Abstract
Although antithymocyte globulin (ATG) had been widely used in hematopoietic stem cell transplantation from unrelated donor due to its ability to prevent acute and chronic graft-versus-host disease (GVHD), the comparative efficacy and safety of ATG-Thymoglobulin (ATG-T) and ATG-Fresenius (ATG-F) in patients undergoing HLA-mismatched allogeneic peripheral blood stem cell transplantation from unrelated donors (UR-PBSCT) has not been evaluated. Retrospective analysis of patients who underwent HLA-mismatched UR-PBSCT between January 2003 and December 2013 and received pre-transplant ATG-T at a total dose of 10 mg/kg or ATG-F at a total dose of 20 mg/kg was performed. Patients who received ATG-T (n = 23) or ATG-F (n = 28) had similar baseline demographic, disease, and transplant characteristics. There were no significant between-groups differences in the probability of acute GVHD (P = 0.721) and chronic GVHD (P = 0.439). ATG-F was associated with nonsignificant trends toward higher disease-free survival at 3-year follow-up compared with ATG-T (45.7 ± 11.1 vs 61.3 ± 9.7 %, respectively, P = 0.07). A significantly greater proportion of ATG-T patients experienced high fever than ATG-F patients (P < 0.01) during ATG infusion. There was no difference in the rate of infection between the two treatment groups. There were less adverse effects comparing ATG-F with ATG-T. ATG-T at a total dose of 10 mg/kg and ATG-F at a total dose of 20 mg/kg had a similar clinical outcome in the setting of HLA-mismatched UR-PBSCT.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hematopoietic stem cell transplantation (HSCT), especially peripheral blood stem cell transplantation (PBSCT) from unrelated donor (UR), steadily increased over the past decades due to the shortage of the matched sibling donor and growing up of UR registries worldwide. Human leukocyte antigen (HLA)-matched URs are considered as the best alternative donors, but there are still many patients undergoing transplantation from HLA-mismatched UR because the probability of identifying a matched UR in the international registries is <75 % [1, 2]. Over the past decades, the outcome of PBSCT from UR has improved in part due to tailored conditioning regimens and better graft-versus-host disease (GVHD) prophylaxis [3]. GVHD prophylaxis is a crucial component of the HSCT protocol, and several T cell antibodies are available for clinical use, such as antithymocyte globulin (ATG). Patients receiving ATG had markedly less acute GVHD and chronic GVHD, and at the same time, survival and relapse were comparable in patients with or without ATG [4–6].
ATG-Thymoglobulin (ATG-T) is collected from rabbits immunized with resting human thymocytes, which create a very broad antibody spectrum on T cells at all stages of maturation [7]. ATG-Fresenius (ATG-F) is raised in rabbits against a human T-lymphoblast cell line, Jurkat cell, which result a more specific antibody spectrum toward activated T cells [8]. Today, although ATG-T or ATG-F had been routinely added to conditioning regimen of UR-HSCT, this tailored transplantation protocol improved the outcome of transplantation in part due to better immunosuppression [9, 10]. There was no standard practice about how to use those two ATG preparations in HSCT from HLA-mismatched UR [11]. It is still unclear whether the ATG-T and the ATG-F would yield different clinical outcomes, especially safety and efficacy, in patients with HLA-mismatched UR-PBSCT.
On the basis of this reason, it is necessary to systematically analyze the clinical outcomes of HLA-mismatched UR-PBSCT patients with ATG-T or ATG-F treatments. From this point of view, we undertook a retrospective study to evaluate the efficacy and side effects between these two ATG preparations on Chinese patients with HLA-mismatched UR-PBSCT in our center. To our knowledge, the present study is the first report to compare the outcomes of those two different ATG in the setting of HLA-mismatched HSCT. After this retrospective analysis of ATG-T and ATG-F applications in HSCT from HLA-mismatched UR, the present results may also help make better choice of different ATG preparations for HSCT in the setting of HLA-haploidentical/mismatched family member.
