Abstract
Two anti-thymocyte globulin (ATG) forms are used in graft-versus-host disease (GVHD) prophylaxis during haploidentical hematopoietic stem cell transplantations (haplo-HSCTs): ATG-thymoglobulin (ATG-T) and ATG-fresenious (ATG-F). However, comparable dosages for haplo-HSCT remain unclear. We compared and evaluated the effects of ATG-T (7.5 mg/kg) or ATG-F (20 mg/kg) dosages in a relatively homogenous population in haplotype HSCT settings. Patients administered ATG-T 7.5 mg/kg (n = 81) or ATG-F 20 mg/kg (n = 35) as part of GVHD prophylaxis during haplo-HSCT were enrolled. Incidence and severity of GVHD, Epstein–Barr virus (EBV) infection, and immune cell recovery were compared using the Mann-Whitney U rank test and chi-square test. Cumulative incidences of GVHD, EBV infection and its subgroups, and relapse mortality were computed; overall survival (OS) was analyzed using the Kaplan-Meier method, with the log-rank test used for univariate comparison. Risk factors for OS were analyzed by the Cox proportional hazards model. Incidence and cumulative incidence of all grades of acute GVHD and subgroups were comparable in both groups (all p > 0.05); however, cumulative incidence of any grade and limited chronic GVHD was significantly higher in the ATG-T group (p = 0.002, p = 0.007, respectively). Cumulative incidences of EBV infections, EBV-DNAemia, and EBV-related diseases were similar; relapse mortality and OS rates were comparable between both groups (all p > 0.05). ATG-T dosage (7.5 mg/kg) appeared comparable to ATG-F dosage (20 mg/kg) for haplo-HSCT. Currently approved ATG-T and ATG-F doses appear efficient to balance the risk–benefit ratio of GVHD, OS, relapse mortality, and EBV infection in haplo-HSCT.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The number of haploidentical hematopoietic stem cell transplantations (haplo-HSCTs) has increased sharply in the past decade. These procedures have benefited from three new methodologies originated from Italy, China, and the USA, and developed further worldwide [1]. Most haplo-HSCT cases in China followed the “GIAC protocol” or modifications of this protocol. The “GIAC protocol” comprises: granulocyte-colony-stimulating factor (G-CSF)-mobilized grafts (G), intensified immunosuppression with cyclosporine using the whole process of conditioning chemotherapy (I), anti-thymocyte globulin (ATG) for in vivo T cell depletion (A), and combined use of bone marrow with peripheral blood stem cells as the source of grafts (C) [2].
ATG plays a pivotal role in haplo-HSCT, especially in the “GIAC protocol” for preventing graft-versus-host disease (GVHD) and increasing engraftment [3]. Nevertheless, its use is associated with an elevated incidence of infections, particularly viral infections, when high doses of ATG are administered. The effect of high doses of ATG on relapse after HSCT is under dispute. Whereas some retrospective studies suggest that ATG increases relapses, randomized studies have not produced such results. Balancing the risk-benefit ratio is the focus of the current study, as several issues remain unresolved regarding the use of ATG in HSCT; in particular, the optimal dosage of ATG has not been precisely identified.
To date, two brands of rabbit ATG, the thymoglobulin (ATG-T, Sanofi, Paris, France) and the anti-human T lymphocyte immunoglobulin (ATG-F, Neovil Biotech, Waltham, MA, USA) have been globally used in haplo-HSCT. Different doses are required in HSCT between the two currently available brands due to the differences regarding the source of the immunizing antigen and the methods used for the development of the antibodies. Additionally, the doses also depend on the donor type (human leukocyte antigen [HLA]-identical sibling, unrelated, haplo-identical donors, etc.) and conditioning regimens (myeloablative, reduced-intensity, low-intensity immunosuppressive conditioning) [4]. For HLA-matched-sibling transplants, a recent randomized study supported the use of ATG-F at a dose of 30 mg/kg [5], whereas both randomized and retrospective studies indicated the use of ATG-T at a dose of approximately 5 mg/kg [6, 7]. For matched-unrelated donor transplants, ATG-F and ATG-T doses are indicated at 60 mg/kg and 4.5–7.5 mg/kg, respectively [4, 8,9,10]. Based on consensus opinion, the European Blood and Marrow Transplant Group recommends 30 mg/kg of ATG-F or 7.5 mg/kg of ATG-T, divided into three doses administered on days 3, 2, and 1 (for 8/8 HLA allele-matched, unrelated donor transplantation) [4]. However, the comparable dosage for these two ATGs for haplo-HSCT remains unidentified.
In this study, we compared and evaluated the effect of the dosages of ATG-T (7.5 mg/kg) or ATG-F (20 mg/kg) in a relatively homogenous population in a haplotype HSCT setting. All patients presented with malignant hematological diseases, had received similar myeloblastic conditioning regimens (MAC), were treated with the same GVHD prophylactic strategy, and had used the same source of grafts. We compared the incidence and severity of GVHD and of Epstein–Barr virus (EBV) infections, and the immune cell recovery between these two ATG-treated groups.
