Abstract
This study aimed to clarify the clinical and prognostic role of body composition and metabolic parameters extracted from baseline 18F-FDG PET/CT in patients with diffuse large B-cell lymphoma (DLBCL). We retrospectively collected the clinicopathological and 18F-FDG PET/CT parameters of 181 DLBCL patients. The indexes of skeletal muscle, subcutaneous adipose tissue, and visceral adipose tissue were calculated using the area measured at the 3rd lumbar level normalized for height. Additionally, the metabolic activity of corresponding muscle and adipose tissue, and maximum standardized uptake value (SUVmax), metabolic tumor volume (MTV), and total lesion glycolysis (TLG) of all lesions were measured. Survival endpoints included progression-free survival (PFS) and overall survival (OS). We identified 75 (41.4%) patients with low skeletal muscle index (sarcopenia), presenting risk factors including male, high β2-microglobulin, low BMI, high visceral adipose tissue index, low SUVmax of skeletal muscle, and high SUVmax of visceral adipose tissue. Male, low BMI, low visceral adipose tissue index, and high SUVmax of subcutaneous adipose tissue were risk factors for low subcutaneous adipose tissue index diagnosed in 105 (58.0%) patients. In total, 132 (79.2%) patients represented low visceral adipose tissue index, associated with younger age, B symptoms, and low BMI. Eastern Cooperative Oncology Group (ECOG) status, sarcopenia, and visceral adipose tissue index were found independently predictive of PFS and OS, while β2-microglobulin was independently predictive of OS. In conclusion, body composition indexes were correlated with both clinical characteristics and 18F-FDG PET/CT metabolic parameters, significantly impacting survival, such that sarcopenia and high visceral adipose tissue index were powerful predictors of poor DLBCL outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common pathological subtype of lymphoma, accounting for approximately 40% of non-Hodgkin lymphomas (NHLs) [1]. Unlike other solid malignancies such as lung cancer and pancreatic cancer, the survival of newly diagnosed DLBCL patients varies greatly. Although the first-line regimen of rituximab plus CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) significantly improves the outcomes of DLBCL patients, nearly one-third of patients still have poor outcomes [2]. The International Prognostic Index (IPI) based on age, lactate dehydrogenase, number of extranodal involvements, Ann Arbor staging, and Eastern Cooperative Oncology Group (ECOG) status is a classic clinical tool for risk stratification and outcome prediction in DLBCL patients. However, the IPI was developed in the era when patients only received chemotherapy (mainly CHOP regimen). With the application of rituximab, the predictive ability of the IPI for patient outcomes has obviously decreased [3]. Therefore, identifying better prognostic indicators for DLBCL is urgently needed.
It has previously been reported that body composition indexes such as muscle and adipose tissue are closely associated with long-term survival in various diseases. Sarcopenia, defined as a progressive and systemic skeletal muscle disease characterized by loss of muscle mass and strength, is prevalent among cancer patients [4,5,6]. The most accepted diagnostic criterion for sarcopenia is the skeletal muscle index, which is derived from the skeletal muscle area as measured at the level of the third lumbar vertebra (L3) on CT images. Studies have shown a significant correlation between sarcopenia and the prognosis of malignancies such as lymphoma, esophageal cancer, and lung cancer [7,8,9]. Similarly, recent evidence has suggested that subcutaneous and visceral adipose tissues were associated with outcomes of patients with malignancy including lymphoma [10, 11]. Furthermore, the indexes of subcutaneous and visceral adipose tissues can be also calculated at the L3 level on CT images.
18F-FDG PET/CT has been incorporated into clinical practice guidelines and consensus of lymphoma, which plays a pivotal role in evaluating clinical staging, treatment response, and prognosis for DLBCL patients [12, 13]. The metabolic parameters provided by 18F-FDG PET/CT, including maximum standardized uptake value (SUVmax), metabolic tumor volume (MTV), and total lesion glycolysis (TLG), have been utilized to predict the survival for lymphoma patients [14, 15]. Meanwhile, whole-body 18F-FDG PET/CT examinations include both PET and CT images at the L3 level, which can be used to not only measure the indexes of skeletal muscle, subcutaneous adipose tissue, and visceral adipose tissue, but also obtain metabolic parameters of body composition indexes including SUVmax of muscle and adipose tissue at the same level. Therefore, this study aimed to utilize 18F-FDG PET/CT to investigate the clinical and prognostic role of metabolic parameters and body composition (muscle and adipose tissue) in DLBCL patients, and identify valid prognostic indicators for DLBCL.