Materials and methods
Patients
The patient database at the Department of Hematology and BMT, Chinese PLA General Hospital, was screened for patients who underwent PBSCT from UR between January 2003 and December 2013. Patients were excluded if the UR was a full-matched donor. A total of 119 patients who underwent allogeneic PBSCT from UR, of whom patients received from HLA-mismatched UR, were enrolled in this study. All the patients treated with conditioning regimens with ATG-T or ATG-F before transplantation. The study protocol was reviewed and approved by the Institutional Review Board of Chinese PLA General Hospital, and written informed consents were obtained from all patients before transplantation.
Peripheral blood stem cell transplantation
Both HLA Class 1 and 2 antigens were determined by high-resolution molecular typing using polymerase chain reaction sequence-specific primers. Donor and recipient pairs were considered mismatch when any difference was found at HLA-A, HLA-B, HLA-C, HLA-DRB1, and HLA-DQ1 loci. Conditioning regimens included modified busulfan (BU)/cyclophosphamide (CY), total body irradiation (TBI)/CY, modified fludarabine (Flu)/CY and Flu/BU, as previously described [12]. All patients received ATG-T or ATG-F during conditioning regimen. In addition to ATG, basic GVHD prophylaxis strategy was attempted by administering cyclosporine A (CsA), methotrexate, and mycophenolate mofetil [12]. GVHD was diagnosed and graded according to standard international criteria [13]. Grade II or higher acute GVHD was treated with methylprednisolone, and the first-line therapy for chronic GVHD was prednisone combined with CsA. Peripheral blood stem cells (PBSCs) were isolated and collected from UR after mobilized with recombinant human granulocyte colony-stimulating factor (Filgrastim, Kirin, Japan). Recipients received unmanipulated fresh PBSCs from UR.
Assessment of safety and efficacy
ATG-T (Sangstat-Genzyme, Cambridge, MA, USA) was given on days −5, −4, −3, and −2 at dose of 2.5 mg/kg/day, and ATG-F (Fresenius Biotech GmbH, Munich, Germany) was administered on days −5, −4, −3, and −2 at a dose of 5 mg/kg/day during conditioning. On the first day, 10 % of the total amount of ATG was slowly infused intravenously over 2 h, and the remaining 90 % was infused within 8–12 h. On the following 3 days, ATG was slowly infused intravenously over 8–12 h. An hour before ATG infusion, all patients were given 40 mg methylprednisolone intravenously, an intramuscular injection of 25 mg promethazine hydrochloride, and 600 mg oral acetaminophen.
The side effects associated with ATG including fever, allergy, shock, liver and kidney dysfunction, serum sickness, and opportunistic infection were assessed by clinical and laboratory evaluations. Physical examination was performed every day from using ATG to 30 days after PBSCT. At the same time, liver function, renal function, electrolyte analysis, blood routine, and urine routine were monitored every other day. Cytomegalovirus (CMV)-DNA and Epstein-barr virus (EBV)-DNA were monitored weekly by real-time PCR. The efficacy evaluation included engraftment, incidence of acute GVHD and chronic GVHD, incidence of relapse, non-relapse-related mortality (NRM), overall survival (OS), and disease-free survival (DFS).
Definition and statistical analysis
The day of PBSCs transfusion was counted as day 0, and all intervals were calculated based on this day. The date of the last follow-up for all surviving patients was April 30, 2014. Myeloid engraftment was defined as the first of three consecutive days with an absolute neutrophil count of at least 0.5 × 109/L. Platelet engraftment was defined as the first day of blood platelet count >20 × 109/L unsupported by platelet transfusion for at least 7 days, as previously described [8]. PCR–STR analysis was used to assess and monitor engraftment and formation of chimerism. NRM was defined as death in continuous complete remission of the primary disease. OS was continuous survival until death from any cause after transplantation. DFS was defined as continuous survival without recurrence of the primary disease after UR-PBSCT.
