Abstract
Rabbit anti-human T lymphocyte globulin (ATLG) and anti-thymocyte globulin (ATG) are commonly used for graft-versus-host disease (GVHD) prophylaxis in allogeneic hematopoietic stem cell transplantation (HSCT). Yet, their efficacy and safety have seldom been compared in hematological malignancies with haploidentical HSCT. A retrospective analysis with 28 ATLG (total dosage, 20–30 mg/kg) and 18 ATG (total dosage, 8–10 mg/kg) patients were performed. The cumulative incidences of chronic GVHD and relapse were comparable between both groups. ATLG showed a trend towards a lower acute GVHD incidence (28.6% vs. 44.4%, P = 0.242) and 3-year non-relapse mortality (10.7% vs. 27.8%, P = 0.160), and had a significantly higher 3-year overall survival (OS, 64.3% vs. 33.3%, P = 0.033) and GVHD-free and relapse-free survival (GRFS, 32.1% vs. 11.1%, P = 0.045) compared with ATG. Multivariate Cox regression analysis demonstrated ATLG was independently associated with a favorable OS (hazard ratio [HR] = 0.37, 95% confidence interval [CI]: 0.16–0.86, P = 0.020) and GRFS (HR = 0.51, 95%CI: 0.26-1.00, P = 0.051). Furthermore, ATLG had a lower risk of fever (25.0% vs. 61.1%, P = 0.014) and hemorrhage cystitis (7.1% vs. 38.9%, P = 0.008) than ATG-T. In conclusion, ATLG confers more survival benefit and a better safety profile than ATG and can be used in hematological malignancies with haploidentical HSCT. Prospective designed trials with a larger sample size are warranted to confirm the results in the future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Combined chemotherapy remains the primary approach for patients with hematological malignancies. However, 20–30% of patients fail to achieve complete remission (CR), and relapse occurs in 50–80% of patients who achieve remission. It is difficult for those patients to achieve complete remission again and they still have an extremely short survival. Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is the only curative treatment with prolonged disease control and greatly increased overall survival (OS) for those patients [1, 2].
Despite a favorable long-term prognosis, HSCT faces various challenges due to graft-versus-host disease (GVHD) that increases post-transplant relapse and infections associated with significant death risk. The incidence of acute GVHD is as high as 40–60% causing mortality rate close to 15%, and the incidence of chronic GVHD (cGVHD) ranges between 35 and 70% [3, 4]. Anti-thymocyte globulins (ATGs) are used as immunomodulatory agents for prevention and treatment of GVHD in allogeneic HSCT and rejection in solid organ transplantation [5,6,7]. ATG depletes T lymphocytes by induction of apoptosis or complement-dependent lysis. It may add immune suppression by modulating surface molecules that mediate leukocyte/endothelium interactions, interfering with dendritic cells properties, inducing B-cell apoptosis, and inducing regulatory T cells and natural killer (NK) T cells [8,9,10]. In haploidentical HSCT (haplo-HSCT) settings, ATGs play a pivotal role in preventing GVHD and increasing engraftment [6, 11].
Two ATG preparations are commonly used in haplo-HSCTs. The traditional ATG, also named as ATG-Thymoglobulin or ATG-T, is a polyclonal immunoglobulin preparation obtained by immunizing rabbits with human thymocytes. Anti-human T lymphocyte globulin (ATLG, also named as ATG-Fresenius or ATG-F), is a highly purified rabbit polyclonal anti-human T-lymphocyte immunoglobulin developed by immunizing rabbits with the Jurkat T-lymphoblast cell line. The distinct source of immune antigens for these two ATG preparations lead to obvious differences in the antigen recognition spectrum, which may cause divergency in immunosuppressive effect and toxicity [12]. In solid organ transplantation, ATLG showed a comparable effect of rejection prevention with ATG-T but a lower risk of cytomegalovirus (CMV) disease, malignancy, and death [13, 14]. Yet, in hematological malignancies with HSCT, it is unclear whether the efficacy and safety differ between these two different rabbit ATG preparations.