Materials and methods
Study cohort
A total of 181 consecutive adult patients with DLBCL were recruited from August 2016 to October 2021. Criteria for selecting the subjects were as follows: (1) pathological diagnosis of DLBCL; (2) underwent baseline 18F-FDG PET/CT scan; and (3) received R-CHOP-based regimen. Patients who did not complete the first-line therapies were excluded in this study. Baseline clinicopathological features were collected, including age, gender, body mass index (BMI), B symptoms (defined as the presence of at least one of the following manifestations: fever, night sweat, and weight loss), serum lactate dehydrogenase (LDH), β2 microglobulin (β2M), absolute lymphocyte count, absolute monocyte count, ECOG performance status (PS), Ann Arbor stage, the number of extranodal sites, International Prognostic Index (IPI), cell of origin (GCB or non-GCB phenotype according to the Hans algorithm), and follow-up data.
18F-FDG PET/CT scan
Before image acquisition, patients were required to fast for at least 6 h and avoid strenuous exercise for 24 h, and the level of fasting blood glucose levels was < 11.0 mmol/L. PET/CT scan was conducted about 60 ± 5 min after intravenous injection of 18F-FDG (3.7 MBq/kg) using Biograph 16 scanner (Siemens Healthineers, Erlangen, Germany). PET images from the skull base to the midthigh were obtained from six or seven bed positions with 2.5 min per bed. A low-dose CT scan was acquired in the same scanner using the following parameters: 120 kV, 75 mA with auto mA, 0.75-mm pitch, 0.5-s tube rotation, and slice thickness of 5 mm. PET images were reconstructed using the ordered subset expectation maximization (OSEM) algorithm and CT-based attenuation correction was applied.
PET metabolic parameters analysis
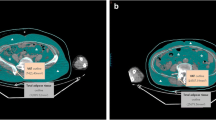
PET/CT images were analyzed by two nuclear medicine physicians who were blinded to the clinical information of the DLBCL patients and worked independently of each other, using the Syngo workstation (Siemens Healthineers, Erlangen, Germany). PET/CT metabolic parameters including SUVmax, MTV, and TLG were recorded for all lesions. TLG was calculated by multiplying SUVmean by MTV, using a threshold of 40% of the SUVmax. The SUVmax values of skeletal muscle, subcutaneous adipose tissue, and visceral adipose tissue were measured at the L3 level (Fig. 1a–c).
Example of SUVmax of skeletal muscle (a), subcutaneous adipose tissue (b), and visceral adipose tissue (c) measured on a cross-sectional PET/CT image at the L3 level and outlined in red, blue, and green, respectively.Example of skeletal muscle area (d), subcutaneous adipose tissue area (e), and visceral adipose tissue area (f) measured on a cross-sectional CT image at the L3 level and outlined in red, blue, and green, respectively
Muscle and adipose tissue measurements
Muscle and adipose tissue measurements were calculated from the CT component of PET/CT using ImageJ software (version 2.9.0). The tissue cross-sectional areas (cm2) at the L3 vertebrae level were determined based on standard Hounsfield units (HU) thresholds, with values of –29 to 150 HU for skeletal muscle, –190 to –30 HU for subcutaneous adipose tissue, and –150 to –50 HU for visceral adipose tissue (Fig. 1d–f). The skeletal muscle area and adipose tissue area were normalized for height (m2) to calculate the indexes of skeletal muscle, subcutaneous adipose tissue and visceral adipose tissue (cm2/m2). According to previous recommendations, sarcopenia is defined as skeletal muscle index < 44.77 cm2/m2 for males and skeletal muscle index < 32.50 cm2/m2 for females [16]. The survival receiver operating characteristic curve (ROC) package was used to determine the appropriate cutoff values of subcutaneous and visceral adipose tissue indexes, which were then divided into high and low groups.