Statistical analyses were performed using IBM SPSS, 20 (SPSS Statistics V20, IBM Corporation, Somers, New York) and R3.1.1 Package. Differences between groups for categorical variables were compared using Chi-square or Fisher’s exact tests. The Mann–Whitney U test was used to compare median values. Rates of OS and DFS were estimated using Kaplan–Meier survival curves and compared using the log-rank test. Cumulative incidence estimates were calculated in a competing risk framework for aGVHD, cGVHD, NRM, and relapse. Deaths were treated as competing events in analyses of GVHD. Deaths of relapse were treated as competing events in analyses of NRM, and deaths of NRM were treated as competing events in analyses of relapse.
Results
Patient characteristics
Until April 30, 2014, one patient in ATG-F group was lost to follow-up after 4 years post-transplantation. Baseline demographic, disease, and transplantation characteristics of patients treated with ATG-T (n = 23) or ATG-F (n = 28) were comparable (Table 1). Single-HLA-locus-mismatched patients were 13 (59.1 %) in the ATG-T group and 22 (78.6 %) in the ATG-F group. Two-loci-mismatched patients were nine (40.9 %) in the ATG-T group and six (21.4 %) in the ATG-F group. Patients were diagnosed as having different diseases, mainly including acute myeloid leukemia (27.5 %; n = 14) and acute lymphoid leukemia (33.3 %; n = 17). Forty-eight patients received myeloablative regimens with modified BU/CY or TBI/CY, except for two acute aplastic anemia patients with Flu/CY regimen in ATG-T group and one old patient with reduced intensity regimen Flu/BU in ATG-F group. The median time from diagnosis to PBSCT was 9 months for both the ATG-T and ATG-F (P = 0.819). The median follow-up was 29 (2.5–67) months and 34 (5.0–47) months for the ATG-T and ATG-F, respectively.
Engraftment and GVHD
Except one patient in ATG-T group who was not evaluable because of early death during conditioning, other 50 evaluable patients were analyzed for both engraftment and GVHD. For the 50 patients who survived more than 1 month after transplantation, all patients achieved myeloid engraftment at a median of 13 days (range 11–18 days) and 15 days (range 11–20 days) in the ATG-T and ATG-F groups, respectively (P = 0.161). Platelet engraftment was achieved at a median of 21 days (range 14–28 days) in the ATG-T group, whereas 24 days (range 16–56 days) in the ATG-F group (P = 0.111). All the 50 recipients achieved full donor chimerism at +28 day after UR-PBSCT.
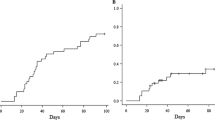
As shown in Fig. 1, both groups had similar incidence and severity of acute GVHD. The overall cumulative incidence of aGVHD was 40.9 ± 10.8 and 35.7 ± 9.3 % in the ATG-T and ATG-F groups, respectively (P = 0.721). The rates of grade II–IV aGVHD were 26.1 ± 10.4 and 26.9 ± 8.9 % in the ATG-T group and ATG-F group, respectively (P = 0.898). A nonsignificant difference in the cumulative incidence of cGVHD between ATG-T group and ATG-F group (37.5 ± 12.7 vs 25.0 ± 9.1 %, respectively, P = 0.439) is shown in Fig. 2. Although the cumulative incidence of extensive cGVHD was lower in the ATG-F compared with the ATG-T group at 2-year follow-up [(4.5 ± 4.5) vs (31.9 ± 12.4) %, respectively], the difference was not statistically significant (P = 0.118).
Non-relapse-related mortality and relapse
There were no significant differences between the ATG-T group and ATG-F group in the 100-day NRM rate [(21.7 ± 8.8) vs (14.3 ± 6.7) %, respectively, P = 0.474] and the 2-year NRM rate [(30.4 ± 9.9) vs (28.6 ± 8.7) %, respectively, P = 0.785]. Relapse rate at 2-year follow-up in ATG-T group and ATG-F group was 17.4 ± 8.1 and 7.6 ± 5.3 %, respectively (P = 0.158).