Materials
Participants
This was a retrospective study enrolling patients with hematological malignancies who received haplo-HSCT at Aerospace Center Hospital, Beijing, China between November 2018 and May 2019. Patients were diagnosed with acute myeloid leukemia (AML), acute lymphoblastic leukemia (ALL), myelodysplastic syndrome (MDS), and lymphoma according to the World Health Organization (WHO) classification of hematopoietic and lymphoid tumors [15]. This study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Ethics Committee of Aerospace Center Hospital, Beijing, China. Written informed consent was obtained from the adult patients and the guardians of children patients.
Conditioning regimens and GVHD prophylaxis
Patients were preconditioned with total body irradiation (TBI) at a total of 12 Gy from days − 10 to -8 or busulfan (2.4 mg/kg/day, from days − 6 to -3) according to patient’s sickness, remission status, previous treatment course, and organ function. All patients were additionally given intravenous drip of cyclophosphamide (1.8 g/m2/day on days − 5 and − 4) for preconditioning.
For GVHD prophylaxis, patients were administered with ATG (2-2.5 mg/kg/day) from days − 5 to -2 or ATLG (5-7.5 mg/kg/day) from days − 5 to -2. The vital signs and serious adverse reactions were closely monitored during treatment. In addition, every patient was administered with cyclosporine A (CSA, 3 mg/kg/day, day − 1, blood trough concentration maintained at levels between 150 and 200 ng/ml), a short cycle of methotrexate (MTX, 15 mg/m2/day intravenously, day + 1, and 10 mg/m2/day on days + 3, +5 and + 11), and mycophenolate mofetil (MMF, 15 mg/kg/day from day − 1).
Supportive treatments included hydration, alkalinization, platelet transfusion when platelet count was less than 20 × 109/L, and red blood cell transfusion when red blood cell count was less than 70 g/L during preconditioning.
Definitions of outcomes
Patients attained complete remission characterized by the existence of less ≤ 5% leukemic cells in the bone marrow as defined by an international panel of experts [16,17,18]. Acute GVHD (aGVHD) was diagnosed and graded according to the consensus criteria [19], while chronic GVHD (cGVHD) was graded as extensive or limited [19]. Neutrophil engraftment was defined at the first of 3 consecutive days with an absolute neutrophil count ≥ 0.5 × 109/L, while platelet engraftment was defined at the first of 7 consecutive days with a platelet count ≥ 20 × 109/L without transfusion. Relapse was defined as the presence of fusion gene positive or minimal residual lesions or extramedullary lesions after transplantation. Cytomegalovirus (CMV)-DNA and Epstein-Barr virus (EBV)-DNA were monitored weekly by real-time polymerase chain reaction (RT-PCR). The detection of CMV and EBV virus copy numbers in peripheral blood indicated viral activation. We monitored morphologic assessment of the bone marrow, minimal residual disease (MRD) detection by flow cytometry, cytogenetic or molecular genetic marker detection, and hematopoietic chimerism testing monthly after neutrophil engraftment.
The survival outcomes were defined as previously reported [20]. Overall survival (OS) was defined as the duration from transplantation to death from any cause. Relapse free survival (RFS) was calculated from the date of transplantation to the date of relapse or death. GVHD-free and relapse-free survival (GRFS) was defined as the time from transplantation to the first occurrence of grade III/IV aGVHD, extensive cGVHD, relapse or death. Non-relapse mortality (NRM) was defined as death without relapse.