Patients and methods
Study design
This was a retrospective study. One hundred and sixteen patients with hematological malignances, including acute myeloid leukemia, acute lymphoblastic leukemia, and myelodysplastic syndrome, who underwent haplo-HSCT using ATG-T or ATG-F as GVHD prophylaxis and Bu/Cy or Bu/Flu as MAC at Shanghai Dao-pei Hospital between November 2007 and June 2015 were enrolled in this study. The cut-off follow-up date was March 2017. Three patients who received haplo-HSCT in 2007 were also enrolled in this study. From 2008, ATG-T 7.5 mg/kg and ATG-F 20 mg/kg were used as standard doses in conditioning regimens in the haplo-HSCT protocol for the patients with hematological malignances. The choice of the type of ATG was at the discretion of the treating physicians.
The study was approved by the Institutional Ethics Committee of Shanghai Dao-pei Hospital. All patients underwent single haplo-HSCT. Written informed consent was provided by the patients or their guardians.
Analysis of HLA-typing
All patients and their donors underwent HLA-A, HLA-B, HLA-Cw, and HLA-DRB1 typing by high-resolution DNA techniques, according to the manufacturer’s instructions (Special Monoclonal Tray-Asian HLA Class I and Micro SSP HLA Class I and II ABDR DNA Typing Tray; One Lambda, Canoga Park, CA, USA). The haplo-type was confirmed for each patient and donor.
Conditioning regimens
Conditioning regimens included two protocols. The Bu/Cy protocol composed of intravenous (i.v.) cytarabine (2.0 g/m2/day, from days −9 to –8), busulfan (3.2 mg/kg/day, from days −7 to −5), cyclophosphamide (1.8 g/ m2/day, from days −4 to −3), methylsmostatin (250 mg/m2/day, from days −3), and ATG (ATG-T 2.5 mg/kg/day, from days −4 to −2 or ATG-F 5 mg/kg/day, from days −5 to −2). The Bu/Flu protocol consisted of the same dosages of cytarabine (from days −14 to −13), busulfan (from days −7 to −5), methylsmostatin (day, day −3), and ATG (ATG-T from days −4 to −2 or ATG-F from days −5 to −2); cyclophosphamide was replaced with fludarabine (30 mg/m2/day, from days −12 to −8).
GVHD prophylaxis
For GVHD prophylaxis, every patient was administered cyclosporine A (CSA, 5 mg/kg/day, i.v., at inception of the conditioning regimen), a short cycle of methotrexate (15 mg/m2/day, i.v., day +1, and 10 mg/m2/day on days +3 and +5), and oral mycophenolate mofetil (MMF, 15 mg/kg/day from days +1 to +14). Oral CSA was initiated when the neutrophil count reached to 1.0 × 109/L. CSA blood trough concentration was initially monitored twice a week, and then weekly until +180 days, to maintain levels between 150 and 200 ng/ml.
Supportive care in the transplantation process
The standard supportive care during the process of transplantation included:
-
(1)
Routine prophylaxis strategy: use of irradiated and leukocyte-depleted blood products; routinely administered caspofungin during the conditioning process; voriconazole post-transplantation for antifungal prophylaxis until neutrophil engraftment; trimethoprim-sulfamethoxazole for Pneumocystis prophylaxis until 6 months post-transplantation; and acyclovir, typically at a dose of 400 mg orally, twice daily, from the date of neutrophil engraftment to 24 months post-transplantation.
-
(2)
Management of cytomegalovirus (CMV) and EBV reactivations: monitoring of CMV and EBV-DNA copies by quantitative polymerase chain reaction (PCR) in peripheral blood samples every week during the first 30 days post-haplo-HSCT and then every other week until 3 months post-transplantation, or until CMV/EBV-DNA copies were undetectable in the peripheral blood. CMV and EBV-DNA copies were also tested by PCR in biopsy specimens when available. PCR was performed according to our previously published study [11]. CMV reactivation was confirmed when PCR indicated ≥ 1000 copies/ml whole blood; then, ganciclovir (500 mg/m2/day, i.v.) was administered until CMV was again undetectable by PCR. The detection threshold of EBV reactivation by PCR was 500 copies/ml whole blood. In cases where the EBV infection was confirmed, a high dose of acyclovir (500 mg/m2/day) was administered. Moreover, the immunosuppressants, particularly CSA and MMF, were withdrawn instantly if GVHD did not concurrently occur.
Mobilization, collection, and infusion of grafts
G-CSF at a dose of 300 μg was administered to the donors at day −5, −4, −3, −2, and −1. Bone marrow stem cells were collected on day 1 and peripheral stem cells on day 2. Infused mixed grafts consisting of bone marrow and peripheral stem cells collected on the same day were used in all patients in both groups.