Survival
The 3-year OS rates for the ATG-T and ATG-F groups were 51.4 ± 10.6 and 66.0 ± 9.3 %, respectively (P = 0.282). As shown in Fig. 3, compared with the ATG-T group, the ATG-F group had a higher DFS rate at 3-year follow-up (45.7 ± 11.1 vs 61.3 ± 9.7 %, respectively), but there was no marked difference (P = 0.07). The causes of death for the 12 patients in ATG-T group were disease relapse (41.7 %; n = 5), infection (25 %; n = 3), GVHD (8.2 %; n = 1), heart failure (8.3 %; n = 1), severe allergic reaction followed with shock (8.3 %; n = 1), and severe serum sickness (8.3 %; n = 1). For the nine patients who died in ATG-F group, two (22.2 %) patients died from disease relapse, five (55.6 %) from infection, one (11.1 %) from GVHD, and one (11.1 %) from heart failure.
Adverse events associated with ATG
Table 2 summarizes the rates of adverse events in the two kind ATG groups. A significantly lower percentage of patients treated with ATG-F experienced fever than patients treated with ATG-T (46.4 vs 91.3 %, respectively, P = 0.001), whereas no patients in the ATG-F group reported a body temperature ≥40 °C, seven patients receiving ATG-T had this adverse reaction (P = 0.002). There were no statistically significant differences between groups in the incidence of serum sickness, CMV antigenemia, opportunistic infection, and elevation of alanine transaminase, total bilirubin, creatinine and urea nitrogen.
Unexpectedly, two patients in the ATG-T group died from ATG-related adverse reactions: one died from shock and acute renal failure due to ATG anaphylaxis and another from severe serum sickness. There were no deaths directly associated with adverse reactions to ATG-F.
Discussion
Unrelated donor, HLA-mismatch, and PBSCs (versus other sources of stem cells) increase the risk of GVHD in HSCT [14, 15]. ATG is often administered as part of the UR-HSCT conditioning regimen to prevent GVHD [9]. This retrospective study is the first to compare efficacy and side effects of ATG-T versus ATG-F at fixed doses in patients undergoing HLA-mismatched UR-PBSCT. Neither acute GVHD, nor chronic GVHD had significant difference between ATG-T and ATG-F groups. There was no survival difference when comparing patients who received ATG-T with those who received ATG-F. These findings suggest that ATG-T and ATG-F at fixed dose as presented in this study have similar GVHD, non-relapse morality, relapse, and survival for HLA-mismatched UR-PBSCT.
Although two retrospective researches compared efficacy of ATG-T and ATG-F in UR-HSCT [16, 17], findings regarding the protective effects of those two different ATG preparations were conflicting. There still was no report to compare ATG-T and ATG-F in HLA-mismatched UR-HSCT. Remberger et al. [16] reported that patients undergoing UR-HSCT in the ATG-T group were less likely to experience severe aGVHD, but the ATG-F group had a significantly lower rate of relapse and a tendency to improve 4-year survival. In that study, the total dose of ATG-T ranges from 8.5 to 23.5 mg/kg (n = 61) and the total dose of ATG-F ranges from 19 to 51.5 mg/kg (n = 26) [16]. Basara et al. [17] compared outcomes in patients with acute myeloid leukemia undergoing UR-HSCT who were treated with ATG-T (total dose range, 5-15 mg/kg; n = 49) and ATG-F (total dose, 45–60 mg/kg; n = 38). Patients treated with ATG-F had a significantly lower rate of cGVHD (despite more extensive use of PBSCT) than those treated with ATG-T, but ATG-T was associated with a significantly lower risk of relapse. Unlike our study, those two studies differed in terms of ATG dose and administration schedule, stem cell source, disease status, and other potentially confounding variables; it is not possible to draw definitive conclusions regarding the relative efficacy of those two different ATG preparations in UR-HSCT, let alone in the setting of HLA-mismatched UR-PBSCT.