Statistical analysis
Continuous variable were presented as median with range and compared using Wilcoxon rank-sum test. Categorical variables were presented as percentages and compared using Pearson chi-square test or Fisher’s exact test. Kaplan-Meier method was adopted to estimate OS, RFS, and GRFS with log-rank test for comparisons between ATG and ATLG groups. Cumulative incidences of aGVHD, cGVHD, relapse, and NRM were estimated accounting for competing risks. Relapse was deemed as the competing risk of NRM, and NRM was the competing risk of relapse. Relapse and death without GVHD were competing risks of GVHD. Cumulative incidences of GVHD, relapse, and NRM were compared using Fine and Gray model [21]. Hazard ratios with 95% confidence interval (CIs) for baseline variables were calculated using univariate Cox regression model or competing risk model, and those with P value < 0.10 were further adjusted by multivariate models. P value < 0.05 was deemed as statistically significant. All analyses were performed by using STATA 16.0 (StataCorp, TX, USA).
Results
Baseline comparisons of both ATG groups
A total of 46 patients, who were diagnosed with AML (n = 33), ALL (n = 6), MDS (n = 3), and lymphoma (n = 4), were included in our analysis. Twenty-eight patients received ATLG and 18 received ATG for GVHD prophylaxis. The median age was 35.5 years (range: 5–64) with no significant difference between ATLG and ATG groups (P = 0.605). CR prior transplantation was achieved in 14 (50.0%) patients of ATLG group and 7 (38.9%) of ATG group. According to hematopoietic cell transplantation comorbidity index (HCT-CI), 8 (28.6%) patients in ATLG group and 5 (27.8%) in ATG group were graded as high risk. Busulfan plus cyclophosphamide were the major preconditioning regimens used in 19 (67.9%) patients of ATLG group and 15 (83.3%) patients of ATG group. There were no significant differences between both groups in recipient sex, median infused mononuclear cells and CD34+ cells, identical blood types of recipient and donor, donor sex, and use of umbilical cord blood (Table 1).
Engraftment and chimerism
After transplantation, one patient in ATLG group died early due to intracranial infection without neutrophil and platelet engraftment. In ATG group, one patient died early due to severe infection without engraftment and 2 experienced primary implantation failure. There were no significant differences of median days to neutrophil engraftment (14 vs. 16, P = 0.551) and platelet recovery (14 vs. 16, P = 0.825) for the rest patients between both groups (Table 2). Bone marrow assessments of 42 patients with successful implantation all indicated morphological CR and full donor chimerism after transplantation. All patients were negative for MRD except 1 in ATLG group showing MRD positivity.
GVHD
In ATLG group, 8 patients developed aGVHD, including 5 grade I/II cases and 3 grade III/IV cases. In ATG group, 8 patients had aGVHD, comprising 4 grade I/II cases and 4 grade III/IV cases. The cumulative incidence of all-grades aGVHD in ATLG group seemed to be lower than ATG group, which, however, was not statistically significant (28.6% vs. 44.4%, P = 0.242, Table 2).
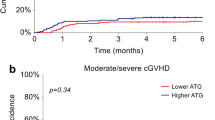
There were 2 and 4 patients in ATLG group experiencing limited and extensive cGVHD, respectively. In ATF group, 2 and 3 patients had limited and extensive cGVHD, respectively. The cumulative incidence of cGVHD did not differ between both groups (21.4% vs. 27.8%, P = 0.603, Table 2).
Viral reactivation and adverse events
CMV-DNAemia occurred in 18 ATLG patients and 14 ATG patients, which were not significantly different (Table 2). EBV-DNAemia was only observed in 2 patients receiving ATG. We documented fever, hypoxemia, and hemorrhage cystitis in 18, 1 and 9 patients, respectively. Compared with ATG group, ATLG group had significantly lower incidences of fever (25.0% vs. 61.1%, P = 0.014) and hemorrhage cystitis (7.1% vs. 38.9%, P = 0.008, Table 2).