Follow-up
The median follow-up time was 35 months, ranging from 1 to 77 months. The endpoint events of follow-up were progression-free survival (PFS) and overall survival (OS). PFS was defined as the interval between the date of the diagnosis and the date of disease progression or death. OS was defined as the interval between the date of the diagnosis and the date of the death. For patients without death, cases were defined as censored at the date of their last follow-up.
Statistical analysis
Data management and analysis were performed using SPSS (version 26.0, IBM Corp) or R (version 4.2.2, R Foundation for Statistical Computing). Continuous variables were expressed as median and range, and categorical variables were expressed as frequencies. Independent-sample t test or Mann–Whitney U test was used to compare continuous variables, and chi-squared test or Fisher’s exact test was used to compare categorical variables. Factors that were significantly associated with skeletal muscle, subcutaneous adipose tissue, and visceral adipose tissue indexes in the univariate analysis were entered into a multiple logistic regression model using a forward selection strategy, respectively. The correlation of indexes was calculated with the Spearman correlation coefficient. The stepwise multivariate Cox proportional hazards regression model was employed to determine independent prognostic factors. Kaplan–Meier method was applied to plot the survival curves, and survival estimates were compared by log-rank tests. When continuous variables were converted into categorical variables, clinical cutoff values were preferred. If not available, the survival ROC package was used to determine the appropriate cutoff value of continuous variables. P < 0.05 was considered statistically significant.
Results
Patients’ clinicopathological and PET/CT findings
A total of 181 patients with DLBCL (GCB vs non-GCB, 68 vs 113) were included in the study, and their characteristics are outlined in Table 1. The study population consisted of 99 males and 82 females, with a median age of 60 years (range, 22–83 years) at disease onset. Most patients were with absent B symptoms (143/181, 79.0%), Ann Arbor stage III–IV (103/181, 71.8%), and less (0–1) extranodal sites (128/181, 70.7%). Furthermore, 37.6% (68/181), 30.4% (55/181), and 42.0% (76/181) patients had elevated serum LDH, β2M, and absolute monocyte count level, while 35.4% (64/181) patients had decreased serum absolute lymphocyte count level.
At the diagnosis of DLBCL, only 9.4% patients (17/181) had ECOG performance status of 2–4, indicating poor physical condition. Based on IPI risk stratification, 66.9% patients (121/181) had low or low–intermediate risk (score 0–2), and 33.1% patients (60/181) had high–intermediate or high risk (score 3–5). Moreover, the median BMI and the indexes of skeletal muscle, subcutaneous adipose tissue, and visceral adipose tissue of all enrolled patients were 22.41 kg/m2 (range, 13.63–34.05 kg/m2), 40.63 cm2/m2 (range, 25.10–64.53 cm2/m2), 40.93 cm2/m2 (range, 1.47–105.38 cm2/m2), and 34.28 cm2/m2 (range, 0.83–115.03 cm2/m2).
All lesions of these 181 patients showed FDG-avid on PET/CT imaging, with median SUVmax, MTV, and TLG of 19.9 (range, 2.0–105.0), 62.1 (range, 1.3–5350.4), and 691.5 (range, 6.9–42,143.0), respectively. Additionally, the median SUVmax of skeletal muscle, subcutaneous, and visceral adipose tissue values were 1.15 (range, 0.50–2.33), 0.37 (range, 0.13–0.96), and 0.64 (range, 0.24–1.61), respectively. More detailed baseline PET/CT metabolic parameters are shown in Table 1.
Factors associated with muscle and adipose tissue indexes
As shown in Table 2, 75 out of 181 patients were diagnosed with low skeletal muscle index (sarcopenia). Male, higher serum β2M level, lower BMI, lower subcutaneous and visceral adipose tissue indexes, higher MTV and TLG, and lower SUVmax of skeletal muscle were correlated with low skeletal muscle index (P < 0.05). Based on the survival ROC analysis, the best cutoff values of subcutaneous and visceral adipose tissue indexes were 44.16 cm2/m2 and 49.60 cm2/m2, respectively. Low vs. high subcutaneous adipose tissue index was observed in 105 vs 76 patients, and low vs high visceral adipose tissue index was in 132 vs. 49 patients, respectively. Male, present B symptoms, elevated serum LDH level, lower BMI and visceral adipose tissue index, higher MTV and TLG, lower SUVmax of skeletal muscle, and higher SUVmax of subcutaneous and visceral adipose tissue were associated with low subcutaneous adipose tissue index (P < 0.05). Besides, lower age, present B symptoms, lower BMI, lower skeletal muscle and subcutaneous adipose tissue indexes, and lower SUVmax of skeletal muscle were associated with low visceral adipose tissue index (P < 0.05).