Monitoring engraftment and chimerism
Neutrophil engraftment was defined at the first of 3 consecutive days with an absolute neutrophil count ≥ 0.5 × 109/L, while platelet engraftment was defined at the first of 7 consecutive days with a platelet count ≥ 20 × 109/L, without transfusion.
Bone marrow chimerism was monitored every month for 6 months after haplo-HSCT. Chimerism was determined by either DNA fingerprinting of short tandem repeats (STRs) or chromosomal fluorescent in situ hybridization (FISH). Chimerism was analyzed by DNA fingerprinting of STR on recipient bone marrow cells in sex-matched donor-recipient pairs; however, in sex-mismatched donor-recipient pairs, chimerism was analyzed by FISH.
Monitoring immunological recovery post-HSCT
From 22 patients in the ATG-T group and 17 patients in ATG-F group, peripheral blood was collected at +30 and +60 days after haplo-HSCT to analyze the percentage of pan-T lymphocytes (CD3+) and its subsets (helper T cell, CD3+CD4+; effector T cells, CD3+CD8+). We evaluated the performance of CD3+, CD3+CD4+, and CD3+CD8+ T lymphocytes by three-color flow cytometry. All experiments were performed in triplicate.
Monoclonal antibodies, including anti-CD3 (peridinin chlorophyll protein-[PerCP]), anti-CD8 (fluorescein isothiocyanate-[FITC]), and anti-CD4 (allophycocyanin-[APC]), were purchased from BD Biosciences (Franklin Lakes, NJ, USA). Briefly, forward and side scatters were used to gate viable populations of cells; then, CD3+, CD3+CD4+, and CD3+CD8+ cells were gained as target cells for fluorescence-activated cell sorting analysis. The percentages of CD3+, CD3+CD4+, and CD3+CD8+ T lymphocytes were calculated as follows: percentage of T lymphocyte subgroup = (percentage of CD3+/CD3+CD4+/CD3+CD8+ T cells in patients—percentage CD3+/CD3+CD4+/CD3+CD8+ T cells in negative controls) × 100%. The absolute counts of CD3+/CD3+CD4+/CD3+CD4+ T cells (× 106/L) = percentage of T lymphocytes subgroup × white blood cell (× 109/L), counted by blood routine test, × 1000.
Definition of GVHD and EBV infections
Acute GVHD (aGVHD) was graded according to previously published criteria [12], while chronic GVHD (cGVHD) was graded as limited or extensive [12]. Methylprednisolone was used as the first-line therapy for both aGVHD and extensive cGVHD. Second-line therapy was at the discretion of the treating physicians.
EBV infections included EBV-DNAemia and EBV-related diseases. EBV-DNAemia was defined as EBV-DNA > 500 copies/mL at two consecutive time-points without any signs or symptoms of EBV-related diseases. EBV-related diseases included probable and proven post-transplantation lymphoproliferative disorders (PTLDs). Probable PTLDs were defined as significant lymphadenopathy, hepatosplenomegaly, or other end-organ manifestations, confirmed by a high EBV-DNA blood load in the absence of tissue biopsy or other documented causes. Proven PTLDs were diagnosed by EBV nucleic acid detection or EBV-encoded protein detection in tissue specimens, and symptoms and/or sign manifestations from the affected organs [13].
Statistical analysis
Continuous variables with non-normal distributions are expressed as the median and range. Differences in clinical characteristics in Table 1 and clinical outcomes in Tables 2, 3, 4, and 5 (except the cumulative incidence of aGVHD, cGVHD, EBV-DNAemia, and EBV-related diseases) between the ATG-T and ATG-F groups were compared using the Mann-Whitney U rank test for continuous data, the chi-square test for categorical data, and the Fisher’s exact test for binomial data. Overall survival (OS) was defined as the time interval from the time of transplantation until the time of death. The cumulative incidence of aGVHD was evaluated within the first 100 days post-transplantation. Relapse was defined as the presence of > 5% marrow blasts and/or reappearance of underlying diseases. Transplant-related mortality (TRM) was defined as mortality for transplant-related complications, including GVHD and infections. Mortality from disease relapse was defined as relapse mortality. The cumulative incidence of aGVHD and cGVHD was estimated by considering the corresponding type of GVHD as an event of interest, and death without GVHD as a competing event. The cumulative incidence of EBV infection and subsets was estimated as an event of interest, and death without EBV infection as a competing event. The cumulative incidence of relapse mortality was estimated as an event of interest and death with TRM as a competing event. The cumulative incidence method was applied to compute the incidence of GVHD, EBV infection and its subsets, and relapse mortality. OS was analyzed using the Kaplan-Meier method, with the log-rank test used for univariate comparison. Risk factors with values of p < 0.05 in univariate analyses were entered into a Cox proportional hazards model to determine their effects on OS. Statistical analysis was performed using SPSS 21.0 software (SPSS, Inc., Chicago, IL, USA) and R software (R version 4.03; The R foundation for Statistical Computing, www.R-project.org). Graphs were made in GraphPad prism 5.0 (GraphPad Software, La Jolla, CA, USA).