Relapse and survivals
As of May 31, 2022, a total of 23 patients (11 ATLG group and 12 in ATG group) died. The median follow-up time of the survivals was 38.6 (range: 36.5–52.9) months. Log-rank test demonstrated a significantly prolonged OS of ATLG than ATG (P = 0.033, Fig. 1). The estimated 3-year OS rate of ATLG group was 64.3%, which was significantly higher than 33.3% of ATG group (Table 2). Relapse occurred in 12 patients of ATLG group and 7 patients of ATG group. The estimated 3-year cumulative incidences of relapse of both groups were comparable (42.9% vs. 38.9%, P = 0.976). ATLG showed a trend towards a lower 3-year NRM than ATG but the difference did not reach statistical significance (10.7% vs. 27.8%, P = 0.160, Table 2). The 3-year RFS rates of ATLG and ATG groups were 46.4% and 33.3%, respectively, which showed no significant difference (Fig. 2). The 3-year GRFS rates of both groups were 32.1% and 11.1%, respectively. Log-rank test suggested GRFS favored ATLG group at a marginal significance (P = 0.045, Fig. 3).
Univariate and multivariate Cox regression analyses
Univariate Cox regression analysis showed unfavorable OS in patients with older age (≥ 35 yeas) and HCT-CI high risk, and prolonged OS in patients receiving ATLG (Table 3). Patients with HCT-CI high risk had a worse RFS that those with intermediate or low risk (HR = 2.45, 95%CI: 1.11–5.38, P = 0.026, Supplementary information: Table S1). ATLG was not significantly associated with RFS (HR = 0.63, 95%CI: 0.30–1.36, P = 0.241). None of the baseline characteristics were significantly associated with cumulative incidences of relapse (Supplementary information: Table S2). Multivariate analysis demonstrated ATLG was independently associated with a favorable OS (HR = 0.37, P = 0.020, Table 3) and nominally associated with a favorable GRFS (HR = 0.51, P = 0.051, Table 3).
Discussion
Derived from rabbits immunized with Jurkat T-lymphoblast cell line, ATLG has a narrower spectrum of antigen recognition and therefore a possible less immunosuppressive effect than ATG [9]. This retrospective study compared the efficacy and safety outcomes of two ATG preparations for haplo-HSCT of hematological malignancies. We documented comparable risks of cGVHD and relapse between ATLG and ATG groups, but observed a tendency of lower aGVHD incidence and 3-year NRM and a significantly higher 3-year OS in ATLG patients. In addition, lower risks of fever and hemorrhage cystitis in ATLG patients were noted. Our results demonstrated that, despite similar efficacy for GVHD prophylaxis, ATLG conferred survival benefit and had better safety profile for hematological malignancy patients undergoing haplo-HSCT.
Recently, several retrospective studies made comparisons of these two different rabbit ATG preparations in terms of GVHD prophylaxis, relapse, survival and safety among hematological malignancy patients under different transplantation settings [22,23,24,25,26,27]. Paiano et al. analyzed 30 patients with various hematologic malignancies who underwent allo-HSCT after reduced intensity conditioning [24]. Both ATGs showed comparable results regarding GVHD, relapse, survival, and infections [24]. In a larger study comprising 110 patients undergoing unrelated donor HSCT, Huang et al. documented a lower probability of cGVHD and a trend towards lower relapse and higher disease-free survival in those receiving ATLG than those receiving ATG [23]. In matched unrelated donor (MUD) setting, Polverelli et al. observed a lower cumulative incidence of moderate-severe cGVHD and a favorable GRFS in ATLG group than ATG group [25]. Yet, cumulative incidences of aGVHD and relapse, treatment-related mortality, and survival probability demonstrated no significant differences in this study. Furthermore, Wang et al. demonstrated no effect of ATLG on relapse and survival but a lower risk of extensive cGVHD compared with ATG in unrelated HSCT [27]. These comparisons were mostly made in unrelated donor or matched related donor settings. Zhou et al. evaluated the effect of ATG preparations in halpo-HSCT settings comprising 35 ATLG patients and 81 ATG patients [28]. They found significantly lower cumulative incidences of any grade cGVHD and limited cGVHD in ATLG group than ATG group. Yet, the relapse mortality and OS were comparable between both groups. Contrarily, our study, also in haplo-HSCT setting, observed no significant difference in preventing GVHD but a favorable OS in ATLG group.