Furthermore, Table 3 displayed the results of multivariate logistic regression analysis, which revealed that sex, serum β2M level, BMI, visceral adipose tissue index, and SUVmax of skeletal muscle and visceral adipose tissue were independent predictors of low skeletal muscle index (P < 0.05). Moreover, sex, BMI, visceral adipose tissue index, and SUVmax of subcutaneous adipose tissue were independent predictors of low subcutaneous adipose tissue index (P < 0.05). Besides, age, B symptoms, and BMI were independent predictors of low visceral adipose tissue index (P < 0.05).
Correlations between BMI, and muscle and adipose tissue indexes
For these 181 DLBCL patients, BMI had a positive correlation with the indexes of skeletal muscle, subcutaneous adipose tissue, and visceral adipose tissue (Rs = 0.57, 0.54, and 0.66; P < 0.001). In addition to that, visceral adipose tissue index also had a positive correlation with skeletal muscle and subcutaneous adipose tissue indexes (Rs = 0.27 and 0.55; P < 0.001). There was no significant correlation between skeletal muscle and subcutaneous adipose tissue indexes (P = 0.607) (Fig. 2).
Survival analysis
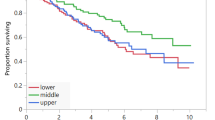
Among the 181 DLBCL patients, 38 patients experienced disease progression, and 32 of those patients died. The 5-year PFS and OS rates were 96.1% and 96.7%, respectively. Univariate Cox regression analysis revealed that age, LDH, β2M, ECOG, IPI, cell of origin, MTV, TLG, sarcopenia, and visceral adipose tissue index were associated with both PFS and OS (P < 0.05, Table 4). Multivariate analysis demonstrated that ECOG (PFS: HR = 3.159, 95%CI = 1.337–7.462, P = 0.009; OS: HR = 3.044, 95%CI = 1.222–7.585, P = 0.017), sarcopenia (PFS: HR = 2.355, 95%CI = 1.150–4.820, P = 0.019; OS: HR = 2.265, 95%CI = 1.041–4.931, P = 0.039), and visceral adipose tissue index (PFS: HR = 1.021, 95%CI = 1.006–1.037, P = 0.008; OS: HR = 1.023, 95%CI = 1.006–1.041, P = 0.008) were independent predictors of both PFS and OS, while serum β2M (HR = 2.963, 95%CI = 1.266–6.937, P = 0.012) was only independently predictive for OS (Table 4).
Kaplan–Meier analyses for PFS and OS are depicted in Fig. 3. The results showed that DLBCL patients with sarcopenia had significantly shorter PFS (P = 0.036) and OS (P = 0.044) than non-sarcopenic patients. Furthermore, the patients with high visceral adipose tissue index had significantly poorer PFS (P < 0.001) and OS (P < 0.001) compared to the low group patients.
Discussion
18F-FDG PET/CT is a routine examination for DLBCL patients, which allows for the acquisition of body composition and metabolic parameters without increasing additional costs or risks. In our study, the incidence of sarcopenia in DLBCL patients was 41.1% which falls within the range of 23.9–55.6% reported in previous studies [17]. Sarcopenia has a complex etiology, with aging as one of the most important factors in the general population [6]. However, possibly due to the relatively advanced age of DLBCL patients in our cohort (median age of 60 years), we did not observe age as a predictive value for sarcopenia, which is inconsistent with previous DLBCL-related studies [18, 19]. Consistent with our previous studies, the results showed that male, lower BMI, and lower metabolic activity of skeletal muscle were associated with the increased probability of sarcopenia [20]. Notably, both metabolic activity and index of visceral adipose tissue were significantly correlated with sarcopenia, probably attributed to the abnormal distribution of adipose tissue and adipose infiltration into skeletal muscle, leading to muscle loss. Furthermore, very little was found in the literature on the question of β2M levels and sarcopenia, but we observed that higher β2M levels indicated the incidence of sarcopenia. The same result was observed in elderly patients with end-stage renal disease [21]. β2M is the light chain subunit of the class I antigen of the major histocompatibility complex and is associated with host immunity. However, the mechanism underlying the correlation between β2M and sarcopenia remains unclear.