Results
Patients characteristics
One hundred and sixteen consecutive patients were enrolled in this study and were classified into two groups according to the ATG brands used in conditioning regimens. Eighty-one patients (69.83%) received ATG-T 7.5 mg/kg in total, while 35 patients (30.17%) received ATG-F 20 mg/kg in total.
The clinical characteristics of these two groups are summarized in Table 1. There were no significant differences in age (p = 0.298), sex of recipients and donors (p > 0.99, p = 0.066, respectively), distribution of disease diagnoses (p = 0.415), disease status (p = 0.295), ABO blood-type comparability between recipients and donors (p = 0.596), CMV and EBV status in recipient/donor pairs before transplantation (p > 0.99, p = 0.180, respectively), or conditioning regimen (p = 0.227) between the two groups. The percentages of cord blood usage as a third-party cell to improve engraftment before stem cell-infusion differed between the ATG-T and ATG-F (p = 0.047) groups, with a higher proportion of patients using cord blood in the ATG-T group (84.0% vs. 65.7%) (Table 1).
Engraftment and chimerism
All patients received completed neutrophil and platelet engraftments. The time to neutrophil and platelet engraftment was comparable between the ATG-T and ATG-F groups (p = 0.319, p = 0.397, respectively). The chimerism on days +30, +90, and +180 was tested on time. All patients in the two groups reached a 100% donor-type chimerism at +30 days post-HSCT.
GVHD
Sixty patients in the ATG-T group and 26 patients in the ATG-F group belonged to any grade of aGVHD, without statistically significant difference (p > 0.99). The patients in the ATG-T group who developed grade I-II and III-IV aGVHD were 50 and 10, respectively; and accordingly, the respective patients in the ATG-F group were 23 and 3. The proportion of subgroups (grades I-II and grades III-IV) aGVHD did not differ significantly between the two groups (p = 0.746) (Table 2).
The cumulative incidence of any grade of aGVHD was comparable between the ATG-T and ATG-F groups (p = 0.930) (Table 2). The difference in cumulative incidence in grades I-II and III-IV aGVHD between the ATG-T and ATG-F groups also did not reach statistical significance (p = 0.459, p = 0.513, respectively). However, the data showed that most patients had a relatively mild aGVHD within 100 days after HSCT (Table 2).
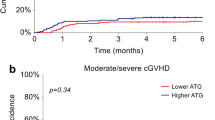
Any grade of cGVHD occurred in 34 patients in the ATG-T group and in 13 patients in the ATG-F group, without significant difference between the groups (p = 0.684). Twenty-four patients developed limited cGVHD, while 10 patients developed extensive cGVHD in the ATG-T group; in the ATG-F group, 10 patients developed limited cGVHD, and three patients developed extensive cGVHD. The proportions of limited and extensive cGVHD in the ATG-T and ATG-F groups were also not statistically significantly different (p > 0.999) (Table 2).
The cumulative incidence of any grade and limited cGVHD after HSCT was higher in the ATG-T group than in the ATG-F group with significant difference (p = 0.002, p = 0.007, respectively) (Table 2). The cumulative incidence of extensive cGVHD after HSCT was similar between the two groups (p = 0.717). From the cumulative incidence of cGVHD and its subsets, we identified that most patients had limited cGVHD and that the skin was the injured tissue (Table 2).
The median time of occurrence of aGVHD was 29 days (95% CI 24.590–33.410) in the ATG-T group and 41 days (95% CI 14.341–67.659) in the ATG-F group. The median time of occurrence of grade I-II aGVHD was 40 days (95% CI 18.442–61.558) in the ATG-T group and 41 days (95% CI 14.341–67.659) in the ATG-F group (data not shown).
EBV infections
Overall, 45 patients (55.6%) in the ATG-T group and 13 patients (37.1%) in the ATG-F group had EBV infections, which included EBV-DNAemia (22 patients in the ATG-T group vs. 4 patients in the ATG-F group) and EBV-related diseases (23 patients in the ATG-T group vs. 9 patients in the ATG-F group). Both the incidence of EBV infections and the proportion in its subgroups were comparable between the two groups (p = 0.105, p = 0.247, respectively) (Table 3).
All 32 cases with EBV-related diseases were probable PTLDs confirmed by positron emission tomography-computed tomography (PET-CT) imaging or tissue biopsy combined with EBV-qPCR. The majority of these patients developed fever, tonsillitis, adenopathy, and high blood EBV-DNA loads; however, EBV-DNA was not detected in the blood of two patients, but it was detected in the intestinal biopsy specimens obtained by colonoscopy in one patient and in the cerebrospinal fluid collected from the lumbar in the other.