These comparisons between two rabbit ATG preparations, performed in different transplantation settings, yielded inconclusive results in terms of GVHD prevention, relapse, and survivals. One possible explanation is the different dosages of ATGs given for GVHD prophylaxis. ATLG was given at a total dosage of 20 mg/kg [23, 24, 27, 28] or 30 mg/kg [25]. Meanwhile, ATG was usually given at a total dosage of 7.5 mg/kg [24, 25, 28] or 10 mg/kg [23, 27]. Boga et al. concluded the recommended total dosage of 30 mg/kg of ATLG might decrease cGVHD compared with a lower 15 mg/kg dosage in matched sibling donor transplantation [29]. Another study conducted by Binkert et al. revealed the 35 mg/kg dosage of ATLG, compared with a higher dosage (60 mg/kg), was associated with a decreased incidence of cGVHD and prolonged overall survival [30]. Oostenbrink et al. found that low dosage of ATG (6–8 mg/kg) had a higher risk of grade III-IV aGVHD compared to 10 mg/kg dosage of ATG [31]. It seems that a total dosage around 30 mg/kg of ATLG is the optimal choice balancing GVHD prevention and survival benefits whereas increasing ATG dosage did not affect cGVHD and survival. In our study, we administered ATLG at a total dosage ranging from 20 to 30 mg/kg and ATG from 8 to 10 mg/kg at the discretion of the physicians to gain a better control of GVHD and achieve a more favorable survival. In overall, ATLG preparation may have positive effect on preventing cGVHD and improve survivals compared with ATG for hematological malignancies undergoing allo-HSCT.
In addition to potential survival benefits, ATLG may be associated with a lower risk of acute ATG-related adverse reactions than ATG. In line with Huang et al.’s study in unrelated donor transplantation settings [23], we found more ATG patients experienced high fever than ATLG patients, which may translate to a better quality of life. In addition, we found a significantly lower incidence of hemorrhage cystitis in ATLG group. These results indicate a lower toxicity of ATLG preparation than ATG preparation.
Active ATG/ATLG refers to the part of ATG/ATLG that can bind to lymphocytes, and the proportion of active ATG/ATLG in the total ATG/ATLG is usually less than 10%. The elimination of active component of ATG/ATLG has significant impact on immune recovery and the clinical outcomes of transplantation by reaching the critical threshold of 1 AU/mL for T-cell reconstitution [32]. Active ATLG is cleared in circulation faster than active ATG, resulting a earlier immune recovery in ATLG group [31, 33, 34]. The delayed immune reconstitution of ATG, leading to increased post-transplantation adverse events and increased risk of death, may be the cause of a lower OS in patients receiving ATG in our study.
Conclusion
In conclusion, we found the rabbit ATLG, compared with ATG, was associated with a favorable survival and less adverse events in patients with hematological malignancies undergoing haplo-HSCT. Yet, the results of our study should be cautiously interpreted since the small sample size and retrospective nature are the major limitations of our study. Prospective designed trials with a larger sample size are warranted to confirm the clinical benefit of ATLG in the future.
Data availability
The data that support the findings of this study are available on request from the corresponding author.