Unlike muscle tissue, the definitions and evaluation methods for adipopenia and adiposity vary greatly across different studies, and a consensus has yet to be reached [22,23,24,25]. It is well known that subcutaneous adipose tissue is lower in men than women [26], as observed in our study. And in a large-scale population study of 59,429 adults conducted in China, a younger age was associated with lower visceral adipose tissue area, while there was no significant difference in subcutaneous adipose tissue area between different age groups. Besides, both subcutaneous and visceral adipose tissue areas were positively correlated with BMI levels [27]. These findings are consistent with our own study. Among them, visceral adiposity is a key driver of cardiovascular disease [28], which may be linked to higher visceral adipose tissue index in older patients. Furthermore, FDG uptake of adipose tissue reflects glucose metabolism during fatty acid synthesis in adipocytes, as a marker of adipose tissue inflammation state [29]. And in our study, the area of subcutaneous adipose tissue decreased as its metabolic activity increased, suggesting a potential self-regulatory phenomenon, which was also observed in visceral adipose tissue [30].
Although our and other studies showed that BMI had positive correlation with the indexes of skeletal muscle, subcutaneous adipose tissue, and visceral adipose tissue, it cannot distinguish between muscle and adipose tissue, making it a simple but imperfect index for evaluating the prognosis of patients with malignancy [24]. Changes in muscle and adipose tissue distribution have important effects on cancer patients, especially skeletal muscle loss and increased visceral adipose tissue, which indicate a worse prognosis in various tumor diseases [24, 31]. Indeed, we found that sarcopenia (low skeletal muscle index) and high visceral adipose tissue index were the true determining factors for worse PFS and OS in patients, rather than BMI. A recent meta-analysis of 12 retrospective studies showed that sarcopenia was still associated with lower survival rates in DLBCL patients, even after adjusting for confounding factors [17]. However, as of now, the effect of subcutaneous and visceral adipose tissue on the survival of DLBCL patients has not yet reached a consensus. In the literature about elderly (> 70 years) DLBCL patients, those with decreased subcutaneous and visceral adipose tissue had shorter OS [32], which was inconsistent with our study. Compared to subcutaneous adipose tissue, visceral adipose tissue produces more pro-inflammatory cytokines and growth factors, affecting cell proliferation and diffusion, as well as response to treatment, promoting inflammation and tumor progression, and leading to a poor prognosis eventually [33,34,35].
ECOG performance status is a practical tool for assessing the overall health status and daily activity ability of cancer patients, commonly used to assist in guiding clinical decisions and prognosis [36]. Our study also showed that ECOG was an independent predictor of prognosis in DLBCL patients, which was in line with previous studies. Prior studies have noted that serum β2M can serve as an important prognostic indicator for DLBCL, and a similar result was observed in the present study. Although the prognostic value of β2M in DLBCL has been repeatedly confirmed, the underlying mechanisms have not been fully elucidated. These findings suggest a role for host immunity in cancer biology, and related biomarkers may provide prognostic information [37, 38].
There are certain limitations to this study. Firstly, this is a retrospective single-center study involving only the Asia cohort, which might limit the extrapolation of our findings to other cohorts. Secondly, sarcopenia is also characterized by a decline in muscle function, such as grip strength and gait speed, and the relationship between these functional parameters and prognosis needs further investigation. Third, due to the lack of consensus on the definition of adipopenia and adiposity, it is unclear whether the cutoff values used in this study could be applicable to other regions and populations. Therefore, further prospective large-scale multicenter studies and validation in other malignancies are required to confirm these results. In spite of its limitations, the study certainly adds to our understanding of the role of 18F-FDG PET/CT in assessing body composition for patients with DLBCL.