There were no statistically significant differences in the cumulative incidence of EBV infections, EBV-DNAemia, or EBV-related diseases between the two groups (Table 3). However, the cumulative incidence of EBV infection and EBV-DNAemia tended to be higher in the ATG-T group (Table 3).
The median time of occurrence of EBV-DNAemia and of EBV-related diseases in the ATG-T and ATG-F groups did not differ significantly (p = 0.312, p = 0.458, respectively) (Table 3). The median peak value of the EBV-DNA copies in the blood and the median time to peak value did not differ significantly between the two groups (p = 0.341, p = 0.421, respectively).
Twenty-three patients (28.4%) in the ATG-T group and 5 patients (14.3%) in the ATG-F group had concomitant CMV and EBV infections. The incidences were not statistically and significantly different between the two groups (p = 0.155) (Table 3).
Immunological recovery
Twenty-seven patients (17 patients in the ATG-T group and 10 patients in the ATG-F group) were evaluable for immunological recovery analysis by assessing CD3+, CD3+CD4+, and CD3+CD8+ lymphocytes. The distribution of EBV infections was as follows: 5/17 patients with EBV-DNAemia, 6/17 patients with EBV-related diseases in the ATG-T group, and 3/10 patients with EBV-related diseases in the ATG-F group. Patients in the ATG-T and ATG-F groups did not differ in immunity recovery at +60 days after HSCT in CD3+ T lymphocytes and its subsets CD3+CD4+ helper T cells and CD3+CD8+ effector T cells (p = 0.270; p = 0.601; p = 0.315, respectively). However, the patients in the ATG-T group had higher absolute counts of total CD3+ T cells and CD3+CD8+ effector T cells at +30 days post-HSCT (p = 0.046, p = 0.083, respectively) (Fig. 1). The CD3+CD4+ helper T cell subset at +30 days after HSCT was comparable in the two groups (p = 0.863) (Table 4).
The counts of T lymphocytes and their subsets at +30 days after haploidentical stem cell transplantation (haplo-HSCT) in the ATG-T and ATG-F groups; the unit of cell count was ×106/L. HCST, haploidentical hematopoietic stem cell transplantations; ATG-T, anti-thymocyte globulin-Thymoglobulin; ATG-F, anti-thymocyte globulin-Fresenius
Survival and relapse mortality
At a median follow up of 64.7 months (range, 2.03–113.8 months), 44 of 116 patients died, with main cause of death TRM (Table 4). Nine patients died of relapse and 21 patients died of TRM in the ATG-T group and 6 patients died of relapse and 8 patients died of TRM in the ATG-F group. The difference in ratio between causes of mortality in the two groups was not statistically significant (p = 0.501). The cumulative incidence of relapse mortality in the ATG-T and ATG-F groups did not differ significantly (9.88% [95% CI, 4.58–17.58%] versus 17.93% [95% CI, 7.12–32.7%], p = 0.196). OS was also similar in the two groups (p = 0.421) (Fig. 2).
The disease status before HSCT, interval between disease initiation and HSCT initiation, and EBV infection status were associated with OS in the univariate analysis; the multivariate analysis confirmed the significance of disease status before HSCT (p < 0.001), interval between disease initiation and HSCT initiation (p = 0.016), and EBV-related disease (p < 0.001) (Table 6).
Discussion
The two brands of rabbit ATG currently used for the prevention of GVHD after HSCT present different pharmacokinetic and pharmacodynamic characteristics because of the differences in their manufacture processes. ATG-T is a polyclonal immunoglobulin G (IgG) obtained from the hyperimmune sera of rabbits immunized with fresh human thymocytes; the rabbit IgGs are attached to the human erythrocytes as adsorbed antibodies and bind to their surface antigens [14]. ATG-Fs derived from rabbits after immunization with homogenous Jur-kat T lymphoblastoid cells and are adsorbed antibodies against human erythrocytes and human placental cells [15]. Therefore, the spectrum of antigens recognized by ATG-F is narrower than that recognized by ATG-T; thus, the doses of ATG-F required for GVHD prophylaxis must be higher than those of ATG-T [14,15,16]. Nevertheless, which ATG type is more suitable during haplo-HSCT or which are the comparable dosages between ATG types is not identified yet and is subject to physician discretion.
In this retrospective study, we analyzed the outcomes achieved using ATG-T 7.5 mg/kg or ATG-F 20 kg/mg in haplo-HSCT in patients with hematological malignancies who were relatively homogenous regarding the graft sources, conditioning regimens, GVHD prophylaxis, and support care strategies. In our study of 116 patients, we did not find any significant differences between the two groups regarding engraftment, chimerism rates, cumulative incidence of relapse mortality, and OS.