References
Apperley J, Niederwieser D, Huang XJ et al (2016) Reprint of: Haploidentical hematopoietic stem cell transplantation: A global overview comparing Asia, the European Union, and the United States. Biol Blood Marrow Transpl 22(3 Suppl):S15–S18
Dohner H, Wei AH, Appelbaum FR et al (2022) Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood 140(12):1345–1377
Shah NR, Leone A, Rothbaum R et al (2019) Cutaneous chronic graft versus host disease in a symmetric distribution. Cureus 11(5):e4614
Zhang F, Zuo T, Yeoh YK et al (2021) Longitudinal dynamics of gut bacteriome, mycobiome and virome after fecal microbiota transplantation in graft-versus-host disease. Nat Commun 12(1):65
Chang YJ, Wu DP, Lai YR et al (2020) Antithymocyte Globulin for Matched Sibling Donor transplantation in patients with hematologic malignancies: a Multicenter, Open-Label, Randomized Controlled Study. J Clin Oncol 38(29):3367–3376
Kumar A, Reljic T, Hamadani M et al (2019) Antithymocyte globulin for graft-versus-host disease prophylaxis: an updated systematic review and meta-analysis. Bone Marrow Transpl 54(7):1094–1106
Walker I, Panzarella T, Couban S et al (2020) Addition of anti-thymocyte globulin to standard graft-versus-host disease prophylaxis versus standard treatment alone in patients with haematological malignancies undergoing transplantation from unrelated donors: final analysis of a randomised, open-label, multicentre, phase 3 trial. Lancet Haematol 7(2):e100–e111
Mohty M (2007) Mechanisms of action of antithymocyte globulin: T-cell depletion and beyond. Leukemia 21(7):1387–1394
Popow I, Leitner J, Grabmeier-Pfistershammer K et al (2013) A comprehensive and quantitative analysis of the major specificities in rabbit antithymocyte globulin preparations. Am J Transpl 13(12):3103–3113
Popow I, Leitner J, Majdic O et al (2012) Assessment of batch to batch variation in polyclonal antithymocyte globulin preparations. Transplantation 93(1):32–40
Lv M, Chang Y, Huang X (2019) Everyone has a donor: contribution of the Chinese experience to global practice of haploidentical hematopoietic stem cell transplantation. Front Med 13(1):45–56
Baron F, Mohty M, Blaise D et al (2017) Anti-thymocyte globulin as graft-versus-host disease prevention in the setting of allogeneic peripheral blood stem cell transplantation: a review from the Acute Leukemia Working Party of the European Society for Blood and marrow transplantation. Haematologica 102(2):224–234
De Santo LS, Della Corte A, Romano G et al (2004) Midterm results of a prospective randomized comparison of two different rabbit-antithymocyte globulin induction therapies after heart transplantation. Transpl Proc 36(3):631–637
Ducloux D, Kazory A, Challier B et al (2004) Long-term toxicity of antithymocyte globulin induction may vary with choice of agent: a single-center retrospective study. Transplantation 77(7):1029–1033
Jordan J, Goldstein JS, Jaye DL, Gurcan M, Flowers CR, Cooper LAD (2018) Informatics approaches to address New challenges in the classification of lymphoid malignancies. JCO Clin Cancer Inf, ;2:CCI.17.00039.
Bernasconi P, Borsani O (2021) Eradication of measurable residual disease in AML: a challenging clinical goal. Cancers (Basel) 13(13):3170
Cheson BD, Bennett JM, Kopecky KJ et al (2003) Revised recommendations of the International Working Group for Diagnosis, standardization of response criteria, treatment outcomes, and reporting standards for therapeutic trials in Acute myeloid leukemia. J Clin Oncol 21(24):4642–4649
Dohner H, Estey E, Grimwade D et al (2017) Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 129(4):424–447
Glucksberg H, Storb R, Fefer A, Buckner C et al (1974) Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation 18(4):295–304
Fei X, Zhang W, Gu Y et al (2024) CLAG combined with total body irradiation as intensive conditioning chemotherapy prior to allogeneic hematopoietic stem cell transplantation in patients with refractory or relapsed acute myeloid leukemia. Ann Hematol 103(1):241–249
Austin PC, Fine JP (2017) Practical recommendations for reporting Fine-Gray model analyses for competing risk data. Stat Med 36:4391–4400