Although studies have demonstrated dose-dependent effects for both ATG-T [18] and ATG-F [19], there is a paucity of data to help determine the optimal dose of each product and the equipotent doses of the two preparations. The immunosuppressive and toxic effects of ATG necessitate the use of an optimal dose to effectively prevent GVHD. In dose-finding studies of ATG-T, a total dose of 6–8 mg/kg achieved a good balance between efficacy and toxicity, while a lower dose (4 mg/kg) had inadequate efficacy and a higher dose (10–15 mg/kg) increased the risk of infection [12–22]. In the present study, all ATG-T-treated patients received a total dose of 10 mg/kg, which is toward the middle of the range of ATG-T doses evaluated in previous research. In an ATG-F dose-finding study, UR-HSCT patients with various hematologic malignancies treated with 30 or 60 mg/kg of ATG-F found comparable rates of aGVHD and cGVHD in the two groups, but significantly lower 2-year NRM and a nonsignificant trend toward better 2-year DFS in the 30 mg/kg group, mainly because of a higher incidence of fatal infections in the higher-dose group [23]. The results of this study suggest that a lower dose of ATG-F, similar to that used in the present study, may be as effective as higher doses in preventing GVHD with a lower risk of adverse effects. In the present study, both ATG preparations were associated with ~40 % incidence of acute GVHD. Similar rates of this complication were reported in studies evaluating ATG-T at doses of 8–10 mg/kg despite a significantly lower percentage of patients receiving PBCST than in the present study [20, 22, 24]. The rate of aGVHD associated with ATG-F in the present study was lower than rates of this complication reported in studies using higher doses of ATG-F [22, 25]. In the present study, ATG-T was associated with a (37.5 ± 12.7) % incidence of 2-year cGVHD, which is consistent with rates of this complication (25–51 %) reported in studies evaluating ATG-T at doses ranging from 7.5 to 10 mg/kg [18, 20–22]. The cumulative incidence of 2-year cGVHD in the ATG-F group in the present study (25.0 ± 9.1 %) was less than that reported in several other studies of ATG-F which included fewer PBSCT patients [22, 25] and was similar to the cGVHD rate in bone marrow transplant patients who received high-dose ATG-F of 60–120 mg/kg [19]. These data suggest that ATG-F at dose of 20 mg/kg is effective in preventing GVHD in HLA-mismatched UR-PBSCT recipients who have increased risk of acute and chronic GVHD.
A major limitation of ATG in preventing GVHD is the risk of infectious complications caused by ATG-related delay in immune reconstitution [26]. The relationship between ATG preparation and dose, time to engraftment, and infection risk remains unclear. It has been suggested that ATG-T may carry a higher risk of infection than ATG-F due to its broader spectrum of activity [19]. The present study contradicts this supposition: Median times to neutrophil and platelet engraftment, and the rate of opportunistic infection (~50 %), were similar in both ATG groups at fixed dose. In the present study, patients who received 20 mg/kg ATG-F had faster neutrophil and platelet engraftment times than those reported for UR-HSCT patients (80 % of whom received PBSCT) who received 60 mg/kg ATG-F in a randomized controlled phase 3 trial [25]. In the phase 3 study, the delayed neutrophil and platelet engraftment associated with ATG-F treatment did not increase the rate of infection [25]. In contrast, a retrospective study found that delayed engraftment was associated with an increased risk of infection in patients receiving ATG-T [20]. Results of the present study suggest that ATG preparations do not differentially affect hematological reconstitution and risk of infection. However, randomized, controlled studies are needed to establish the relative effects of different ATG preparations and doses on time to engraftment and infection risk in HLA-mismatched UR-PBSCT.
Results of the present support suggest that ATG-F may be associated with a lower risk of acute ATG-related adverse reactions, which may be serious and cause considerable discomfort for the recipients [27]. ATG administration frequently causes high fever and chills. A longer profusion time (12 vs 4 h), similar to that used in this study for both ATG treatments, has been shown to substantially attenuate the serious acute side effects associated with ATG. In a previous retrospective comparative study that used median doses of ATG-T and ATG-F similar to the fixed doses in the present study, chills and high fever were more common following administration of ATG-T than ATG-F [16]. The present study corroborates this finding as ATG-F was associated with a significantly lower incidence of chills and high fever than ATG-T, which may translate to improved quality of life, and recipients of ATG-F group had better physical fitness to tolerate transplantation procedure. In addition, it is worthwhile to note that both deaths related to ATG treatment in the present study (shock associated with anaphylaxis and serum sickness) occurred in the ATG-T group.