Our results were in line with those of Paiano et al. [17]. They reviewed 30 patients who received allogeneic HSCT for hematologic malignancies with Bu/Flu as the conditioning regimen. Patients alternatively received total ATG-T 7.5 mg/kg or total ATG-F 25 mg/kg. They found no significant differences in terms of 3-year OS (45.7% vs. 46.7%) or relapse incidence (40% vs. 33.3%) between the two groups.
The incidence of GVHD is the most interesting aspect of our comparative study. The probable cumulative incidences of any grade of aGVHD and cGVHD in our study were higher than those reported by Paiano et al. [17]. We inferred that the differences in HLA consistency in donor/recipient pairs and the free time from immunosuppressants after HSCT were the major reasons for this discrepancy between the two studies. Two-thirds of the enrolled population received HLA-identical or not HLA-matched HSCT; 100% of patients continued immunosuppressive post-transplant therapy, which was associated with a lower cumulative incidence of any grade of aGVHD and cGVHD in Paiano et al.’s study. In our study, all patients underwent haplo-HSCT, and more than half of the patients discontinued all immunosuppressants within 30 days after haplo-HSCT for prophylaxis from relapses or viral infections. Moreover, the cumulative incidence of any grade of cGVHD was higher in the ATG-T group than in the ATG-F group due to the increase of the cumulative incidence of the limited cGVHD. We thought that the duration of immunosuppression achieved by 20 mg/kg of ATG-F is longer than that achieved by 7.5 mg/kg of ATG-T. Fortunately, the limited and controlled cGVHD may improve the graft-versus-leukemia effect and reduce relapse.
Another point of interest in our study was the viral infections, particularly the EBV infection. ATG treatment is considered a risk factor for PTLD development [18, 19]. Before 2000, the mortality rate (84.6%) after HSCT was attributed to PTLDs, the most serious consequence of EBV infections; even in the rituximab era, the mortality due to EBV infection was higher than 72% (95% CI 54–96%) in patients with three risk factors for EBV-PTLD [20, 21]. Thus, monitoring and pre-treatment of the EBV infection in the early phase is crucial. However, studies that directly compare different ATG preparations and dosages for the risk of EBV infections are rare. In our study, we compared the incidence of EBV infections and its subsets. The cumulative incidence of EBV infections and EBV-DNAemia tended to be higher in the ATG-T group, with a p value near 0.05. This may imply that the immunosuppression achieved by 7.5 mg/kg ATG-T is more effective than that achieved by 20 mg/kg ATG-F in early phase after HSCT. Our prior study about EBV-CTL reconstitution in haplo-HSCT patients treated with 7.5 mg/kg ATG-T for GVHD prophylaxis also confirmed that the low 30-day level of EBV-CTLs after haplo-HSCT is comparable with that in healthy persons [11]. Low levels of EBV-CTLs are not good for the control of EBV infection. Fortunately, the immunological recovery delay resulted in only a minor increase in EBV-DNAemia but not in EBV-related diseases; the former is easy to control by reducing or tampering the immunosuppression.
It is interesting to mention that the absolute counts of total CD3+ T cells at +30 days post-HSCT were higher in the ATG-T group than in the ATG-F group (p = 0.046). The CD3+CD8+ effector T cells were higher in the ATG-T group at +30 days after HSCT (p = 0.083); however, the difference did not reach statistical significance due to the small sample size in our study. Our observation of relatively higher CD3+ and CD8+ T cell levels was also supported by Alexandersson and colleagues [22]. They noted that the higher CD8+ T cell levels in EBV infections were inferred to the immune response against EBV infections. In line with their findings, in the 27 patients involved in the immune cell recovery analysis in our study, EBV infections appeared in 11/17 patients in the ATG-T group and in 3/10 in the ATG-F group.
Our findings about early T lymphocyte recovery and EBV infection were supported by few studies on immunorecognition and pharmacokinetic aspects. Mensen et al. [23] confirmed the comparable outcomes between a lower dose of ATG-T (6–8 mg/kg) and a higher dose of ATG-F (20–60 mg/kg) in HSCT. They did not observe any significantly delayed T cell subset reconstitution between low dosages of ATG-T 5–10 mg/kg and high dosages of ATG-F 20–60 mg/kg. Although the incidence of EBV reactivation was similar in the ATG-T and ATG-F groups, the EBV copy counts of > 104 copies/105 peripheral blood mononuclear cells, and the occurrence of PTLD were only found in the ATG-T group. Oostenbrink et al. [24] compared two rabbit ATG products, ATG-Genzyme (ATG-T, total 6–10 mg/kg), and ATG-Fresenius (ATG-F, total 45–60 mg/kg), in 58 pediatric acute leukemia patients (n = 42 ATG-T; n = 16 ATG-F) with HSCT from an unrelated donor. Their study confirmed that ATG-T 6–10 mg/kg and ATG-F 45–60 mg/kg were comparable in terms of immunity reconstitution and clinical outcomes.
