistance exercise in men receiving androgen deprivation therapy for prostate cancer. J Clin Oncol 21(9):1653–1659
Holmes MD, Chen WY, Feskanich D, Kroenke CH, Colditz GA (2005) Physical activity and survival after breast cancer diagnosis. JAMA 293(20):2479–2486
Meyerhardt JA, Giovannucci EL, Holmes MD, Chan AT, Chan JA, Colditz GA et al (2006) Physical activity and survival after colorectal cancer diagnosis. J Clin Oncol 24(22):3527–3534
Nader GA, von Walden F, Liu C, Lindvall J, Gutmann L, Pistilli EE et al (2014) Resistance exercise training modulates acute gene expression during human skeletal muscle hypertrophy. J Appl Physiol (Bethesda, MD, 1985) 116(6):693–702
Figueiredo VC, Roberts LA, Markworth JF, Barnett MPG, Coombes JS, Raastad T et al (2016) Impact of resistance exercise on ribosome biogenesis is acutely regulated by post-exercise recovery strategies. Physiol Rep 4(2):e12670
Galvão DA, Nosaka K, Taaffe DR, Spry N, Kristjanson LJ, McGuigan MR et al (2006) Resistance training and reduction of treatment side effects in prostate cancer patients. Med Sci Sports Exerc 38(12):2045–2052
Courneya KS, Segal RJ, Mackey JR, Gelmon K, Reid RD, Friedenreich CM et al (2007) Effects of aerobic and resistance exercise in breast cancer patients receiving adjuvant chemotherapy: a multicenter randomized controlled trial. J Clin Oncol 25(28):4396–4404
Schmitz KH, Ahmed RL, Hannan PJ, Yee D (2005) Safety and efficacy of weight training in recent breast cancer survivors to alter body composition, insulin, and insulin-like growth factor axis proteins. Cancer Epidemiol Biomarkers Prev 14(7):1672–1680
Hanson ED, Wagoner CW, Anderson T, Battaglini CL (2016) The independent effects of strength training in cancer survivors: a systematic review. Curr Oncol Rep 18(5):31
Adamsen L, Quist M, Andersen C, Møller T, Herrstedt J, Kronborg D et al (2009) Effect of a multimodal high intensity exercise intervention in cancer patients undergoing chemotherapy: randomised controlled trial. BMJ (Clin Res Ed) 339:b3410
Quist M, Rorth M, Zacho M, Andersen C, Moeller T, Midtgaard J et al (2006) High-intensity resistance and cardiovascular training improve physical capacity in cancer patients undergoing chemotherapy. Scand J Med Sci Sports 16(5):349–357
Brown JC, Schmitz KH (2015) Weight lifting and appendicular skeletal muscle mass among breast cancer survivors: a randomized controlled trial. Breast Cancer Res Treat 151(2):385–392
Galvão DA, Taaffe DR, Spry N, Joseph D, Newton RU (2010) Combined resistance and aerobic exercise program reverses muscle loss in men undergoing androgen suppression therapy for prostate cancer without bone metastases: a randomized controlled trial. J Clin Oncol 28(2):340–347
Nilsen TS, Raastad T, Skovlund E, Courneya KS, Langberg CW, Lilleby W et al (2015) Effects of strength training on body composition, physical functioning, and quality of life in prostate cancer patients during androgen deprivation therapy. Acta Oncol (Stockholm, Sweden) 54(10):1805–1813
Schmitz KH, Courneya KS, Matthews C, Demark-Wahnefried W, Galvão DA, Pinto BM et al (2010) American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc 42(7):1409–1426
Brown JC, Schmitz KH (2014) The prescription or proscription of exercise in colorectal cancer care. Med Sci Sports Exerc 46(12):2202–2209
Jones LW, Eves ND, Peppercorn J (2010) Pre-exercise screening and prescription guidelines for cancer patients. Lancet Oncol 11(10):914–916
Jones LW (2011) Evidence-based risk assessment and recommendations for physical activity clearance: cancer. Appl Physiol Nutri Metabol 36(Suppl 1):S101–S112
Panje CM, Glatzer M, Siren C, Plasswilm L, Putora PM (2018) Treatment options in oncology. JCO Clin Cancer Inform 2:1–10
Funding
The authors have no funding to disclose.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors have no competing interests.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
All authors read and approved the final manuscript for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Figueiredo, V.C., McCarthy, J.J. Targeting cancer via ribosome biogenesis: the cachexia perspective. Cell. Mol. Life Sci. 78, 5775–5787 (2021). https://doi.org/10.1007/s00018-021-03888-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-021-03888-6