Conclusion
Body composition indexes of muscle and adipose tissue were associated with clinical features and 18F-FDG PET/CT metabolic parameters. The comprehensive analysis of body composition and metabolic parameters using 18F-FDG PET/CT has shed light on their impact on the survival of DLBCL patients, and identified sarcopenia and high visceral adipose tissue index as independent risk factors for poor DLBCL prognosis.
Data availability
Data are available based on the reasonable request to the corresponding author.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A et al (2021) Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71:209–249. https://doi.org/10.3322/caac.21660
Sehn LH, Salles G (2021) Diffuse large B-cell lymphoma. N Engl J Med 384:842–858. https://doi.org/10.1056/NEJMra2027612
Ruppert AS, Dixon JG, Salles G, Wall A, Cunningham D, Poeschel V et al (2020) International prognostic indices in diffuse large B-cell lymphoma: a comparison of IPI, R-IPI, and NCCN-IPI. Blood 135:2041–2048. https://doi.org/10.1182/blood.2019002729
Chen LK, Liu LK, Woo J, Assantachai P, Auyeung TW, Bahyah KS et al (2014) Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc 15:95–101. https://doi.org/10.1016/j.jamda.2013.11.025
Meza-Valderrama D, Marco E, Dávalos-Yerovi V, Muns MD, Tejero-Sánchez M, Duarte E et al (2021) Sarcopenia, malnutrition, and cachexia: adapting definitions and terminology of nutritional disorders in older people with cancer. Nutrients 13. https://doi.org/10.3390/nu13030761
Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T et al (2019) Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 48:16–31. https://doi.org/10.1093/ageing/afy169
Fang P, Zhou J, Xiao X, Yang Y, Luan S, Liang Z et al (2023) The prognostic value of sarcopenia in oesophageal cancer: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle 14:3–16. https://doi.org/10.1002/jcsm.13126
Yang M, Shen Y, Tan L, Li W (2019) Prognostic value of sarcopenia in lung cancer: a systematic review and meta-analysis. Chest 156:101–111. https://doi.org/10.1016/j.chest.2019.04.115
Zilioli VR, Albano D, Arcari A, Merli F, Coppola A, Besutti G et al (2021) Clinical and prognostic role of sarcopenia in elderly patients with classical Hodgkin lymphoma: a multicentre experience. J Cachexia Sarcopenia Muscle 12:1042–1055. https://doi.org/10.1002/jcsm.12736
Shin DY, Kim A, Byun BH, Moon H, Kim S, Ko YJ et al (2016) Visceral adipose tissue is prognostic for survival of diffuse large B cell lymphoma treated with frontline R-CHOP. Ann Hematol 95:409–416. https://doi.org/10.1007/s00277-015-2571-0
Besutti G, Massaro F, Bonelli E, Braglia L, Casali M, Versari A et al (2021) Prognostic impact of muscle quantity and quality and fat distribution in diffuse large B-cell lymphoma patients. Front Nutr 8:620696. https://doi.org/10.3389/fnut.2021.620696
Zelenetz AD, Gordon LI, Chang JE, Christian B, Abramson JS, Advani RH, et al. NCCN Guidelines® insights: B-cell lymphomas, Version 5.2021. J Natl Compr Canc Netw. 2021;19:1218–30. https://doi.org/10.6004/jnccn.2021.0054
Barrington SF, Mikhaeel NG, Kostakoglu L, Meignan M, Hutchings M, Müeller SP et al (2014) Role of imaging in the staging and response assessment of lymphoma: consensus of the International Conference on Malignant Lymphomas Imaging Working Group. J Clin Oncol 32:3048–58. https://doi.org/10.1200/jco.2013.53.5229
Ceriani L, Martelli M, Zinzani PL, Ferreri AJ, Botto B, Stelitano C et al (2015) Utility of baseline 18FDG-PET/CT functional parameters in defining prognosis of primary mediastinal (thymic) large B-cell lymphoma. Blood 126:950–956. https://doi.org/10.1182/blood-2014-12-616474