Our study has some limitations. Our study was a retrospective observational study that analyzed 110 patients who received haplo-HSCT at a single center; moreover, the physicians were responsible for the choice of the type of ATG. Although the study population was relatively homogenous in the transplantation process, the lack of a rigorous study design and the small sample size were major limitations. Therefore, our observations should be confirmed by further larger and randomized studies.
In conclusion, 7.5 mg/kg ATG-T seemed to be comparable to 20 mg/kg ATG-F for haplo-HSCT. The currently approved doses of ATG-T (2.5 mg/kg/day for 3 days) and ATG-F (2.5 mg/kg/day for 4 days) may be appropriate and may efficiently balance the risk–benefit ratio of GVHD, OS, relapse mortality, and EBV infection after haplo-HSCT with the “GIAC protocol.”
References
Kanakry CG, Fuchs EJ, Luznik L (2016) Modern approaches to HLA-haploidentical blood or marrow transplantation. Nat Rev Clin Oncol 13:10–24
Lv M, Chang Y, Huang X (2019) Everyone has a donor: contribution of the Chinese experience to global practice of haploidentical hematopoietic stem cell transplantation. Front Med 13:45–56
Lu DP, Dong L, Wu T, Huang XJ, Zhang MJ, Han W, Chen H, Liu DH, Gao ZY, Chen YH, Xu LP, Zhang YC, Ren HY, Li D, Liu KY (2006) Conditioning including antithymocyte globulin followed by unmanipulated HLA-mismatched/haploidentical blood and marrow transplantation can achieve comparable outcomes with HLA-identical sibling transplantation. Blood 107:3065–3073
Baron F, Mohty M, Blaise D, Socié G, Labopin M, Esteve J, Ciceri F, Giebel S, Gorin NC, Savani BN, Schmid C, Nagler A (2017) Anti-thymocyte globulin as graft-versus-host disease prevention in the setting of allogeneic peripheral blood stem cell transplantation: a review from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation. Haematologica 102:224–234
Kröger N, Solano C, Wolschke C et al (2016) Antilymphocyte globulin for prevention of chronic graft-versus-host disease. N Engl J Med 374:43–53
Soiffer RJ, Lerademacher J, Ho V et al (2011) Impact of immune modulation with anti-T-cell antibodies on the outcome of reduced-intensity allogeneic hematopoietic stem cell transplantation for hematologic malignancies. Blood 117:6963–69670
Baron F, Labopin M, Blaise D, Lopez-Corral L, Vigouroux S, Craddock C, Attal M, Jindra P, Goker H, Socié G, Chevallier P, Browne P, Sandstedt A, Duarte RF, Nagler A, Mohty M (2014) Impact of in vivo T-cell depletion on outcome of AML patients in first CR given peripheral blood stem cells and reduced-intensity conditioning allo-SCT from a HLA-identical sibling donor: a report from the Acute Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant 49:389–396
Kumar A, Mhaskar AR, Reljic T, Mhaskar RS, Kharfan-Dabaja MA, Anasetti C, Mohty M, Djulbegovic B (2012) Antithymocyte globulin for acute-graft-versus-host-disease prophylaxis in patients undergoing allogeneic hematopoietic cell transplantation: a systematic review. Leukemia 26:582–588
Bashir Q, Munsell MF, Giralt S, de Padua Silva L, Sharma M, Couriel D, Chiattone A, Popat U, Qazilbash MH, Fernandez-Vina M, Champlin RE, de Lima MJ (2012) Randomized phase II trial comparing two dose levels of thymoglobulin in patients undergoing unrelated donor hematopoietic cell transplantation. Leuk Lymphoma 53:915–919
Finke J, Bethge WA, Schmoor C, Ottinger HD, Stelljes M, Zander AR, Volin L, Ruutu T, Heim DA, Schwerdtfeger R, Kolbe K, Mayer J, Maertens JA, Linkesch W, Holler E, Koza V, Bornhäuser M, Einsele H, Kolb HJ, Bertz H, Egger M, Grishina O, Socié G, ATG-Fresenius Trial Group (2009) ATG-Fresenius trial group. Standard graft-versus-host disease prophylaxis with or without anti-T-cell globulin in haematopoietic cell transplantation from matched unrelated donors: a randomised, open-label, multicentre phase 3 trial. Lancet Oncol 10:855–864
Zhou L, Lu DP (2019) Immune reconstitution of HLA-A*0201/BMLF1- and HLA-A*1101/LMP2-specific Epstein Barr virus cytotoxic T lymphocytes within 90 days after haploidentical hematopoietic stem cell transplantation. Virol J 16:19
Glucksberg H, Storb R, Fefer A, Buckner CD, Neiman PE, Clift RA, Lerner KG, Thomas ED (1974) Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation 18:295–304
Styczynski J, van der Velden W, Fox CP et al (2016) Sixth European Conference on Infections in Leukemia, a joint venture of the Infectious Diseases Working Party of the European Society of Blood and Marrow Transplantation (EBMT-IDWP), the Infectious Diseases Group of the European Organization for Research and Treatment of Cancer (EORTC-IDG), the International Immunocompromised Host Society (ICHS) and the European Leukemia Net (ELN). Management of Epstein-Barr Virus infections and post-transplant lymphoproliferative disorders in patients after allogeneic hematopoietic stem cell transplantation: Sixth European Conference on Infections in Leukemia (ECIL-6) guidelines. Haematologica 101:803–811