Conclusion
The present study, which constitutes the first comparison of two commonly used ATG preparations administered at fixed doses in HLA-mismatched UR-PBSCT patients, suggests that ATG-F is effective as ATG-T but less adverse effects. However, this study had limitations inherent in a retrospective design with a relatively small number of patients. There is a need for well-designed and sufficiently powered randomized clinical trials to establish the optimal timing, dose and schedule, and to compare the efficacy and safety, of ATG preparations in HLA-mismatched UR-PBSCT.
References
Valcárcel D, Sierra J, Wang T, Kan F, Gupta V, Hale GA, et al. One-antigen mismatched related versus HLA-matched unrelated donor hematopoietic stem cell transplantation in adults with acute leukemia: Center for International Blood and Marrow Transplant Research results in the era of molecular HLA typing. Biol Blood Marrow Transplant. 2011;17(5):640–8.
Cheuk DK. Optimal stem cell source for allogeneic stem cell transplantation for hematological malignancies. World J Transplant. 2013;3(4):99–112.
Anasetti C, Logan BR, Lee SJ, et al. Peripheral-blood stem cells versus bone marrow from unrelated donors. N Engl J Med. 2012;367(16):1487–96.
Bacigalupo A. Matched and mismatched unrelated donor transplantation: is the outcome the same as for matched sibling donor transplantation? Hematology Am Soc Hematol Educ Program. 2012;2012:223–9. doi:10.1182/asheducation-2012.1.223.
Pidala J, Tomblyn M, Nishihori T, et al. ATG prevents severe acute graft-versus-host disease in mismatched unrelated donor hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2011;17(8):1237–44.
Sheng Z, Ma H, Pang W, Niu S, Xu J. In vivo T-cell depletion with antithymocyte globulins improves overall survival after myeloablative allogeneic stem cell transplantation in patients with hematologic disorders. Acta Haematol. 2013;129(3):146–53.
Gaber AO, Monaco AP, Russell JA, Lebranchu Y, Mohty M. Rabbit antithymocyte globulin (thymoglobulin): 25 years and new frontiers in solid organ transplantation and haematology. Drugs. 2010;70(6):691–732.
Mueller TF. Thymoglobulin: an immunologic overview. Curr Opin Organ Transplant. 2003;8:305–12.
Kumar A, Mhaskar AR, Reljic T, et al. Antithymocyte globulin for acute-graft-versus-host-disease prophylaxis in patients undergoing allogeneic hematopoietic cell transplantation: a systematic review. Leukemia. 2012;26(4):582–8.
Penack O, Fischer L, Gentilini C, et al. The type of ATG matters: natural killer cells are influenced differentially by Thymoglobulin Lymphoglobulin and ATG-Fresenius. Transpl Immunol. 2007;18(2):85–7.
Ayuk F, Diyachenko G, Zabelina T, et al. Anti-thymocyte globulin overcomes the negative impact of HLA mismatching in transplantation from unrelated donors. Exp Hematol. 2008;36(8):1047–54.
Huang WR, Li HH, Gao CJ, et al. Unmanipulated HLA-mismatched/haploidentical peripheral blood stem cell transplantation for high-risk hematologic malignancies. Transfusion. 2012;52(6):1354–62.
Sullivan KM. Graft-versus-host disease. In: Blume KG, Forman SJ, Appelbaum FR, editors. Thomas’ hematopoietic Cell transplantation. 3rd ed. Malden: Blackwell Publishing Ltd; 2004. p. 633–64.