Sasanelli M, Meignan M, Haioun C, Berriolo-Riedinger A, Casasnovas RO, Biggi A et al (2014) Pretherapy metabolic tumour volume is an independent predictor of outcome in patients with diffuse large B-cell lymphoma. Eur J Nucl Med Mol Imaging 41:2017–2022. https://doi.org/10.1007/s00259-014-2822-7
Zeng X, Shi ZW, Yu JJ, Wang LF, Luo YY, Jin SM et al (2021) Sarcopenia as a prognostic predictor of liver cirrhosis: a multicentre study in China. J Cachexia Sarcopenia Muscle 12:1948–1958. https://doi.org/10.1002/jcsm.12797
Xu XT, He DL, Tian MX, Wu HJ, Jin X (2022) Prognostic value of sarcopenia in patients with diffuse large B-cell lymphoma treated with R-CHOP: a systematic review and meta-analysis. Front Nutr 9:816883. https://doi.org/10.3389/fnut.2022.816883
Nakamura N, Hara T, Shibata Y, Matsumoto T, Nakamura H, Ninomiya S et al (2015) Sarcopenia is an independent prognostic factor in male patients with diffuse large B-cell lymphoma. Ann Hematol 94:2043–2053. https://doi.org/10.1007/s00277-015-2499-4
Lanic H, Kraut-Tauzia J, Modzelewski R, Clatot F, Mareschal S, Picquenot JM et al (2014) Sarcopenia is an independent prognostic factor in elderly patients with diffuse large B-cell lymphoma treated with immunochemotherapy. Leuk Lymphoma 55:817–823. https://doi.org/10.3109/10428194.2013.816421
Yuan H, Tan X, Sun X, He L, Li D, Jiang L (2023) Role of (18)F-FDG PET/CT and sarcopenia in untreated non-small cell lung cancer with advanced stage. Jpn J Radiol 41:521–530. https://doi.org/10.1007/s11604-022-01369-9
Kim JK, Choi SR, Choi MJ, Kim SG, Lee YK, Noh JW et al (2014) Prevalence of and factors associated with sarcopenia in elderly patients with end-stage renal disease. Clin Nutr 33:64–68. https://doi.org/10.1016/j.clnu.2013.04.002
Choi H, Park YS, Na KJ, Park S, Park IK, Kang CH et al (2021) Association of adipopenia at preoperative PET/CT with mortality in stage I non-small cell lung cancer. Radiology 301:645–653. https://doi.org/10.1148/radiol.2021210576
Ebadi M, Tandon P, Moctezuma-Velazquez C, Ghosh S, Baracos VE, Mazurak VC et al (2018) Low subcutaneous adiposity associates with higher mortality in female patients with cirrhosis. J Hepatol 69:608–616. https://doi.org/10.1016/j.jhep.2018.04.015
Fujiwara N, Nakagawa H, Kudo Y, Tateishi R, Taguri M, Watadani T et al (2015) Sarcopenia, intramuscular fat deposition, and visceral adiposity independently predict the outcomes of hepatocellular carcinoma. J Hepatol 63:131–140. https://doi.org/10.1016/j.jhep.2015.02.031
Ebadi M, Martin L, Ghosh S, Field CJ, Lehner R, Baracos VE et al (2017) Subcutaneous adiposity is an independent predictor of mortality in cancer patients. Br J Cancer 117:148–155. https://doi.org/10.1038/bjc.2017.149
Iliodromiti S, Celis-Morales CA, Lyall DM, Anderson J, Gray SR, Mackay DF et al (2018) The impact of confounding on the associations of different adiposity measures with the incidence of cardiovascular disease: a cohort study of 296 535 adults of white European descent. Eur Heart J 39:1514–1520. https://doi.org/10.1093/eurheartj/ehy057
Zeng Q, Wang L, Dong S, Zha X, Ran L, Li Y et al (2021) CT-derived abdominal adiposity: distributions and better predictive ability than BMI in a nationwide study of 59,429 adults in China. Metabolism 115:154456. https://doi.org/10.1016/j.metabol.2020.154456
Neeland IJ, Ross R, Després JP, Matsuzawa Y, Yamashita S, Shai I et al (2019) Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: a position statement. Lancet Diabetes Endocrinol 7:715–725. https://doi.org/10.1016/s2213-8587(19)30084-1
Reijrink M, de Boer SA, Antunes IF, Spoor DS, Heerspink HJL, Lodewijk ME et al (2021) [(18)F]FDG uptake in adipose tissue is not related to inflammation in type 2 diabetes mellitus. Mol Imaging Biol 23:117–126. https://doi.org/10.1007/s11307-020-01538-0