Mohty M (2007) Mechanisms of action of antithymocyte globulin: T-cell depletion and beyond. Leukemia 21:1387–1394
Popow I, Leitner J, Majdic O et al (2012) Assessment of batch to batch variation in polyclonal antithymocyte globulin preparations. Transplantation 93:32–40
Popow I, Leitner J, Grabmeier-Pfistershammer K et al (2013) A comprehensive and quantitative analysis of the major specificities in rabbit antithymocyte globulin preparations. Am J Transplant 13:3103–3013
Paiano S, Roosnek E, Tirefort Y et al (2015) Comparing two types of rabbit ATG prior to reduced intensity conditioning allogeneic hematopoietic SCT for hematologic malignancies. Bone Marrow Res 2015:980924
Brunstein CG, Weisdorf DJ, DeFor T, Barker JN, Tolar J, van Burik J, Wagner JE (2006) Marked increased risk of Epstein–Barr virus-related complications with the addition of antithymocyte globulin to a nonmyeloablative conditioning prior to unrelated umbilical cord blood transplantation. Blood 108:2874–2880
Landgren O, Gilbert ES, Rizzo JD, Socié G, Banks PM, Sobocinski KA, Horowitz MM, Jaffe ES, Kingma DW, Travis LB, Flowers ME, Martin PJ, Deeg HJ, Curtis RE (2009) Risk factors for lymphoproliferative disorders after allogeneic hematopoietic cell transplantation. Blood 113:4992–5001
Gil L, Styczyński J, Komarnicki M (2012) Strategy of pre-emptive management of Epstein-Barr virus post-transplant lymphoproliferative disorder after stem cell transplantation: results of European transplant centers survey. Contemp Oncol (Pozn) 16:338–340
Styczynski J, Gil L, Tridello G et al (2013) Infectious Diseases Working Party of the European Group for Blood and Marrow Transplantation. Response to rituximab-based therapy and risk factor analysis in Epstein Barr Virus-related lymphoproliferative disorder after hematopoietic stem cell transplant in children and adults: a study from the Infectious Diseases Working Party of the European Group for Blood and Marrow Transplantation. Clin Infect Dis 57:794–802
Alexandersson A, Koskenvuo M, Tiderman A, Lääperi M, Huttunen P, Saarinen-Pihkala U, Anttila VJ, Lautenschlager I, Taskinen M (2019) Viral infections and immune reconstitution interaction after pediatric allogenic hematopoietic stem cell transplantation. Infect Dis (Lond) 51:772–778
Mensen A, Na IK, Häfer R, Meerbach A, Schlecht M, Pietschmann ML, Gruhn B (2014) Comparison of different rabbit ATG preparation effects on early lymphocyte subset recovery after allogeneic HSCT and its association with EBV-mediated PTLD. J Cancer Res Clin Oncol 140:1971–1980
Oostenbrink LVE, Jol-van der Zijde CM, Kielsen K et al (2019) Differential elimination of anti-thymocyte globulin of fresenius and genzyme impacts t-cell reconstitution after hematopoietic stem cell transplantation. Front Immunol 10:315
Acknowledgments
We thank Editage (www.editage.cn) for the English language editing.
Funding
This research was funded by an award to Ling Zhou from the Shanghai City Health Bureau (item number: 20124209).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. The entire haploidentical hematopoietic stem cell transplantation process was performed by Zhi-yong Gao, the Director of the Hematology Department in Shanghai Dao-Pei Hospital. Material preparation, data collection, and analysis were performed by Ling Zhou. The first draft of the manuscript was written by Ling Zhou, and all authors contributed to previous versions of the manuscript. Ling Zhou wrote the final manuscript, and all authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving human participants
Our research was an observational study on patients that underwent haplo-HSCT with ATG as GVHD prophylaxis. The study was approved by the Institutional Ethics Committee of Shanghai Dao-pei Hospital and was performed in accordance with the ethical standards in the Declaration of Helsinki (1964) and its later amendments.
Informed consent
Written informed consent was provided by the patients or their guardians.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhou, L., Gao, Zy. & Lu, Dp. Comparison of ATG-thymoglobulin with ATG-Fresenius for Epstein-Barr virus infections and graft-versus-host-disease in patients with hematological malignances after haploidentical hematopoietic stem cell transplantation: a single-center experience. Ann Hematol 99, 1389–1400 (2020). https://doi.org/10.1007/s00277-020-04014-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-020-04014-5