Shin SH, Yoon JH, Yahng SA, et al. PBSC vs BM grafts with myeloablative conditioning for unrelated donor transplantation in adults with high-risk ALL. Bone Marrow Transplant. 2014;49(6):773–9.
Pidala J. Graft-vs-host disease following allogeneic hematopoietic cell transplantation. Cancer Control. 2011;18(4):268–76.
Remberger M, Svahn BM, Hentschke P, Lofgren C, Ringden O. Effect on cytokine release and graft-versus-host disease of different anti-T cell antibodies during conditioning for unrelated haematopoietic stem cell transplantation. Bone Marrow Transplant. 1999;24(8):823–30.
Basara N, Baurmann H, Kolbe K, et al. Antithymocyte globulin for the prevention of graft-versus-host disease after unrelated hematopoietic stem cell transplantation for acute myeloid leukemia: results from the multicenter German cooperative study group. Bone Marrow Transplant. 2005;35(10):1011–8.
Bacigalupo A, Lamparelli T, Bruzzi P, et al. Antithymocyte globulin for graft-versus-host disease prophylaxis in transplants from unrelated donors: 2 randomized studies from Gruppo Italiano Trapianti Midollo Osseo (GITMO). Blood. 2001;98(10):2942–7.
Schleuning M, Gunther W, Tischer J, Ledderose G, Kolb HJ. Dose-dependent effects of in vivo antithymocyte globulin during conditioning for allogeneic bone marrow transplantation from unrelated donors in patients with chronic phase CML. Bone Marrow Transplant. 2003;32(3):243–50.
Remberger M, Svahn BM, Mattsson J, Ringden O. Dose study of thymoglobulin during conditioning for unrelated donor allogeneic stem-cell transplantation. Transplantation. 2004;78(1):122–7.
Duval M, Pedron B, Rohrlich P, et al. Immune reconstitution after haematopoietic transplantation with two different doses of pre-graft antithymocyte globulin. Bone Marrow Transplant. 2002;30(7):421–6.
Meijer E, Cornelissen JJ, Lowenberg B, Verdonck LF. Antithymocyteglobulin as prophylaxis of graft failure and graft-versus-host disease in recipients of partially T-cell-depleted grafts from matched unrelated donors: a dose-finding study. Exp Hematol. 2003;31(11):1026–30.
Ayuk F, Diyachenko G, Zabelina T, et al. Comparison of two doses of antithymocyte globulin in patients undergoing matched unrelated donor allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2008;14(8):913–9.
Remberger M, Storer B, Ringden O, Anasetti C. Association between pretransplant Thymoglobulin and reduced non-relapse mortality rate after marrow transplantation from unrelated donors. Bone Marrow Transplant. 2002;29(5):391–7.
Finke J, Bethge WA, Schmoor C, et al. Standard graft-versus-host disease prophylaxis with or without anti-T-cell globulin in haematopoietic cell transplantation from matched unrelated donors: a randomised, open-label, multicentre phase 3 trial. Lancet Oncol. 2009;10(9):855–64.
Waller EK, Langston AA, Lonial S, et al. Pharmacokinetics and pharmacodynamics of anti-thymocyte globulin in recipients of partially HLA-matched blood hematopoietic progenitor cell transplantation. Biol Blood Marrow Transplant. 2003;9(7):460–71.
Pihusch R, Holler E, Muhlbayer D, et al. The impact of antithymocyte globulin on short-term toxicity after allogeneic stem cell transplantation. Bone Marrow Transplant. 2002;30(6):347–54.
Acknowledgments
The authors wish to acknowledge all the physicians, nurses, and supporting personnel for their dedicated care of patients in this study. This study was supported by the Capital Health Research and Development of Special (No. 2011-5001-07).
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Huang, W., Zhao, X., Tian, Y. et al. Outcomes of peripheral blood stem cell transplantation patients from HLA-mismatched unrelated donor with antithymocyte globulin (ATG)-Thymoglobulin versus ATG-Fresenius: a single-center study. Med Oncol 32, 32 (2015). https://doi.org/10.1007/s12032-014-0465-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-014-0465-y