Basara N, Baurmann H, Kolbe K et al (2005) Antithymocyte globulin for the prevention of graft-versus-host disease after unrelated hematopoietic stem cell transplantation for acute myeloid leukemia: results from the multicenter German cooperative study group. Bone Marrow Transpl 35(10):1011–1018
Huang W, Yu L, Cao T et al (2016) The efficacy and safety of rabbit anti-thymocyte globulin vs rabbit anti-T-lymphocyte globulin in peripheral blood stem cell transplantation from unrelated donors. Leuk Lymphoma 57(2):355–363
Paiano S, Roosnek E, Tirefort Y et al (2015) Comparing two types of rabbit ATG prior to reduced intensity conditioning allogeneic hematopoietic SCT for hematologic malignancies. Bone Marrow Res 2015:980924
Polverelli N, Malagola M, Turra A et al (2018) Comparative study on ATG-thymoglobulin versus ATG-fresenius for the graft-versus-host disease (GVHD) prophylaxis in allogeneic stem cell transplantation from matched unrelated donor: a single-centre experience over the contemporary years. Leuk Lymphoma 59(11):2700–2705
Remberger M, Svahn BM, Hentschke P et al (1999) Effect on cytokine release and graft-versus-host disease of different anti-T cell antibodies during conditioning for unrelated haematopoietic stem cell transplantation. Bone Marrow Transpl 24(8):823–830
Wang L, Kong P, Zhang C et al (2023) Outcomes of patients with hematological malignancies who undergo unrelated donor hematopoietic stem cell transplantation with ATG-Fresenius versus ATG-Genzyme. Ann Hematol 102(6):1569–1579
Zhou L, Gao ZY, Lu DP (2020) Comparison of ATG-thymoglobulin with ATG-Fresenius for Epstein-Barr virus infections and graft-versus-host-disease in patients with hematological malignances after haploidentical hematopoietic stem cell transplantation: a single-center experience. Ann Hematol 99(6):1389–1400
Boga C, Yeral M, Gereklioglu C et al (2018) Effects of two doses of anti-T lymphocyte globulin-Fresenius given after full-match sibling stem cell transplantation in acute myeloblastic leukemia patients who underwent myeloablative fludarabine/busulfan conditioning. Hematol Oncol Stem Cell Ther 11(3):149–157
Binkert L, Medinger M, Halter JP et al (2015) Lower dose anti-thymocyte globulin for GvHD prophylaxis results in improved survival after allogeneic stem cell transplantation. Bone Marrow Transpl 50(10):1331–1336
Oostenbrink LVE, Jol-van der Zijde CM, Kielsen K et al (2019) Differential Elimination of Anti-thymocyte Globulin of Fresenius and Genzyme impacts T-Cell reconstitution after hematopoietic stem cell transplantation. Front Immunol 10:315
Admiraal R, van Kesteren C, Jol-van der Zijde CM et al (2015) Association between anti-thymocyte globulin exposure and CD4 + immune reconstitution in paediatric haemopoietic cell transplantation: a multicentre, retrospective pharmacodynamic cohort analysis. Lancet Haematol 2(5):e194–203
Burkhalter F, Schaub S, Bucher C et al (2016) A comparison of two types of rabbit antithymocyte globulin induction therapy in immunological high-risk kidney recipients: a prospective Randomized Control Study. PLoS ONE 11(11):e0165233
Huang W, Zhao X, Tian Y et al (2015) Outcomes of peripheral blood stem cell transplantation patients from HLA-mismatched unrelated donor with antithymocyte globulin (ATG)-Thymoglobulin versus ATG-Fresenius: a single-center study. Med Oncol 32(2):465
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
RL and JW conceived and designed the study. ZT, QM, YY, HG, and YW collected the data. ZT, QM, and YY analyzed the data and drafted the manuscript. All authors revised the manuscript approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Ethics Committee of Aerospace Center Hospital, Beijing, China.
Patient approval statement
Written informed consent was obtained from the adult patients and the guardians of children patients.
Conflict of interest
The authors have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tian, Z., Man, Q., Yang, Y. et al. Comparison of rabbit ATLG and ATG for GVHD prophylaxis in hematological malignancies with haploidentical hematopoietic stem cell transplantation. Ann Hematol 103, 1729–1736 (2024). https://doi.org/10.1007/s00277-024-05724-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-024-05724-w