Van de Wiele C, Van Vlaenderen M, D’Hulst L, Delcourt A, Copin D, De Spiegeleer B et al (2017) Metabolic and morphological measurements of subcutaneous and visceral fat and their relationship with disease stage and overall survival in newly diagnosed pancreatic adenocarcinoma : metabolic and morphological fat measurements in pancreatic adenocarcinoma. Eur J Nucl Med Mol Imaging 44:110–116. https://doi.org/10.1007/s00259-016-3525-z
Okumura S, Kaido T, Hamaguchi Y, Kobayashi A, Shirai H, Yao S et al (2017) Visceral adiposity and sarcopenic visceral obesity are associated with poor prognosis after resection of pancreatic cancer. Ann Surg Oncol 24:3732–3740. https://doi.org/10.1245/s10434-017-6077-y
Camus V, Lanic H, Kraut J, Modzelewski R, Clatot F, Picquenot JM et al (2014) Prognostic impact of fat tissue loss and cachexia assessed by computed tomography scan in elderly patients with diffuse large B-cell lymphoma treated with immunochemotherapy. Eur J Haematol 93:9–18. https://doi.org/10.1111/ejh.12285
Crudele L, Piccinin E, Moschetta A (2021) Visceral adiposity and cancer: role in pathogenesis and prognosis. Nutrients 13. https://doi.org/10.3390/nu13062101
Doyle SL, Donohoe CL, Lysaght J, Reynolds JV (2012) Visceral obesity, metabolic syndrome, insulin resistance and cancer. Proc Nutr Soc 71:181–189. https://doi.org/10.1017/s002966511100320x
Lutz CT, Quinn LS (2012) Sarcopenia, obesity, and natural killer cell immune senescence in aging: altered cytokine levels as a common mechanism. Aging (Albany NY). 4:535–46. https://doi.org/10.18632/aging.100482
Neeman E, Gresham G, Ovasapians N, Hendifar A, Tuli R, Figlin R et al (2019) Comparing physician and nurse Eastern Cooperative Oncology Group Performance Status (ECOG-PS) ratings as predictors of clinical outcomes in patients with cancer. Oncologist 24:e1460–e1466. https://doi.org/10.1634/theoncologist.2018-0882
Chen Y, Neelapu S, Feng L, Bi W, Yang TH, Wang M et al (2016) Prognostic significance of baseline peripheral absolute neutrophil, monocyte and serum β2-microglobulin level in patients with diffuse large b-cell lymphoma: a new prognostic model. Br J Haematol 175:290–299. https://doi.org/10.1111/bjh.14237
Chen H, Zhong Q, Zhou Y, Qin Y, Yang J, Liu P et al (2022) Enhancement of the International prognostic index with β2-microglobulin, platelet count and red blood cell distribution width: a new prognostic model for diffuse large B-cell lymphoma in the rituximab era. BMC Cancer 22:583. https://doi.org/10.1186/s12885-022-09693-z
Funding
This work was supported by the fund from the National Natural Science Foundation of China (81971645), Guangdong Provincial People’s Hospital (KY0120211130), and Guangdong Provincial Key Laboratory of Artificial Intelligence in Medical Image Analysis and Application (2022B1212010011).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethics approval
All procedures performed in studies involving human participants were approved by the ethics committee at Guangdong Provincial People’s Hospital, and with the principles of the 1964 Declaration of Helsinki and its later amendments.
Informed consent
Informed consent was waived due to the retrospective nature of this study.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, Y., Chen, Z., Tan, X. et al. Role of body composition and metabolic parameters extracted from baseline 18F-FDG PET/CT in patients with diffuse large B-cell lymphoma. Ann Hematol 102, 2779–2789 (2023). https://doi.org/10.1007/s00277-023-05379-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-023-05379-z